Introduction
Since 2010, the Ministry of Health and Social Protection and Colciencias in Colombia, alongside with a group of experts have been drafting evidence based clinical practice guidelines for critical health issues for the Colombian population. One of the evidence based integrated guidelines (GAI in Spanish) drafted in 2011, were the Integrated Care Guidelines for the Prevention, Early Detection and Treatment during Pregnancy, Childbirth and Puerperium complications. These guidelines, included a section dedicated to recommendations for pregnancy related infections, specially toxoplasmosis.
Toxoplasmosis, may lead to severe neurological impairment, and compromise the visual health of the infant and (in most cases) is caused by a primary infection in an immunocompetent pregnant woman. There are several programs worldwide focused in detection during pregnancy2-4. For more than a decade there was a debate over the efficacy of prenatal treatment; however recent publications that include a systematic review comparing cohorts in Europe, North America and South America 6, as well other observational studies8, indicate there is a benefit in prenatal treatment to prevent serious conditions in the infant. On the other hand, findings indicate that in South America, the infection is more severe than in Europe or North America, not only because of the higher frequency of cases but because there are more severe clinical presentations and a higher mortality rate6,7, given the higher virulence of the strains found in this geographical area8-11.
In Colombia, more than half of pregnant women (50-60%) have anti-Toxoplasma antibodies, suggesting there is a high exposition and circulation of the parasite in the country 12,11. It is expected that between 0.6 to 3% of women acquire the infection during pregnancy. The risk is higher for adolescents, with a risk for seroconversion of 1.5% and lower for pregnant women 35 or older, with seroconversion risk of 0.7%1. There is information, about the differences in the frequencies of congenital infection in Colombia. For example, Florencia (Caquetá) and Armenia (Quindío) have the highest percentages (3 and 6/1.000 born alive, respectively); whilst Cucuta and Riohacha had the lowest percentages (0.5 and 0.7/1.000 born alive respectively). For the first time an epidemiological study on congenital toxoplasmosis, could indicate the relationship between high and low frequency of congenital toxoplasmosis risk markers and high or low rainfall levels in each city14.
The recommendations contained in this section of the Integral Care Guidelines (ICG) for Pregnancy, Childbirth, and Puerperium are addressed to clinical care personnel who provide care to women in the prevention, early detection, and care of toxoplasmosis in pregnancy, childbirth, or puerperium across the different levels of health care (family doctors, general practitioners, rural physicians, specialists in Obstetrics and Gynecology, Internal Medicine, Infectious Diseases, Pediatrics, Neonatology, Nursing professionals and other health professionals). It also targets, indirectly, administrative decision makers, both in hospital related contexts and insurers, health care payers and health policies makers.
We included information for 9 clinical questions related to the infection management during pregnancy:
1. What are the risk factors for transmission of toxoplasmosis during pregnancy?
2. What are the recommendations for the primary prevention of Toxoplasma infection during pregnancy?
3. What is the recommended follow-up of a seronegative pregnant woman? How should it be monitored?
4. Which Toxoplasma antibody test should be ordered first?
5. What are the recommended confirmatory tests for toxoplasmosis?
6. Is ultrasound recommended to determine the severity of fetal involvement in a fetus with positive tests for Toxoplasma infection?
7. What is the secondary prevention scheme (prevention of fetal transmission) recommended for women diagnosed with a pregnancy acquired infection?
8. What are the recommended tests for establishing the diagnosis of congenital infection in the newborn?
9. What is the recommended therapy for newborns diagnosed with congenital infection?
During the outlining of this clinical practice guideline, the group that developed the guidelines (CDG) for the ICG for prevention, early detection, and treatment of complications during pregnancy, childbirth or puerperium followed the criteria contained in the methodological guidelines for the elaboration of ICG that are a part of the General System of Social Security in Colombian Health and were developed by the Ministry of Health and Social Protection along with Colciencias, the Center for Studies and Research in Health of the Fundación Santa Fe de Bogota and Harvard Public Health School. University15. The information generated during the different stages of the process was published in the web pages of the Ministry of Health and Social Protection, as well as in the GDG webpage:
http://gpc.minsalud.gov.co/gpc_sites/Repositorio/Conv_500/ GPC_embarazo/gpc_embarazo.aspx.
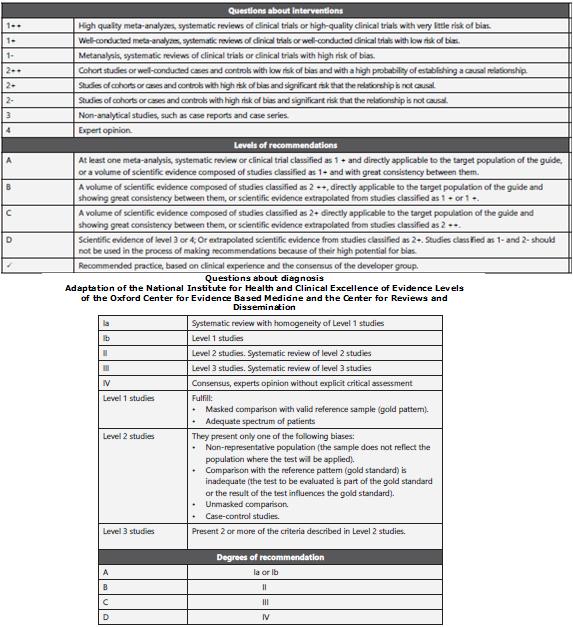
The guidelines were developed through systematic review of literature, two panels of evaluation, gradation of evidence by using SIGN classification (Scottish Intercollegiate Guidelines Network), discussion panel with users, patients, external evaluation and socioeconomical evaluation15
Clinical questions and recommendations included in the section
What are the risk factors for the transmission of toxoplasmosis during pregnancy?
Toxoplasmosis is a highly preventable disease and studies on risk factors for infection during pregnancy have been able to identify variables associated with its acquisition. However, the epidemiology of toxoplasmosis varies from country to country. Being aware of risk factors may suggest recommendations for prevention and education programs.
Summary and description of the body of evidence
Socio-demographic risk factors
Age: There is evidence that the prevalence of toxoplasmosis increases with age. In Colombia, the National Health Study of 1980 found that the prevalence increased from 32% in <10 years to 65% in people aged 60 years or over 13. A study in Taiwan found a significant difference in antibodies against T. gondii among adults (28.3%) and children (18.7%), with a higher frequency of seropositivity (38.1%) in the 50-59 age group, and the lowest frequency (7.7%) in the 1-9 age group16. The increase in seroprevalence with age is a predictable result due to the increasing duration of exposure to T. gondii
Gender: no evidence has been found about significant differences in the prevalence of antibodies against T. gondii between men and women. It is worth stating that the increase in the risk of seropositivity in men found in a 134-person's prevalence study was explained by the authors as caused by carelessness at the time of cleaning and food preparation17.
Race: there is evidence from European studies that have found differences in the prevalence of T. gondii amongst native and immigrant populations; However, this difference can be explained by geographic and epidemiological factors of infection acquisition rather than by ethnic or genetic factors of the host18. In a study conducted in Norway, foreign women had a higher incidence of toxoplasmosis than native women (0.60 and 22.6%, respectively). On the other hand, in France, such prevalence was higher among native women of France in comparison with immigrant women18.
Area of residence: the incidence of toxoplasmosis differs even within the same country. Annual mean rainfall is a factor that has recently been associated with these differences. In the 1988 National Health Study in Colombia, the highest prevalence was found in the Atlantic coast region (63%), while the lowest was found in the Central region (36%)13. This same heterogeneity was identified in the first multicentric Colombian study for neonatal toxoplasmosis19, which found 3 different prevalence levels in the country: low prevalence (Riohacha and Cucuta), intermediate (Bogota, Barranquilla and Bucaramanga) and high prevalence cities (Armenia and Florencia). Differences in prevalence were associated with average rainfall levels (intensity), but not with temperature or height above sea level. This factor is not surprising since it is already known that in humid conditions, the oocyst is preserved for long periods of time19. On the other side, pregnant women living in Oslo had T. gondii faction incidence 5 times higher than those pregnant women living outside Oslo, a fact attributed to the greater proportion of foreign women living in the city. The highest prevalence rates (13.4%) were found in counties, where there is a mild climate and the close to the coastline and the lowest prevalence (6.7%) was found in the counties where there was a dry climate with cold winters and hot summers20. Similarly, a study in Chile found a progressive increase in the seroprevalence of toxoplasmosis at higher altitudes in comparison to the regions in the country with lower altitude; This phenomenon is probably related to geographic conditions and the type of meat consumption21.
Biological risk factors
Pregnancy: in Colombia´s 1980 National Health Study, there was a higher seroprevalence of T. gondii in the subsample of pregnant women compared to the rest of the population13. Cellular immunity plays a major role in the resistance of the host to infection by T. gondii; A Th1 cytokine profile is necessary for protection and control of the infection. The production of progesterone during pregnancy and an increased expression in the HLAG molecule, which inhibits the induction of natural killer cells, are important factors in preventing fetal rejection by the mother and lead to a reduction of immune cellular functions; Therefore, in pregnant women, there is evidence of immunophysiological factors that would increase the susceptibility to T. gondii infection or other intracellular organisms22.
Number of births: There is evidence that women with children have a higher prevalence of T. gondii infection that increases proportionally to the increase in the number of pregnancies. In a study of risk factors in Brazil, a history of childbirth had an odds ratio (OR) of 14 (confidence interval [CI] 95% 2.8-68) after controlling for age 23. In another study performed in Sweden, there was an increased risk of T. gondii as the number of births increased. This can be explained by the same reduction factor in the immune response derived from the increased expression of the HLAG molecule during pregnancy24.
Genetic predisposition: there is evidence of an association between human leukocyte antigen (HLA) and susceptibility to T. gondii infection. Among Caucasians, the frequency of DQ3 HLADQ gene alleles was significantly higher in infants with hydrocephalus infected with Toxoplasma (78%) than in infected infants without hydrocephalus (48%) or in normal controls. Although there is no significant association between HLA antigens, the total absence of HLA-B51 antigen in the mothers of patients with ocular toxoplasmosis was observed. The HLA characterization performed in these patients revealed an increase in the frequency of HLA-Bw62 antigen in patients with severe ocular impairment, indicating a possible relationship between the severity of ocular toxoplasmosis and an immunogenic factor. Recently, polymorphisms have been identified in the gene encoding an adenosine triphosphate (ATP) transporter protein, the ABCA4 subfamily A, and an increased probability of ocular and cerebral disease, likewise polymorphisms in the COL2A1 gene, which codifies collagen type II (predominant in ocular tissue), are associated with an increased probability of ocular involvement in children with Congenital toxoplasma infection.
Immunodeficiency: there is evidence of the relationship between the severity of infection by T. gondii and the immune status of the infected person. While toxoplasmosis in immunocompetent adolescents or adults is usually asymptomatic, in immunocompromised individuals it causes significant morbidity and mortality. Immunosuppression caused by acquired immunodeficiency syndrome, therapies for malignant diseases, transplants or lymphoproliferative disorders may result in the reactivation of a pre-existing latent toxoplasma infection, leading to broad clinical spectrum expressions, especially in the central nervous system (CNS), where it produces intracerebral lesions. Toxoplasmosis is a common opportunistic infection in patients with advanced human immunodeficiency virus (HIV); HIV positive pregnant women had higher rates of T. gondii positive titers (21.1%) compared to HIV seronegative women (13.1%). T. gondii infection can cause serious complications in pregnant women infected with HIV, giving rise to congenital defects, spontaneous abortion, fetal death, mental retardation, blindness, epilepsy, etc.26.
Risk factors linked to lifestyle
Exposure to cats: the risk of acquiring toxoplasmosis associated to cat contact varies from region to region. The transmission of cat secreted oocysts to humans depends on age, nutritional status and level of risk of acquiring the infection by the cat itself27. Among cats, seroprevalence is high, varying between 21 and 87% in Latin America28,29.
In Colombia, a study with 170 cats from Bogota and Armenia (Quindío) found an infection prevalence of 45%; However, there were large differences in seroprevalence among the 137 cats in Bogota (35%) compared to the 33 cats studied in Armenia (84%). In 15 animals in which Toxoplasma was isolated, 3 were classified as type I, 1 type II and 11, a combination of type I and type 11110. Likewise, a seroepidemiological study performed in Bangladesh found a significant difference in the prevalence of antibodies against T. gondii between those with and without household cats. (24 vs. 11%, respectively, p = 0.01)30. A study in Illinois, USA, also reported an association between cats infected with T. gondii and an increased risk of human infections due to soil contact as a probable mechanism of transmission17. In contrast, the European multicentric study of risk factors did not find a strong association between having cat and T. gondii infection31. In fact, the association between cats and human toxoplasmosis is difficult to evaluate by epidemiological studies because the soil is the main reservoir of the infection, not cats. Although gardening activity was not associated with seropositivity, there was a non-significant lower seroprevalence in gardeners who always wore gloves compared to those who did not use them.
Recently, a serological test that identifies antibodies by exposure to oocysts has been developed, which would help to identify if the infection was acquired by contact with cats or oocysts in water, and if results are negative for these oocyst proteins, undercooked meat could be considered the source of infection32.
Contaminated food: intake of cysts through infected meat is an important source of T. gondii infection in humans. In Colombia, it has been found that the risk for Toxoplasma infection with the intake of raw or half-cooked meat has a 13.2-fold higher risk33. In a multicentric European study, it was reported that undercooked meat accounted for between 30% and 63% of infections in different parts of the European continent. T. gondii has been detected in even 1 out of 67 ready-to-eat cured meat samples in the UK, suggesting that curing methods do not kill all tissue cysts.
The prevalence of cysts in the tissues of different kinds of nimal meat varies considerably; The highest one was reported in sheep (23.9%) and porcine (12-15%), and the lowest in cattle (0-10%) and hen (0.3-8%). Pork has been the meat most commonly associated with the transmission of toxoplasmosis by food. In a 1994, in a study of 47 pig farms in Illinois, USA. 17% were contaminated with Toxoplasma oocysts. Antibodies against T. gondii were also detected in the serum of 6.9% of horses slaughtered for food in North America. In addition, a significant difference was found in the seroprevalence of T. gondii among poorly and adequately cooked meat recipients (19.5 and 9.6%, respectively).
In Colombia, a study of 180 meat samples for human consumption obtained in the 3 departments of the Eje Cafetero, 52.7% were positive for T. gondii.
Pork meat had the highest level of contamination (70%), with a higher prevalence in Manizales (80%), followed by beef in Armenia with 80%, and, finally, chicken meat in Pereira had the highest prevalence (70%) compared to chicken meat in other cities. There were no positive samples for chicken in Manizales34.
Drinking raw water: In pregnancy, drinking tap or unfiltered ater increases the risk of infection, when compared to the consumption of bottled or filtered water. The first outbreak of toxoplasmosis affecting the largest number of people in the world was reported in 1955 and it was associated with the municipal water supply of a reservoir in the city of Victoria in the province of British Columbia, in Canada32. Of the 94 individuals associated with the outbreak of acute cases living in the capital district of the region, 83 (88%) lived in the area served by a water distribution system. The incidence rate of acute infection among persons residing in the area served by the reservoir was 3times higher than areas served by other sources (relative risk 3.53, 95% CI, 1.88-6.63), and acute T. gondii infection in 3,812 pregnant women was associated with consumption of unboiled municipal water consumption32. Drinking beverages made with unboiled water was an important risk factor for T. gondii infection in pregnant women in Armenia, Colombia33. Therefore, the consumption of unboiled water a risk factor for Toxoplasma infection, and the consumption of bottled or filtered water reduces the risk33. As in the case of Giardia or Cryptosporidium, chlorination is not sufficient to remove Toxoplasma in treated waters, and filtration is required to reduce transmission. A study done in France found that 1 in 6 samples of water had Toxoplasma deoxyribonucleic acid content, and there was a proposal for the detection of the messenger of ribonucleic acid in water using polymerase chain reaction (PCR) with reverse transcriptase assay to monitor drinkable water sources and to be able to prevent contamination from the parasite in the population35.
What are the recommendations for primary prevention of Toxoplasma infection during pregnancy?
Primary prevention strategies such as health education arise as elements of vital importance to avoid Toxoplasma infection; Its success depends on the modification of habits and lifestyles that are permeated by strong cultural components.
Summary and description of the body of evidence
A cohort study involving 27,827 pregnant women portrayed the experience in an obstetrics clinic during 1979-2001 in the prevention of Toxoplasma infection36. In phase 1 (1979-1982), no specific advice was given, while in phase 2 (1983-1990), women received a list of written instructions on primary prevention plus a full explanation by an attending physician. In the third phase (1991-2001), women received a leaflet withfurther explanation of congenital toxoplasmosis and a verbal repetition of recommendations during prenatal classes in the middle of the gestational period. There was a sustained decrease in seroconversion rates over the three periods, from 1.43, 0.53 to 0.09%. A reduction of 63% in the incidence of seroconversion between phase 1 and 2 and of 92% between the first and third phases was calculated. (Level of evidence 2-)
A study including several successive cross-sectional studies included 8,267 patients in 4 obstetrical rooms in Poland. During the 1991-1997 interval, researchers, using questionnaires administered by trained interviewers, measured the knowledge and serological status of 4,311 women, between 1991-1992. Later, between 1995-1996, knowledge during pregnancy and postpartum was evaluated in 1,246 women as well as the serological status, knowledge and behavior in 2,710 women in 1997. The prevalence of Toxoplasma antibodies dropped during the 1991 period -1992 to 1997, going from 58.9 to 44.0%. Knowledge considered as "good" increased from 24.3 to 45.3%. Among women analyzed after childbirth, the knowledge about prevention measures increased from 45.5 to 80.3%. However, the lack of knowledge did not predict "unhealthy" behavior, and about half of the women who thought they had insufficient knowledge adopted correct behaviors. Among the 2,710 women surveyed in 1997, 60% had heard of toxoplasmosis on TV or women's magazines, compared with less than the 40% through radio, newspapers and providers of health services. (Level of evidence 3). A controlled clinical study was conducted for 432 pregnant women from an urban and rural health agency in Ontario, Canada38. In some prenatal education classes, a 10-minute module based on the knowledge of toxoplasmosis, the effect of the disease on the fetus and the prevention strategies were randomly provided. Preventive measures focused on cat hygiene, food hygiene and personal hygiene. In the control group, toxoplasmosis was not specifically mentioned unless the patient was asked about the disease. Pre- and postclass questionnaires were conducted with respect to demographic information, knowledge, and specific behaviors. The sample was determined retrospectively among the women who completed both questionnaires. Of the 432 women who answered the previous questionnaire, only 285 (66%) answered the questionnaire after the classes. The response time varied between 5 weeks and 5 months. A scale was developed that scored in favor of preventive behaviors. At the end of the study, women in the intervention group had a better score than those in the control group regarding cat hygiene (p <0.05). There is also an improvement in the cooking behavior of more kinds of meat. The high loss of patients and the absence of an outcome based on Toxoplasma infection limits the usefulness of this study. (Level of evidence 2+)
An unpublished ECC included 5,023 pregnant women part of a prenatal care clinics group in 7 departments in the Lyon, France area39. The effects of providing a 20-page booklet with 4 pages mainly aimed at modes of transmission and prevention measures coupled with an audio recording covering the most frequently asked questions during pregnancy, specially toxoplasmosis related questions were assessed. At the beginning of the study, no significant differences were found between the 2 arms of the study regarding levels of knowledge or behavior nor significant differences between the 2 groups in demographic or social aspects were found. In the 2 adjusted regression models, no significant association was found between group assignment and follow-up behaviors. Consumption of cooked beef "always" versus "less than always" was marginally associated with the study arm (OR 1.21, p 0.08). Handwashing "always" after contact with soil, raw meat or unwashed vegetables also showed no association with intervention (OR 1.01, 95% CI 0.8-1.2). However, other variables such as baseline knowledge of the behaviors related to toxoplasmosis (OR 1.2, 95% CI 1.0-1.4), smoking (OR 1.3, 95% CI 1, 0-1,6) and alcohol consumption (OR 1.2, 95% CI 1.0-1.5) were associated with behavioral changes. Only 0.43% of the pregnant women presented seroconversion for toxoplasmosis: 13 / 2,591 (5 / 1,000) in the experimental group and 4 / 1,358 (3 /1,000) in the control group (p=0.35). (Level of evidence 1-).
Interpretation and discussion of evidence
Evidence assessment on the effectiveness of education during pregnancy for the prevention of toxoplasmosis presents weaknesses, given the methodological problems found in the studies. Although the Canadian and French studies were clinical trials with randomized interventions and control groups, there are serious limitations on the conclusions due to the losses in the follow-up and the outcomes used. The highest quality study on educational strategies in France found no significant changes in risk behavior despite detailed and continuous information39. Only one study allows to provide information in brochures that include simple and precise measurements.
The GDG considered that toxoplasmosis prevention recommendations should include topics such as personal hygiene (hand washing), hygiene in food processing (cleaning of surfaces, proper washing of vegetables), in the preparation of meat and Its adequate cooking, as well as the use of filtered or boiled water, per the risk factors identified for Toxoplasma infection. It should be emphasized that chlorination is insufficient for the elimination of oocysts.
Clinical recommendation
B As part of prenatal control, it is advisable to make recommendations to the patients regarding: the consumption of well-cooked meats, consumption of safe water and hygienic food handling, hand washing after gardening, manipulation of animals (Cats), to prevent Toxoplasma infection.
What is the recommended follow-up for a seronegative pregnant woman? How should she be monitored?
The seronegative woman for Toxoplasma is the primary goal of pregnancy screening programs. These pregnant women are susceptible to infection, and are the ones who must have a serological follow-up to detect if the infection has been acquired. In Europe, there are programs that include monthly monitoring (France), quarterly (Austria) or no monitoring (Denmark), and it is necessary to establish if these programs are applicable to the Colombian context.
Summary and description of the body of evidence
A systematic review of cohort studies of 1,438 mothers and children diagnosed during prenatal and neonatal screening programs evaluated the effect of treatment on vertical transmission and clinical manifestations of toxoplasmosis before the age of 1 5. Analyzes were adjusted per gestational age at the time of maternal seroconversion and other variables such as geographic latitude. A reduction in transmission was found related to the time elapsed between seroconversion and initiation of treatment: If treatment was started within the first 3 weeks of seroconversion, the risk was estimated at OR 0.48 (95% CI 0.28 -0.80); Risk ratio increasing to 0.6 (95% CI 0.4-1.0) if treatment was started at week 4 or between week 5 and 8 (OR 0.6, 95% CI 0.36-1, 0). As for the effect on the reduction of symptoms in the child, the publication only included the results of the analysis were presented in 550 European live infected infants, not including the American cohorts, and no evidence was found that the prenatal treatment reduced the risk of Clinical manifestations (OR 1.11, 95% CI 0.61-2.02). The non-inclusion of the American cohorts was ince in Colombia and Brazil, tomography was used for the diagnosis of brain lesions, while ultrasound was used in Europe (Level of evidence 2 ++).
Analysis of the unpublished results of the Systematic Review on Congenital Toxoplasmosis included 691 children, including the American cohorts excluded in the previously presented analysis40. There was a decrease in the risk of congenital infection related to the time of initiation of treatment:the estimated risk for the presentation of clinical signs in the newborn with prenatal treatment was 0.4 (95% CI 95 -0.9) with spiramycin initiated 4 weeks after seroconversion; A risk of 0.6 (95% CI 0.4-0.99) if it was initiated after 4 weeks of seroconversion, was also considered (Level of evidence 2 ++) An ECC for 276 patients evidenced that most immunoglobulin M (IgM) tests by ELISA and ISAGA obtained sensitivities over 98% for the detection of seroconversions, in comparison to any other test41 (Level of evidence 1 b).
Interpretation and discussion of evidence
The analysis and direct comparison of the results of the European screening programs were not able to conclude whichcis the best strategy, given the geographic heterogeneity forcthe risk of infection42. Clustered data from systematic reviewscof observational cohorts across continents shows that the benefit of prenatal treatment to reduce transmission is only obtained if treated prior to seroconversion week 4; After this period of infection, there is uncertainty as to whether there is any benefit to the treatment. For this reason, seronegative follow-ups in seronegative with more than four weeks' intervals between tests would not allow cases to be detected early enough to obtain a significant treatment effect. Regarding the test of choice for such follow-up, the ELISA or SAGA test for anti-Toxoplasma IgM detection allows detection of infection 15 days after the infection is acquired and in this case, it is recommended as first line test. It is necessary to highlight that there must be a quality control of the tests to guarantee seroconversion detection.
Which are the Toxoplasma antibody tests that should be ordered first?
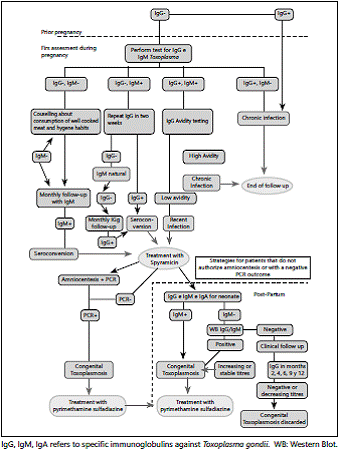
There are numerous tests for the diagnosis of toxoplasmosis during pregnancy; the common goal is to differentiate if he is infection acquired during pregnancy or before pregnancy. Antibodies detection as markers of recent infection can be performed with tests based on specific IgM detection methods such as immunofluorescence (IF), immunoassay,chemiluminescence, Western blot (WB) or immunoagglutinon. Unlike what happens in other diseases, IgM, although a marker of acute disease, can remain positive for several months, making difficult the differentiation between acute and old infection. Strategies can also be used to distinguish a chronic infection from an acute infection by measuring the avidity of immunoglobulin G (IgG), the presence of specific antibody isotypes such as IgM, immunoglobulin A (IgA), immunoglobulin E (IgE), or Differential agglutination (AC / HS). Thus, it is necessary to make recommendations for practice that allows to select the most appropriate strategy for the detection of infection acquired during pregnancy (Fig. 1).
Summary and description of the body of evidence
Identification of chronic toxoplasmosis
A systematic review of 11 studies of diagnostic tests found that the sensitivity of IgG antibodies in pregnant women with a history of toxoplasmosis was extremely high. Of these studies, 6 that included 3096 patients included information that allowed to correctly identify the diagnosis and the characteristics of the population. The sensitivity of the tests for detecting IgG is greater than 95%, with a specificity greater than 94% 43.
IgGs against Toxoplasma usually appear after the fourth week post infection, and persist for a lifetime. It is observed that its usefulness for the identification of an old infection can be greater before pregnancy or early in the gestation. It is considered that IgG negative women are susceptible to acute infection (Level Level of evidence 1a).
Accuracy of the tests used for the diagnosis of acute toxoplasmosis in pregnant women
In a multicentric study41, 276 patients 'serum (pregnant and non-pregnant women) in which acute toxoplasmosis, with less than 3 months' difference, 3 to 12 months' difference IgG, IgM, and more than 12 months' difference was diagnosed. Diagnostic results were evaluated in a single serum sample with tests for specific anti-Toxoplasma IgM, IgA, IgG, and IgE antibodies and different combinations of anti-body assays. The reference test was a seroconversion of specific IgG or the Sabin-Feldman staining test. 195 combinations were assessed (IgM first line assays, IgM, IgA other AC / HS or IgG avidity second line). All ELISA, IgM and ISAGA assays except one showed> 98% sensitivities. The sensitivity of IgA was lower, with a significant variation (50-90%). IgE had a very low sensitivity (54%). The specificity of IgM and IgA to detect an infection acquired in the past was> 92%, except for 2 IgA ELISA assays. IgM and IgA tests were used to discriminate acute infection from convalescence with a specificity of 40%. No test combination of any kind could discriminate acute infections that occurred 3-12 months prior. Excellent diagnostic outcomes were achieved with the sequential use of highly sensitive IgM assays combined with methods that examined IgG avidity with specificities around 96%, while sensitivity was maintained around 96%, considering a period of recent infection < 16 weeks. IgA or IgE assays were less suitable than IgM for acute infection (Level of evidence lb).
Interpretation and discussion of evidence
The selected studies coincide in showing that the choice test for diagnosis during pregnancy is a combination of tests (performed under the principle of ELISA, chemiluminescence or fluorometry or ISAGA) for IgM together with IgG tests n a first stage of diagnosis. Anti-Toxoplasma IgG and IgM anti-Toxoplasma negative IgG tests at the onset of gestation indicate a chronic (prior or former) infection and discard the risk of infection during pregnancy for the pregnant woman.
A negative IgG test with a positive IgM test requires a new sample to differentiate between seroconversion or natural IgM. These patients should have a second IgG test. A second positive IgG test is evidence of recent seroconversion. Patients with negative IgG (and, therefore, with natural IgM) should have monthly follow-up with IgG. The IgM test should not be repeated because this result is not expected to change.
A positive IgM test with positive IgM requires acute phase confirmation with avidity tests for anti-Toxoplasma IgG. This combination of tests must be performed before week 16, period in which the test combination performance is higher. The IgA ISAGA test can be recommended to support the diagnosis when the pregnant woman is tested after week 16 and a result of high avidity cannot rule out infections during the onset of gestation (Note: some persons do not develop IgA because possess a genetic deficiency to produce this immunoglobulin, for this reason an IgA negative test does no discard a recent toxoplasmosis). An IgG test against positive Toxoplasma before pregnancy or in a previous pregnancy precludes the possibility of infection during pregnancy in the mother.
Clinical recommendation
A. In cases where the infection status is unknown, it is recommended to perform IgG and IgM tests on pregnant woman during their first prenatal control to determine the presence of Toxoplasma infection.
B. It is recommended that women with positive IgG and IgM be tested for avidity to confirm the antigenicity of the infection if the pregnancy is less than 16 weeks, and IgA if greater than 16 weeks.
C. It is recommended that women with negative IgG and IgM be followed monthly following the criteria established by this guideline.
• It is recommended that women with IgG negative and IgM positive repeat the test for IgG in 2 weeks to document acute seroconversion or presence of natural IgM.
• I It is recommended that a woman who considers becoming pregnant be tested for Toxoplasma IgG test to identify her prior infection status with the parasite.
What are the recommended confirmatory tests for toxoplasmosis?
When seroconversion or results of recent Toxoplasma infection tests (low avidity IgG, IgM and IgA both positive) are found in pregnant women, it is necessary to know if the fetal infection is already present. This transmission varies per gestational age at the time of infection, and it is necessary to underline that there are asymptomatic infections that do not present echographic alterations. Until recently, experts advised amniocentesis to change the mother's treatment, from spiramycin to pyrimethamine- sulfadiazine. However, several observational studies have failed to demonstrate that treatment with pyrimethamine- sulfadiazine is better than only with spiramycin.
Summary and description of the body of evidence
Specific Immunoglobulin M in fetal blood or amniotic fluid °
A cohort of evaluation of diagnostic tests with 134 pregnant women found that the IgM test obtained a sensitivity of 47% and a specificity of 95% for the detection of fetal infection, the lowest operational characteristics reported in the literature44 (Level of evidence Ib).
A study evaluating diagnostic tests that included 127 pregnant women found that the test sensitivity in amniotic fluidand fetal blood was 88.2% (15/17) and 87.5% (14/16), respectively. Specificities were estimated between 86.3 and 100% 44-46 (Level of evidence Ib).
Another study evaluating diagnostic tests that included 127 French women (1985-1993) who acquired the infection during pregnancy and received prenatal diagnosis found that the sensitivity of the tests for amniotic fluid and fetal blood was 88, 2% (15/17) and 87.5% (14/16), respectively. The specificities ranged from 86.3 to 100%. The results support theJorge A. Cortés, et a discarding of cordocentesis with regards to amniocentesis (Level of evidence II).
Isolation of Toxoplasma gondii in fetal blood or amniotic fluid
A study evaluating diagnostic tests in 134 pregnant women found that the sensitivity of T. gondii isolation by inoculation in mice in the fetal blood ranged from 31 to 73%. (Level of evidence Ib) A study of diagnostic tests with 280 serum in which T. gondi i was isolated by means of inoculation of mice in the amniotic fluid, found that sensitivity was estimated in 52% 47 (Level of evidence Ib).
PCR
A prospective multicentric study of diagnostic tests in 270 cases, 75 of them with confirmed infection, found that the sensitivity of CRP was 64% 48. (Level of evidence Ib).
In the diagnostic tests studies that included 593 cases, it was found that the sensitivity of the PCR oscillated between 69 and 71%, and the specificity was between 96 and 99%. The PCR sensitivity seemed to increase with the age of the seroconversion, but was not influenced by maternal treatment49 (Level of evidence Ib).
In a study evaluating diagnostic tests with 110 cases, it was estimated that the PCR sensitivity oscillated between 70% to 97%, while the specificity ranged from 93% to 100%. By combining the PCR diagnosis with T. gondii isolation in the amniotic fluid by mouse inoculation, the sensitivity increased to 91 and 94%, and the specificity to 97 and 99% 47 (Level of evidence II).
Interpretation and discussion of evidence
The sensitivity of Toxoplasma deoxyribonucleic acid amplification tests by PCR in the amniotic fluid to determine fetal infection, ranges from 67 to 80% (conventional PCR), with specificities close to 100%. Cordocentesis is excluded because it is risky and offers low sensitivity for diagnosis. From a practical point of view, amniocentesis may be unnecessary in the third trimester, considering the high rates of transmission of the infection to the fetus (> 66%).
GDG does not recommend amniocentesis before week 18. Like any invasive procedure, performing an amniocentesis should be accompanied by an assessment of the balance between risks (preterm delivery, bleeding, etc.) and benefits. The decision of the woman should be recorded in the clinical history, and the signature of informed consent is recommended.
Clinical recommendation
B. It is suggested to offer amniocentesis as an alternative the diagnosis of fetal infection through and PCR in the second trimester of gestation; The final decision must be agreed and recorded in the clinical history. A negative result does not rule out congenital infection
B. The use of cordocentesis as a confirmatory test for toxoplasmosis infection is not recommended
• It is recommended to carry out rigorous quality controls in the centers that perform different diagnostic tests based on amniotic fluid for Toxoplasma infection.
Is the use of ultrasound recommended to determine the severity of fetal involvement with positive tests for Toxoplasma infection?
Fetal infection by Toxoplasma may or may not be detected in an ultrasound. The detection of morphological alterations suggests the presence of brain lesions that could be compatible with Toxoplasma infection and severe CNS involvement.
Summary and description of the body of evidence
A systematic review that included 7 studies with 25,036 womenfound that the quality of fetal anomaly detection withthe use of ultrasound depends on the system of organs compromised,with higher detection rates for the CNS and not forskeletal and cardiac anomalies80. (Level of evidence Ib)
A study of diagnostic tests that included 162 children withfollow-up for 15 to 71 months found that 27 of them hadconfirmed congenital toxoplasmosis and no echographiclesions, and reported a normal neurological developmentduringfollow-up61. (Level of evidence Ib)
A study of diagnostic tests with 286 cases 52 found that combinedprenatal tests (serology, amniocentesis, echography)allowed the diagnosis of 77% of cases of congenital toxoplasmosis.( Level of evidence II)
Interpretation and discussion of evidence
Ultrasound monitoring allows the detection of CNS disorders. The absence of echographic alterations is correlated with a lower probability of post-natal neurological impairment. The absence of cerebral changes in the ultrasound does not discardan ocular involvement.
Clinical recommendation
The follow up of the pregnant woman with fetal morphology ultrasound is recommended to define the severity and involvement of the fetus in the presence of positive tests for Toxoplasma infection.
It is recommended that follow-up ultrasound for these patients be performed by specialized staff trained in identifying fetal morphological alterations.
What is the recommended secondary prevention scheme (prevention of fetal transmission) for women diagnosed with pregnancy acquired infection?
The purpose of infection detection programs during pregnancy is to detect and treat cases, as well as to reduce the transmission of infection and severe damage to the fetus and the newborn. For many years, spiramycin has been used as t first line treatment in the programs developed by the Government of France. However, its efficacy was questioned by a systematic review which did not allow the conclude as to whether there was any evidence of its benefit. The European Multicentre Study on Congenital Toxoplasmosis of the European Union encouraged several collaborative studies among several countries to collect evidence to determine whether prenatal treatment of toxoplasmosis was useful. Although the studies are observational, the outcomes have been consistent and allow for recommendations based on the best available evidence.
Summary and description of the body of evidence
A systematic review included 22 European cohorts (550 infants) and 4 cohorts from other countries (141 children) to assess the effect of spiramycin and pyrimethamine- sulfadiazine on the risk of transmission to the child. Treatment with spiramycin (3 g / day until delivery) was found to be effective for prevention of transmission if it occurred within the first 3 weeks after seroconversion (OR 0.48, 95% CI 0, 2-0.8). There was a tendency to benefit from the treatment when performed between 3 and 5 weeks after seroconversion (OR 0.64, 95% CI 0.4-4.0). No beneficial effect was found if the treatment was administered between 5 to 8 weeks after seroconversion or after 8 weeks of seroconversion, an adjusted OR of 0.42 (95% CI 0.2-0.9) was estimated for risk of clinical symptoms in the child if prenatal treatment with spiramycin (3 g day until birth) was administered within the first 4 weeks after seroconversion, and an adjusted OR of 0.64 (95% CI 0.4-0.99) if it occurred after this time. In the European cohorts, this protective effect was less marked (OR 0.68, 95% CI 0.3-1.5 with treatments prior to 5 weeks of seroconversion, and OR 0.87, 95% CI 0, 4-1,8 after 5 weeks of seroconversion). With respect to drugs or protocols other than spiramycin, in Austria, for example, an initial protocol with pyrimethaminesulfadiazine is used for the onset of treatment. No differences were found between protection with spiramycin or pyrimethamine sulphadiazine5 (Level of evidence 2 ++).
An observational study involving 293 patients evaluated prenatal versus spiramycin treatment in women with seroconversion or infants with congenital toxoplasmosis infection. Pyrimethamine vs. No treatment6. The therapy reduced severe neurological sequelae (OR 0.23, 95% CI 0.07-0.70), but no differences were observed between Spiramycin and Pyrimethamine- Sulfadiazine (OR 0.77, 95% CI 0.204-2.849). (Level of evidence 2 ++)
Interpretation and discussion of evidence
In the identified evidence, there were no controlled clinical for transmission of Toxoplasma infection to the fetus; However, observational studies are consistent in demonstrating a benefit of prenatal treatment with spiramycin, which has not been shown to be superior to the pyrimethamine-sulfadiazine regimen. Treatment should be initiated early after the diagnosis of acute toxoplasmosis, ideally, within 4 weeks of seroconversion. No studies were found to evaluate other antibiotics other than those analyzed in the systemic review for prenatal treatment of toxoplasmosis.
Clinical recommendation
B. Pharmacological treatment with spiramycin (3 g / day for the rest of the pregnancy) is recommended for infection confirmed by Toxoplasma in the pregnant woman.
• In the case of confirmation of fetal transmission of toxoplasmosis (PCR tests or ultrasounds suggesting neurological involvement), it is recommended to change to pyrimethamine plus sulfadiazine plus folinic acid
What tests are recommended to establish the diagnosis of congenital infection in the newborn?
The newborn of a mother with infection during pregnancy requires a diagnostic evaluation to confirm or rule out congenital infection. As the infection, may be asynchronous and the clinical signs are nonspecific, serological tests should be used to differentiate between maternal or prophylactic antibodies. Likewise, the role of the detection tests of deoxyribonucleic acid of the parasite in the diagnosis of the newborn should be reviewed.
Summary and description of the body of evidence
Serological tests immunoglobulin M
A study of diagnostic tests in 105 patients, estimated the specificity of IgM in cord blood sample to be 78%. The IgM ISAGA test had the highest sensitivity estimates, varying from 54 to 73%. Increased sensitivity was found with the IF sequence, ELISA and then ISAGA53 (Level of evidence II).
A study of diagnostic tests with 126 cases found that the sensitivity of the IgM test in umbilical cord blood at birth oscillated between 28% with ELISA and 91% with SAGA. Beyond birth, a lower specificity of IgM was reported (96%). The lowest sensitivity was found in the IF tests, ranging from 10 to 27% 54 (Level of evidence II).
Two studies, one of diagnostic tests with 593 cases and another one of diagnostic tests with retrospective evaluation in 294 pregnant women, found a positive association between IgM sensitivity and the age of the mother in seroconversion. There was no association between the test results and the mother's treatment49,55 (Level of evidence lb).
Immunoglobulin A + immunoglobulin M serologic tests A retrospective evaluation of diagnostic tests including 294 children of mothers with seroconversion found that IgM in umbilical cord blood was positive in 80% of cases when pregnant women were not treated, and 35% when receiving treatment (P = 0.01), while cord IgA was positive in 78 and 61% cases, respectively (P = 0.45) 47,53,55-57. (Level of evidence 1 b) A study of diagnostic tests involving 14 laboratories of the European Community biomedical program evaluated immunological methods for the postnatal diagnosis of congenital toxoplasmosis. 55 newborns with persistent anti-Toxoplasma IgG and 50 infants without anti-Toxoplasma IgG in the first year of life were analyzed. The ISAGA test had a specificity below 90%, while the ELIFA test was 100%; The sensitivity for these 2 tests was 64.2 and 56.7%, respectively. When the results were combined for IgM and IgA with standardized methods and for IgG and IgM with comparative immunological profiles methods (CIP), the sensitivity increased but the specificity remained like the combination of IgA-IgM58 (Level of evidence 1 b).
A study of diagnostic tests in 894 patients comparing IgM and IgA found that both tests were more specific in neonatal blood (IgM: 98%; IgA: 100%) than umbilical cord results (IgM: 85%, IgA: 88 %). The sensitivity for IgM and IgA in neonatal blood was 61 and 60%, respectively) and in umbilical cord blood (67 and 54%, respectively). The combination of IgM and IgA resulted in an overall sensitivity increase of 73% and specificity of 98% 47,53,55-57. (Level of evidence 1 b)
A study evaluating diagnostic tests comparing WB against serology in 175 children, 36 infected and 139 non-infected, estimated a sensitivity of 85% in the third month of life of this test. Likewise, when combined with serology, the sensitivity of WB increased to 94%, with specificity of 100% (95% CI 98.7-100) 47,53,55-57. (Level of evidence 1b)
Western blot
In a study evaluating diagnostic tests, 48 infants were included: 27 with confirmed diagnosis of congenital toxoplasmosis and 21 with suspicion and no manifestation of congenital infection.
The relationship between immunoblot diagnosis and other methods was evaluated, finding that the sensitivity, specificity and positive predictive values in immunoblot for IgG were 92.6, 89.1, and 92.4%, respectively69. (Level of evidence 1 b)
A study of diagnostic tests in 3-month-old patients evaluated a cohort of 165 pregnant women in their relationship to prenatal care. The study found a sensitivity with WB of 85% 60. (Level of evidence 1b)
A study of screening diagnostic tests in Colombia with 200 patients evaluated the WB test for the diagnosis of congenital toxoplasmosis with a new criterion determined by band densitometry or immunodensitometry which diagnosed 91% of the cases compared to not using it with 72%. This method made possible to compare if in the child's serum there were antibodies with a different specificity to those from mother so that it was possible to differentiate the antibodies passively transmitted by the mother and those produced by the child, eliminating the problem posed by IgG61 measurement(Level of evidence II)
PCR
A diagnostic test study with 94 patients evaluated the role of PCR in placental tissue for the diagnosis of congenital toxoplasmosis, finding sensitivity of 61% and specificity of 92% 62. When the isolation of T. gondii in placental tissue was investigated by mouse inoculation and cell culture for cell culture, sensitivity was 30%, and specificity ranged from 98% to 100%. (Level of evidence 1 b)
Interpretation and discussion of evidence
The use of the IgM + IgA combination for postnatal diagnosis was reported in 7 studies demonstrating consistent improvements in sensitivity across all studies. The sensitivity of the combination of IgA + IgM at birth varied from 55 to 89%. After birth, it varied from 63 to 94%. Although there is good evidence that the use of anti-Toxoplasma IgM and IgA tests have adequate sensitivity and specificity for the detection of congenital toxoplasmosis, the seronegativity of these tests does not rule out the diagnosis of congenital toxoplasmosis. GDG considered that both tests should be used simultaneously. In case of negative results in both tests, the WB test can increase sensitivity. Another way to identify patients with congenital toxoplasmosis is to monitor IgG titers. In uninfected children, maternal IgG titers should disappear between 6 and 10 months of age, indicating transplacental passage, while ascending titers are indicative of congenital infection.
Clinical recommendation
A. The simultaneous measurement of IgG, IgM and IgA anti-Toxoplasma is recommended for diagnosis of congenital infection in the newborn.
A. When IgA and IgM anti-Toxoplasma are negative in serum, use of Western blot is recommended.
• For IgG positive and negative results in the 3 tests (IgM, IgA and Western blot), the decision to defer postnatal treatment should be cautiously made after complete clinical, radiologic, and laboratory evaluation (head ultrasonography may be used). Monthly follow up of the newborn for 6 months is recommended, and then every 3 months up to the first year with IgG to discard congenital infection.
What is the recommended medicine for newborns diagnosed with Congenital infection?
When a newborn has a confirmed diagnosis of congenital toxoplasmosis it requires specific treatment for one year. The aim of the treatment in symptomatic cases is to reduce ocular and neurological sequelae and mortality. In the asymptomatic children, the aim is to prevent the appearance of retinochoroiditis lesions or the development of hydrocephalus. According to studies of children without treatment (natural history of the disease), the risk varies per the geographical area. In European and North American series4, more than 82% of untreated children suffered from retinal damage in adolescence, and in children with neurological symptoms at birth, 85% had delayed psychomotor development, 81% seizures, 70% Motor impairment, 60% loss of vision, 33% hydrocephalus or microcephaly, and 14% hearing loss at 4 years of age. In some South American cohorts, eye injuries appear more rapidly, for example, in southern Brazil, in the first months of life, 80% of newborns already suffer from retinochoroiditis lesions, 50% of which were active63.
Summary and description of the body of evidence
A multicentric study included 120 children with congenital toxoplasmosis treated for one year with pyrimethamine sulfadiazine (pyrimethamine 2 mg / kg on the first day and then continued with 1 mg / kg / day [not to exceed 15 mg / day] until one year + sulfadiazine: 100 mg / kg / day distributed in 2 doses, until completing one year) and evaluated if there were differences in cognitive, neurological and ocular outcomes compared to 120 historical controls. It was found that most of the treated children had normal cognitive performance at the one year follow-up. 72% of children with major neurological impairment had normal cognitive scores and no auditory involvement4. (Level of evidence 3)
In relation with the use of transfontanelar ultrasound in the first evaluation of the newborn to identify the presence of intracerebral calcifications, a study of diagnostic tests that included 44 patients, it was found that both methods (ultrasound and cerebral tomography) had very good diagnostic agreement (K> 0.8)64. (Level of evidence lb)
Interpretation and discussion of evidence
There is no evidence to support the recommendation based on randomized controlled trials. GDG together with the group of experts concluded that all symptomatic and asymptomatic children with paraclinical or clinical criteria compatible with toxoplasmosis, as well as all those with confirmation of fetal infection during pregnancy, regardless of if their mother received treatment, should be treated with a pyrimethamine sulfadiazine/ folic acid protocol for one year. Complete clinical, radiologic, and laboratory evaluation should be done at birth even for asymptomatic infants (head ultrasonography may be used). In asymptomatic children with negative IgM and IgA and western blot at first examinations, the decision to defer postnatal treatment should be cautiously made and follow-up should be performed to confirm or rule out T. gondii infection and proceed accordingly. Pyrimethamine is administered: 1 mg/ kg every 12 hours on the first 2 days, followed by 1 mg/kg per day for 1 year, and sulfadiazine at doses of 50 mg/kg every 12 h and folic acid (10 mg 3 times/week) up to 1 week after suspension of pyrimethamine. Several studies have presented other combinations of pyrimethamine-sulfadoxine, besides the one included in the summary of the evidence65-68, thus:
• Pyrimethamine + sulfadoxine tablets (1 tablet = 500 mg sulfadoxine and 25 mg pyrimethamine). It is administered dissolved and based on the dose of sulfadoxine 25 mg/kg every 8 days. A loading dose of 50 mg/kg of sulfadoxine is administered on the first day, and then 25 mg/ kg in weekly single doses up to the first year of life along with 7.5 mg/ day (a half-tablet of 15 mg) of folinic acid. There is a controversy over the use of this combination, given the risk of adverse effects and doubts about the therapeutic levels during administration at weekly intervals.
• Clindamycin 1 mg/kg /day orally plus clindamycin 30 mg/kg/ day intramuscularly divided into 3 doses during the acute phase and then administered orally at 20 mg/kg/day divided into 3 doses (clindamycin suspension 75 mg = 5 Ml) as a maintenance dosage until the first year of life.
Clinical recommendations
• All symptomatic and asymptomatic congenitally infected children should be treated for Toxoplasma infection with pyrimethamine plus sulfadiazine (1 mg kg / day once daily plus 100 mg / kg / day, twice daily for one year) plus Folic acid.
• In case of adverse effects and / or limitations to the firstchoice treatment, and per physician´s criteria clindamycin, sulfadoxine or azithromycin may be used as an alternative along with pyrimethamine and folinic acid.