Introduction
Fish and fish products are an important source of animal protein, easily digestible, as well as rich in minerals and vitamins. The Pacific region straddling Colombia and Ecuador has a number of important fishing ports that have enabled the development of industrial and artisanal fishing activities1 According to the Food and Agriculture Organization of the United Nations (FAO), average annual fish consumption per capita in Colombia and Ecuador is 4.73 kg and 5.6 kg, respectively1,2. Compared to countries such as Spain (38 kg / year), and Japan (54 kg / year), and even average consumption in Latin América in general (18 kg / year), consumption in Colombia and Ecuador is notably low. In recent years, with the introduction of the Mediterranean diet and the benefits of fish consumption for the prevention of cardiovascular diseases now well documented, fish consumption has been increasing. However, the intake of these products has been connected to various infections in humans when fish is consumed that is minimally processed (i.e. raw, semi-raw, in sushi, salted or marinated)3. In some Latin American countries such as Brazil, Chile, México and Perú, most of the infections related to these types of fish preparation are associated with the presence of parasites in fish, a widespread phenomenon and particularly difficult to eliminate in raw and unprocessed fishery products4.
The nematodes of the family Anisakidae are of particular concern to public health. These parasites cause anisakiasis, a zoonosis caused mainly by the genera Anisakis (Dujardin, 1845) and Pseudoterranova5. In humans, Anisakid larvae can cause gastric, allergic or gastro-allergic symptoms. Allergic reactions, (hypersensitivity type I), are the most common and caused mainly by the genus Anisakis, while gastric symptoms are more commonly associated with the genus Pseudoterranova, causing pseudoterranovosis6.
Human anisakidosis has been reported in more than 26 countries on five continents including, Holland, Italy, Egypt, New Zealand, Canada, the United States, Brazil, Peru, and Chile7-14. The majority of reported cases have occurred in Spain, Japan and other Asian countries, where epidemiological studies have indicated that anisakiasis (caused by the genus Anisakis) is more frequent in coastal populations. In countries such as Brazil, Chile, Peru and Colombia, anisakidosis is considered an emerging public health risk and one that could increase with the introduction of a Mediterranean or Japanese diet15.
The World Health Organization (WHO) and the FAO have established regulations, recommendations and guidelines to prevent and minimize the negative effect of anisakid nematodes on human health16. For its part, the European Community has specific sanitary regulations for foods of animal origin which are applicable to fishery products in relation to minimizing infection by anisakid parasites17. In Latin America, countries such as Nicaragua, Mexico and Ecuador follow some of these measures for the prevention and control of fish parasites that affect human health18,19. However, Colombia does not have clear legislation in this regard since these pathogens have not been fully identified.
Given that the process of globalization has popularized the consumption of raw or undercooked fish across various regions of the world and that fish parasitized with anisakids have been recorded in countries such as Chile, Peru, Argentina, Brazil, Colombia and Venezuela. Thus, the objective of this study was to determine the presence of anisakid nematodes in fish species that are sold commercially in the fishing ports of Colombia and Ecuador.
In addition, it is important to note that, in Colombia and Ecuador, no cases of anisakidosis in humans have been described to date, though this is most likely due to a lack of clinical and epidemiological information, and consequently an underreporting of the parasitosis as an emerging disease.
Materials and methods
Study area
Commercial fish species of particular economic importance were collected from the following fishing ports (Figure 1):
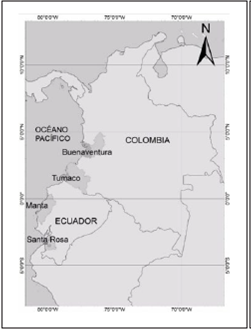
Figure 1 Location of the sampling sites in the Pacific coast of Colombia (Buenaventura and Tumaco) and Ecuador (Manta and Santa Rosa).
Ecuadorian Pacific: Manta (0 ° 57’00 “S 80 ° 42’58” W) and Santa Rosa (3 ° 27’08 “S 79 ° 57’42” W). These ports concentrate a catch of 23,000 tons of fish per year (1).
Colombian Pacific: Buenaventura (3 ° 52’38 “N 77 ° 01’36” W) and Tumaco (1 ° 48’24 “N 78 ° 45’53” W), the main ports of the Colombian Pacific coast where 53.89% of the catch comes from artisanal fishing and 46.11% from industrial fishing, which contributed a combined total of 1,099,568 tons of fish between 1998 and 20131.
Sampling
The samples were gathered from collection centers, points of sale, or supplied by fishermen in the region according to availability and the species’ economic importance. The samples were stored individually in labeled bags, kept in a refrigerator with ice and then transferred to the laboratory.
The study focused on the identification of anisakids in the viscera of the fish. In most cases, there was not access to the musculature of the fish due to its greater economic value. The nematodes were fixed in warm formalin at 4% (v / v) and immediately transferred to alcohol at 70% (v / v) until their identification by taxonomy. A subsample was stored directly in 96% alcohol (v / v) for subsequent identification by molecular biology.
Morphological identification of nematodes
The nematodes were clarified in gradual solutions of glycerin 20. The observation of their internal structures was carried out under an optical microscope with a built-in clear camera (Leica DM750). Nematodes of the Anisakidae family were identified up to a generic level, taking into account the characteristics described by Shiraki (1974). Images were taken with magnifications of 40x, 100x and 400x (Application Suite LAS v 3.8).
The morphologically identified anisakids were separated, counted and grouped by host. Finally, the percentage of infection for each host was calculated.
Molecular identification
DNA extraction: Thirty-eight anisakid nematodes were processed in the parasitology laboratory in the Faculty of Medicine at the Universidad de Chile. The larvae were sectioned into three and the individual extraction of each section of the nematodes was performed using the Invitrogen kit (Genomic DNA Mini Kit), following manufacturer instructions. The DNA was eluted in elution buffer and kept at -20°C until use.
Primers: Specific primers were used for six anisakid species: Anisakis physeteris (Baylis, 1923); Pseudoterranova decipiens (Krabbe, 1878); Anisakis simplex sensu stricto (Rudolphi, 1809); Contracaecum osculatum (Rudolphi, 1802); Hysterothylacium aduncum (Rudolphi, 1802) and Anisakis pegreffii (Bell-Rouget & Biocca, 1955), (Integrated DNA Technologies)33.
Identification by multiplex PCR: A multiplex PCR was performed, using six specific primers and a universal primer21. The final reaction volume was 25μl: water (9.38μl); buffer 10x (2.5 μl) (HotMaster ™ Taq); dNTPs (0.63 L) (0.2 mM), for each specific primer (1 μl), universal primer (1 μl), HotMaster Taq DNA Polymerase (0.5 μl) (5PRIME) and genomic DNA (5 μl). The thermocycler program (Applied biosystems 2720) consisted of 30 cycles of initial denaturation at 95°C for three minutes, followed by 30 cycles of denaturation at 95°C for 30 seconds, hybridization at 52°C for 30 seconds, extension at 72°C for 45 seconds, and a final extension at 72°C for seven minutes. All products were subjected to electrophoresis in 2% (w / v) agarose gel. As a positive control, genomic DNA of L3 larvae of A. simplex, A. pegreffii and P. decipiens was used, supplied by Dr. Hiroshi Yamasaki of the National Institute of Infectious Diseases in Tokyo, Japan.
Results
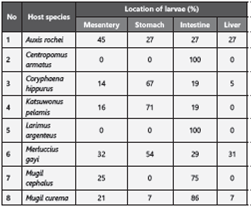
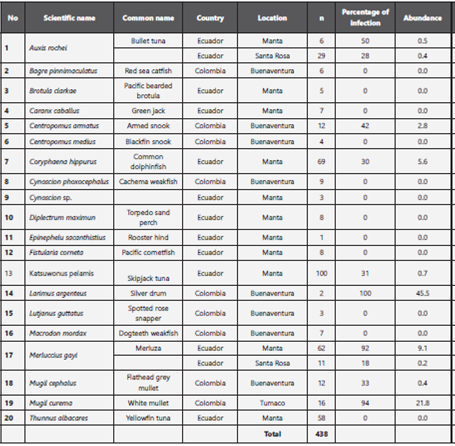
A total of 438 commercial fish destined for human consumption were collected and grouped into 20 species. Of those twenty, eight were found to be parasitized by anisakid nematodes with a percentage of infection of between 18 and 100%, and an average abundance between 0.4 and 45.5 (Table 1).
Table 1: Percentage of infection of anisakid nematodes isolated from fish for human consumption from fishing ports in Colombia and Ecuador.
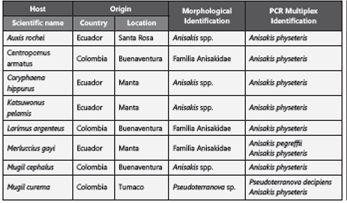
The nematodes were isolated mainly from the intestine (Table 2) and their morphological characteristics examined, resulting in the identification of the Anisakis and Pseudoterranova genera, both of the Anisakidae family (Table 3), with 90% identified as from the Anisakis genus.
Table 3: Morphological and molecular identification of larvae of the Anisakidae family and their hosts in the Colombian and Ecuadorian Pacific.
The type I (Figure 2) and type II (Figure 3) larvae of the Anisakis genus were identified under an optical microscope. Type I larvae are characterized by a rounded posterior and the presence of a mucron (Figures 2c and 2d), while type II larvae present a conical termination with the absence of a mucron (Figures 3c and 3d).
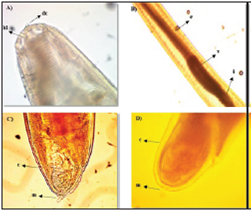
Figure 2 Larva (L3) type I of the Anisakidae family. A. Front end, dc: cuticular tooth, ld: dorsal lip (40x). B. Middle section, e: esophagus, v: ventricle, i: intestine (20x). C and D. Posterior end, m: mucron, c: cuticle (40x).
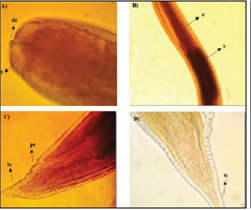
Figure 3 Larva (L3) type II of the Anisakidae family. A. Front end, dc: cuticular tooth, ld: dorsal lip (40x). B. Middle section, e: esophagus, v: ventricle (20x). C and D. Posterior end, tc: conical termination, pa: anal pore (40x).
The larvae present characteristics of the Anisakis genus; a whitish color, transverse grooves along the entire length of the body that become more pronounced towards its posterior end, (Figures 2b, 3 c and 3d), a well-formed mouth composed of three lips surrounding the cuticular tooth, (Figures 2a and 3a), an elongated ventricle with a direct connection to the intestine and well-aligned along the longitudinal axis of the nematode (Figures 2b and 3b).
Multiplex PCR identification confirmed at the species level 26 of the 38 larvae analyzed. Anisakis physeteris species were identified in eight hosts and P. decipiens and A. pegreffii were each identified in one host. Anisakis physeteris was also reported in hosts already parasitized by P. decipiens and A. pegreffii (Table 3).
The specific primers were able to detect P. decipiens isolated from the M. curema (White mullet), using an amplification product with an expected weight of 370 bp, A. pegreffii with 672 bp, isolated from M. gayi (Merluza) and A. physeteris with 143 bp, isolated from A. rochei (Bullet tuna), C. armatus (Armed snook), C. hippurus (Common dolphinfish), K. pelamis (Skipjack tuna), L. argenteus (Silver drum), M. gayi (Merluza), M. cephalus (Flathead grey mullet) and M. curema (White mullet) (Figure 4).

Figure 4 Molecular identification of anisakid nematodes by Multiplex PCR. Line 1: 370 bp, P. decipiens (M. curema). Line 2: 672 bp, A. pegreffii (M. gayi) and Lines 3 to 9: 143 bp, A. physeteris (A. rochei, C. armatus, C. hippurus, K. pelamis, L. argenteus, M. gayi, M. cephalus and M. curema). Line L: molecular weight marker of 100 bp.
Discussion
In the present study we have verified the presence of anisakid nematodes in fish for human consumption that inhabit the waters of the Ecuadorian and Colombian Pacific. Results found a high percentage of infection of Anisakis and Pseudoterranova parasites, the main causative agents of allergic and gastric anisakidosis respectively6. We identified third instar larvae (L3) based on the structural characteristics of the ventricle and the shape of the posterior end of the larvae22. In fish such as Merluccius sp., Centropomus armatus and Mugil sp. - species that are widespread across the Pacific Ocean from the coast of California in the United States to southern Chile23 - infection with anisakidos could be related to feeding habits. These species inhabit coastal and estuarine waters and they have been classified as detritivores, iliophages, herbivores, omnivores, phytophages and zooplanktons24, feeding behaviors that favor parasitic infections4.
In recent years, these fish species have recorded high rates of capture by artisanal fisheries and constitute one of the main sources of protein as an economically important species on the Pacific coast25. The identification of these fish species parasitized by Anisakis and Pseudoterranova can be used to develop measures for the prevention and control of human anisakidosis in Ecuador and Colombia; a disease that can be classified as emergent and easy to manage but whose spread must be addressed.
The location of the larvae is reported in this study, recorded as parasitizing the mesentery, stomach, intestine and liver. Our interpretation is that these results are related to the time that elapsed between the capture of the fish and the parasitological review. Most of the fish were reviewed fresh and it is known that the larvae of the anisakids require a minimum time of thirty minutes to migrate from the digestive system of the fish to the muscle26. Our results are similar to those of studies conducted in European countries, where hake (merluza) has a high commercial value and where A. pegreffii and A. simplex s.s. have been identified in viscera and muscle without significant differences in the values of prevalence between the two species of Anisakis26. These findings may be of economic interest for Ecuador and Colombia, providing information about the likely locations of larvae in order to eliminate them and therefore develop more effective and sanitary processing of the fish caught in the maritime waters of these countries.
The molecular characterization by PCR multiplex enabled the identification of the nematode A. physeteris in the fish species A. rochei, C. armatus, C. hippurus, K. pelamis, L. argenteus, M. gayi and M. cephalus, as well as A. pegreffii in M. gayi and P. decipiens in M. curema. However, it must be taken into account that the use of other molecular methods such as sequencing could vary these results given that these parasites are a complex of species and their taxonomy changes constantly. Nevertheless, these results concur with comparable studies by different authors who previously reported A. physeteris parasitizing C. hippurus fish in Peru with a prevalence of 33.33%27-29 and in the Mugil sp. fish in Chile where P. decipiens was isolated and identified, a parasite associated with cases of gastric infection in that country5,30. However, in other investigations, A. typica has been recorded in the same hosts examined in our study, as in the A. rochei and K. pelamis fish in Indonesia31,32 and the C. hippurus fish in the Indian Ocean33,34.
Hake (merluza) is considered a potential host for all Anisakis species31. We report A. pegreffii and A. physeteris for this species, while in Chile it is associated with cases of pseudoterranovosis and in Colombia there is the report of Contracaecum sp. parasitizing the fish Merluccius sp. from the Caribbean Sea5,9,30,32.
Additionally, the mugilidae constitute the family of fish with the highest number of anisakidos reports. We identified P. decipiens in Lisa fish, as in Chile, Venezuela and Peru, where it has been reported as the causative agent of several cases of anisakidosis4,27,30,35. Other authors report this species as a host for Contracaecum sp. in Colombia24,25,36-38 and A. pegreffii in the Yellow Sea and in the Mediterranean Sea31,39. However, our bibliographic research did not find any reports of anisakids for the C. armatus and Larimus argenteus fish suggesting our study may be the first report of parasitosis in these species by A. physeteris. Similarly, we did not find reports of anisakidos in Ecuador.
Although in the majority of human cases of anisakidosis where molecular biology has been performed A. simplex s.s. has been identified as the main etiologic agent40, in Italy A. pegreffii is recognized as the main cause of gastric anisakiasis with the molecular larvae isolated from three clinical cases being identified by molecular techniques41. It is important to note that in Japan, where parasitized fish species are widely studied, it is recognized that there are maritime areas of sympatry between A. simplex s.s. and A. pegreffii, and hybrid individuals have been recorded, while in the western Mediterranean A. simplex s.s. is not present, and A. pegreffii has been found to parasitize in a broader range of fish species40. Such variations underline the importance of conducting further research into fish from the Pacific coast to ascertain whether there are fish parasitized by A. simplex in addition to the A. pegreffii nematodes identified by this study.
We can not rule out that the absence of clinical cases diagnosed in Ecuador and Colombia may be related to a lack of knowledge on the part of physicians about anisakiasis and parasitic allergies, and its confusion with other diseases that present similar clinical pictures.
Our research constitutes the first report of the species A. pegreffii, A.physeteris and P. decipiens in Colombia and Ecuador, complementing the studies of geographical distribution worldwide where the Anisakis genus is the most reported in the Mediterranean while Anisakis simplex sensu stricto and H. aduncum are more frequent in the North Atlantic, and Pseudoterranova in the northeastern Atlantic, with no distribution of a particular species apparent in the north of the Pacific Ocean as we have reported.