Introduction
Candida albicans as the most important cause of fungal infections in immune compromised patients can be the reason for different infections from simple mucosal infections to the lethal forms1. Candidiasis is one of the most common fungal infections, which is caused by Candida species especially by C. albicans. Topical azole drugs are usually used for treatment. Continuous use of these drugs has been associated with adverse effects2 and development of resistant C. albicans isolates3,4. Drug resistant C. albicans isolates and the adverse effects of current treatments, have encouraged the scientists to find the new natural herbal antifungal agents in different systems of traditional medicines5-9.
S. khuzistanica has been traditionally used as an antiseptic and analgesic agent10. Some biological activities of S. khuzistanica such as immune stimulatory action11, neuro-protective effect12, improving the fertility in rats13, inhibitory effects against diabetic nephropathy14, protective effects against toxicity of malathion15 and cyclophosphamide16, increasing the opioid analgesic tolerance to morphine17, efficacy in treatment of IBD (inflammatory bowel Disease)12, antioxidant18, anti-diabetic19,20, antinociceptive17, and anti-parasitic effects21,22were confirmed in different investigations.
The antifungal activity of S. khuzistanica essential oil against Cryptococcus neoformans23 , Aspergillus flavus, A. niger, Penicillium sp., Fusarium sp., Alternaria sp., Rhizopus sp. and Mucor sp24 were reported. In this study, we evaluated the anti-candidal activity of S. khuzistanica essential oil against clinical C. albicans, which were isolated from vaginal samples of woman suffering from recurrent candidiasis. S. khuzistanica essential oil related mechanisms were also determined by evaluating its ability to germ tube formation and observing the structures of cell by scanning electron microscope (SEM) and transmission electron microscopy (TEM) in presence of essential oil and carvacrol.
Materials and methods
Plant materials, extraction of essential oil and GC, GC-MS analysis
Satureja khuzistanica aerial parts at full flowering stage were collected from Lorestan Province, Iran in June 2014 and were authenticated under herbarium number of 168-1 by Agricultural Department of Medicinal Plant Research Center of Barij, Kashan, Iran by Dr. H. Hosseini. The dried aerial parts of S. khuzistanica were grinded and subjected to hydro-distillation by Clevenger type apparatus for 3 h. The yellow essential oil was separated and kept in dark vial at cold place (4 °C) until the analysis.
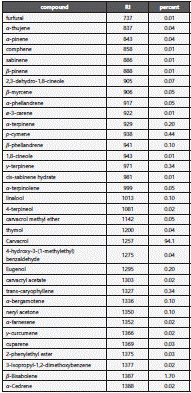
The chemical composition of S. khuzistanica essential oil was analyzed using Gas chromatography (GC) and Gas chromatography-Mass Spectra (GC-MS). The GC and GC-MS analyses were conducted on Agilent technology (HP) 6890 with capillary column of HP-1MS (30 m × 0.25 mm, film thickness 0.25 μm) and Agilent technology (HP) 6890 that was coupled with 5973 network mass selective detector system, respectively. The oven temperature program was initiated at 40 °C, held for 1 min, then raised up to 230 °C at a rate of 3 °C /min, held for 10 min. Helium was used as carrier gas at a flow rate of 1.0 ml/min with a split ratio equal to 1/50 injector. The detector and injector temperatures were 250 and 230 °C, respectively. Components of essential oil were identified by comparison with Retention Indices (RI) relative to homologous series of n-alkanes (injected in conditions equal to sample) and by computer search using libraries of Wiley 275.L and Wiley 7n.1, as well as comparison of the fragmentation pattern of the mass spectra with data published in the literature25.
Microbial strains
This study was conducted on 30 clinical isolates of C. albicans that were isolated from vaginal samples of women suffering from candidiasis. C. albicans isolates were identified according to the results of positive germ tube formation, growth on chloramphenicol sabouraud dextrose agar, chlamydospore formation, color of colony, carbohydrate absorption and fermentation tests. C. albicans ATCC 10231 was used as control strains.
Anti-candidal activities evaluation of essential oil
By using the CLSI methods including disc diffusion (M44-A2) and micro broth dilution (M27-A3) assays, the anti-candidal activities of essential oil, and synthetic carvacrol (Merck) were determined on clinical isolates of C. albicans. The turbidity of each C. albicans suspension was adjusted to 85% at 530 nm (1×106 CFU/ml) by spectrophotometer. Suspensions were cultured on Sabouraud dextrose agar using a sterile cotton swab. Subsequently, filter discs (6 mm in diameter) (Padtan Teb Co, Tehran, Iran) were saturated with different concentrations of essential oil (0.5, 0.75, 1, 1.5 μl) and synthetic carvacrol (0.473, 0.945 μl). Clotrimazole, fluconazole and amphotericin B discs (Rosco Diagnostica A/S, Taastrupgaardsvej 30, DK-2630, Taastrup) were used as positive controls. The plates were incubated at 37 °C for 48 h. The inhibition zone diameters were determined and reported in millimeter ±Standard Deviation (mm±SD)26.
The minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) values of essential oil, clotrimazole and carvacrol were determined by micro broth dilution assay. The compounds were two-fold serially diluted. 100 μl of dilutions were poured in 96- well micro titer plates. MOPS-buffered RPMI 1640 was used as broth media. 100 μl of diluted candidal suspensions (104 CFU/ml) was added to each well and incubated at 35±2 ºC. MIC values were defined as the lowest concentrations of compounds that inhibited the growth after 48 h. MFC values were the first concentrations that showed no growth on Sabouraud dextrose agar27. The experiments were replicated three times and means±standard deviation (SD) were used for drawing the graphs and analysis by Graphpad prism 6. ONE-Way ANOVA test was used to compare the difference between compounds with pvalues at level of 0.05.
Germ tube inhibition test
For germ tube inhibition assay, cell suspension from overnight cultured Sabouraud dextrose agar with C. albicans ATCC 10231 was prepared and the turbidity was adjusted to obtain a density of 1.0×106 CFU/ml. S. khuzistanica essential oil and carvacrol were diluted in DMSO. Then, 10 μl of diluted essential oil or carvacrol were added to 990 μl of yeast suspension to obtain the concentrations equal to 1/8, 1/4, 1/2 and 1 times MIC value. Control suspension (without essential oil or carvacrol) was included as positive control. After treatment, 0.5 ml of sheep serum was added to yeast sediment, the tube was incubated for 2-3 h in a water bath with temperature of 35±2 °C. A drop of this suspension was placed on Neobar slide. The germ tube formation was evaluated and the results of three independent experiments were presented as means±SD28.
Observation of treated C. albicans cells by TEM and SEM
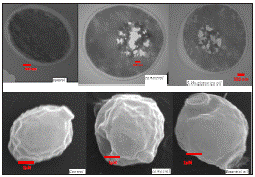
For investigating the effect of S. khuzistanica essential oil and carvacrol in liquid phase by TEM, C. albicans cell suspension (1×106 CFU/ml) was inoculated in RPMI containing the MIC value of S. Khuzistanica essential oil or carvacrol (mixed with 0.5% Tween 80). The flasks were incubated at 30 ºC and 180 rpm for 24 h. The treated C. albicans cells and control cells were separated from the media by centrifugation at 8000 rpm for 10 min. The treated C. albicans cells were fixed with 2.5% glutaraldehyde in phosphate buffer for 30 min and washed three times in 0.1 M phosphate buffer solution (pH 7.2). Each suspension was serially dehydrated with ethanol 25%, 50%, 75%, 90%, and 100%, respectively. Then, the cells were lyophilized. The pellet was post-fixed in 1% osmium tetraoxide for 30 min, washed with phosphate buffer solution (pH 7.2), serially dehydrated in ethanol and embedded in Epon-Araldite resin for making the blocks of the cells pellet. Ultra-thin sections of the cells were stained with gold-palladium and observed under a Philips transmission electron microscope (EM 208) at 100 KV and direct magnification of 180,000×29.
For SEM, a thin serially dehydrated film of cells with ethanol was smeared on a glass cover slip. Samples were stored in desiccators until they were coated with gold-palladium sputter for 200-seconds (Nano Structured coating co. Iran). SEM micrographs were performed using TESCAN VEGA3S electron microscope, at 20 KV.
Results
Chemical Composition of S. khuzistanica essential oil
The extraction yield for essential oil was 4.9% (v/w). The GC and GC-MS analysis of S. khuzistanica essential oil showed the presence of 34 different components, representing 98.4% of total essential oil composition. Carvacrol (94.1%), β-bisabolene (1.7%) were the main components of S. khuzistanica essential oil, followed by p-cymene (0.44%) and γ-terpinene (0.34%) (Table 1).
Antifungal activity of S. khuzistanica essential oil and carvacrol
In disc diffusion method, different concentrations of S. khuzistanica essential oil (0.5, 0.75, 1 and 1.5 μl/disc) and carvacrol (0.48, 0.95 μl/disc) showed that the inhibition zone diameters (mm) of essential oil or carvacrol were increased dose dependently. 1.5 μl/disc of S. khuzistanica essential oil had higher inhibition zone diameters on clinical isolates of C. albicans than that of ketoconazole (10 μg/disc), and clotrimazole (10 μg/disc). The inhibition zone diameters of 1 μl/disc S. khuzistanica essential oil was higher than amphotericin B (50 U/disc) but was lower than ketoconazole (10 μg/disc), and clotrimazole (10 μg/disc). The inhibition zone diameter of 0.75 μl/disc S. khuzistanica essential oil was equal to amphotericin B. The comparison of inhibition zone diameters of S. khuzistanica essential oil showed that there is no significant difference between 0.47 μl/disc carvacrol (equal to amount of carvacrol in 0.5 μl S. khuzistanica oil) and 0.5 μl/disc essential oil, but the inhibition zone diameter of 0.945 μl/disc of carvacrol (equal to amount of carvacrol in 1 μl essential oil) was higher than that of 1 μl/disc S. khuzistanica essential oil (Figure 1).
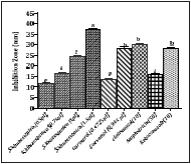
Figure 1 The anti-candidal activity evaluations of compounds against clinical isolates of C. albicans by disc diffusion method. Significant at the level of 0.05 and the higher inhibition zone diameter to the lower level were shown by the letter a, b, c, d, e.
The antifungal activity evaluation of S. khuzistanica essential oil and carvacrol by micro broth dilution assay by assessing their MIC and MFC values showed insignificant difference between the MIC and MFC values of S. khuzistanica essential oil and MIC value of carvacrol (P>0.05). Furthermore, the MFC value of carvacrol had significant difference with its MIC value (Figure 2).
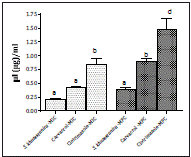
Figure 2 The antifungal activity of S. khuzistanica essential oil and carvacrol (μl/ml) in comparison with clotrimazole (μg/ml) against clinical isolates of C. albicans by micro broth dilution assay. Significant at the level of 0.05 and the lower value to higher level were shown by the letter a, b, c, d, respectively.
Evaluation the inhibitory effects of essential oil and carvacrol on germ tube formation exhibited that the germ tube inhibitory effect of S. khuzistanica essential oil was stronger than that of carvacrol (Figure 3).
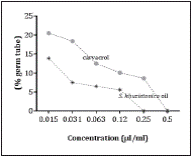
Figure 3 The inhibitory effects of S. khuzistanica essential oil and carvacrol on germ tube formation
In order to observe the caused cytological changes by S. khuzistanica essential oil and carvacrol, the prepared samples were observed by SEM and TEM. Figure 4 exhibits the untreated (control), treated with essential oil or carvacrol, respectively. In SEM observation, minimal changes were observed on the structure of treated cells. Some bubbles and notches were observed on cell membranes of treated C. albicans cells. In TEM images, significant ultra-structural modifications were observed in response to S. khuzistanica essential oil and carvacrol in cytoplasm structures. Huge punctures were observed in treated cells with S. khuzistanica essential oil and carvacrol. In fact, S. khuzistanica essential oil and carvacrol formed huge pits in cytoplasm in comparison with untreated C. albicans cells.
Discussion
C. albicans is the cause of superficial or life threatening systemic infections. In two different kinds of infections, the pathogenicity of C. albicans is determined by some virulence factors such as germ tube formation. Germ tube formation by C. albicans has been reported as critical factor in attachment and pathogenesis of its strains30. Development of novel therapies against surface colonization of C. albicans by Aloe vera extract31, Ferulago capillaris essential oil32, and Rosmarinus officinalis essential oil33 has been the subject of some investigations. According to the results of this study, S. khuzistanica essential oil showed significant anti-candidal effects on clinical C. albicans and inhibitory effects on germ tube formation. The germ tube inhibitory effects of S. khuzistanica essential oil was related to its main component “carvacrol” according to the results of our study. Due to the higher germ tube inhibitory effect of S. khuzistanica essential oil than that of carvacrol, it can be concluded that the other minor compounds of essential oil along with carvacrol may involve in germ tube inhibitory effects of S. khuzistanica essential oil. For example, p-cymene alone as no efficient antimicrobial agent, can enhance the antimicrobial activity of carvacrol34.
The results of anti-candidal activity evaluation of carvacrol on clinical isolates of C. albicans was different by two methods. In disc diffusion method, the inhibition zone diameter of carvacrol was higher than S. khuzistanica essential oil. In broth dilution assay, carvacrol showed the lower anti-candidal effects against C. albicans. Although, carvacrol is a known antifungal agent35,36, but the quota of carvacrol in S. khuzistanica essential oil with higher than 90% carvacrol (94.1%) has not been the subject of any investigation. Due to lower anti-candidal activity of pure carvacrol than that of S. khuzistanica essential oil with 94.1% carvacrol, other minor components of essential oil may exhibit synergistic effects with carvacrol.
Investigation on cellular structures of C. albicans by TEM and SEM images showed that cytoplasmic membranes of C. albicans cells were intact in presence of S. khuzistanica essential oil and carvacrol, while some huge punctures were observed in cell cytoplasm in TEM images. Although, the fungicidal activity of carvacrol through the disruption of ergosterol synthesis has been reported37; but our results showed the cell’s membranes were intact in presence of carvacrol or essential oil. Therefore, the anti-candidal activity of S. khuzistanica essential oil was related to changes in the cytoplasmic structures of C. albicans cells. The results of microscopic observations were in conformity with the results of anti-candidal activities.
Our results have provided the new evidences implicating that S. khuzistanica essential oil had promising anti-candidal activity against clinical isolates of C. albicans by inhibition of germ tube formation and destruction of cytoplasmic structures. Furthermore, it needs further formulations for evaluating the efficacy of S. khuzistanica essential oil against C. albicans related infections in animal models and human studies.