Introduction
Acute cholecystitis and cholangitis dominate the biliary tract infections, which are usually secondary to predisposing factors leading to bacteraemia or sepsis and are associated with high rates of morbidity and mortality, especially in geriatric patients with co-morbid illness or even with delayed diagnosis and treatment1 .The prime reason for biliary tract infections are the ascending infection due to the reflux of duodenal contents and also the blood-borne infection or infection spreading through the portal-venous channels. The other predisposing conditions causing biliary tract infections include critical illnesses such as trauma, burns, sepsis, HIV infection, immunosuppression, diabetes, non-biliary surgery and childbirth2 .
The diagnosis of biliary tract infections mainly depends on the signs and symptoms along with increased WBC count and C-reactive protein and abnormal liver function tests3 . Imaging the biliary tract generally detects either the presence of an obstruction; the cause of the obstruction, and or the level at which the obstruction is occurring1 Successful management depends on drainage of the infected bile along with effective antibiotic therapy4 .
The microbial aetiology of biliary tract infections consists predominantly of bacterial aetiology (Enterobacteriaceae family) followed by fungal group (Candida species and Aspergillus fumigatus) and very rarely viral in origin (Hepatotropic viruses and HIV). The common bacteriological agents are gram negative bacteria - Escherichia coli, Klebsiella pneumoniae, Citrobacter freundii, Salmonella typhi and gram-positive bacteria - Enterococcus spp5-7. Fungal organisms such as yeasts - Candida albicans and non albicans Candida species including Candida glabrata, Candida tropicalis and molds - Aspergillus fumigatus have lower isolation rates8 . The data on the aetiology and antimicrobial resistance profile of biliary tract infections are limited. With the emergence of multidrug resistant pathogens and change in the microbiological spectrum of biliary tract infections, there is a need for the empirical antimicrobial therapy in every clinical setting which would narrow down treatment options thereby allowing optimised strain-specific antimicrobial therapy. Selection of empirical therapy should be influenced by the resistance pattern of the organisms, patient’s prior antimicrobial exposure and the infective aetiology. Therefore, the present study throws light on the microbial aetiology of biliary tract infections and their antimicrobial resistance profile.
Methods
The present retrospective study was carried out from January 2011 to December 2016 at the Enteric Diseases Division, Department of microbiology, Kasturba Medical College, Manipal. Bile samples were collected from adult patients with biliary tract infections admitted to a tertiary care hospital in Manipal, Karnataka, India. Bile samples were processed as per standard procedure. The samples were processed within one hour of collection and inoculated on 5% sheep blood agar, Mac Conkey’s Agar & enrichment was done on bile broth. Bile broth sub-culture was done after overnight incubation on 5% sheep blood agar and Mac Conkey’s Agar. The inoculated plates were incubated at 370 C for 18 to 24 hours. The plates were observed for their growth and types of colonies and were processed for the identification of the microbial aetiology (bacteria and yeast) using MALDI-TOF (BioMerieux) along with their antimicrobial susceptibility testing by VITEK -AST systems (BioMerieux) respectively. The antibiotics evaluated were as follows: for Enterobacteriaceae the antibiotics were amikacin (30 µg), amoxicillin (30 µg), ampicillin (10 µg), cefaperazone/sulbactum (75/30 µg), cefotaxime (30 µg), cefuroxime (30 µg), ciprofloxacin (5 µg), co-trimoxazole (25 µg), gentamicin (10 µg), meropenem (10 µg), piperacillin/ tazobactum (100/10 µg). For Pseudomonas aeruginosa the antibiotics tested were amikacin (30 µg), aztreonam (30 µg), cefaperazone/sulbactum (75/30 µg), ceftazidime (30 µg), ciprofloxacin (5 µg), gentamicin (10 µg), meropenem (10 µg) and piperacillin/tazobactum (100/10 µg). For Enterococcus spp. ampicillin (10 µg), chloramphenicol (30 µg), ciprofloxacin (5 µg), erythromycin (15 µg), gentamicin (30 µg), linezolid (30 µg), piperacillin/tazobactum (100/10 µg) teicoplanin(30 µg), tetracycline (30 µg), ticarcillin/clavulanic acid (75/100 µg) and vancomycin (30 µg). The colistin susceptible isolates by VITEK- 2 were confirmed for their minimum inhibitory concentrations (MIC) of colistin (Sigma-Aldrich) using microbroth dilution method.
The specimens received for anaerobic culture were processed for gram stain and were cultured on 5% sheep blood agar, Neomycin blood agar, Phenyl ethyl Alcohol agar and Robertson’s cooked meat broth as per the standard guidelines9 . The inoculated plates were incubated into Whitley A35 Anaerobic workstation (Don Whitley Scientific, Shipley, UK). The anaerobes were identified by gram stain, aerotolerance (on chocolate agar incubated at 370 C in CO2 incubator), special potency identification discs (Vancomycin 5μg, Kanamycin 1000μg and Colistin 10 μg) and biochemical reactions such as catalase, nitrate reduction, growth in presence of 20% bile, esculin hydrolysis. The Vitek 2 automated system (BioMerieux) and MALDI-TOF (BioMerieux) were used for species identification.
The minimum inhibitory concentrations (MIC) of anaerobes were determined by E test method for Metronidazole (range 0.016-256 µg/mL). The results were interpreted as per Clinical Laboratory Standards Institute (CLSI) guidelines10. B. fragilis ATCC 25285 was used as reference strain for quality control. Categorisation of the bacterial isolates as multidrug resistant (MDR) and pandrug resistant (PDR) were given according to the definitions specified by CDC, which stated MDR as acquired nonsusceptibility to at least one agent in three or more antimicrobial categories and PDR as nonsusceptibility to all agents in all antimicrobial categories11.
Results
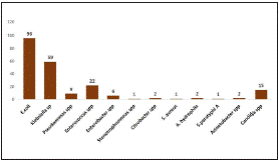
Out of 307 bile samples sent for aerobic culture and susceptibly testing, 187 (60.91%) were found to be culture positive and the remaining 120 (39.08%) were sterile. Of the 187 bile culture positives, 151 (80.74%) showed monomicrobial aetiology and the remaining 36 (19.25%) were polymicrobial. A total of 216 pathogens were isolated out of which Escherichia coli (44.4%, n=96) was predominant, followed by Klebsiella pneumoniae (27.3%, n=59). Microbial aetiology of biliary tract infections is depicted in Figure 1. Among the 15 Candida species, non -C. albicans Candida (NCAC) species were predominant (73.3%, n=11) than C. albicans (26.6%, n=4). The NCAC species were C. tropicalis (n=5); C. famata (n=2); C.glabrata (n=2); C. kefyr (n=1), and C. krusei (n=1) respectively.
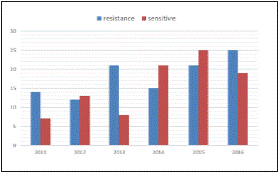
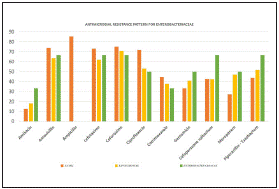
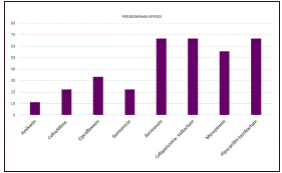
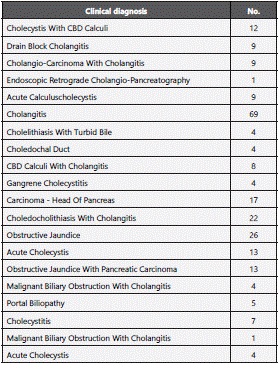
Antimicrobial susceptibility pattern over the years (Figure 2) shows overall resistance to the antibiotics and the increase in emerging pan drug (PDR)/ multi drug resistant (MDR) pathogens. Among the 201 bacterial pathogens tested for their antimicrobial susceptibility, 108 (53.73%) isolates were resistant and the remaining 98 (46.26%) were susceptible to drugs tested12. ESBL producing strains of E.coli and Klebsiella spp. were also reported in this study wherein 8 isolates of Klebsiella spp. and a single E.coli were PDR and susceptible to colistin (MIC =1 mcg/ml). All the MDR isolates were also found to be susceptible to meropenem. The co-resistance harboured among the ESBL positive E. coli and K. pneumoniae belonged to the cephalosporins, penicillins, quinolones, monobactams, beta-lactam inhibitors, aminoglycosides and Trimethoprim/ sulfamethoxazole. All the Candida species were susceptible to fluconazole with the exception of C. glabrata and C. krusei being intrinsically resistant to fluconazole. Antimicrobial resistance pattern of Enterobacteriaceae, Pseudomonas aeruginosa and Enterococcus spp. are depicted in (Figure 3,4,5). Clinical information of the patients with biliary tract infections are depicted in Table 1.
Of the 14 samples received for anaerobic culture, significant growth was observed in 5 (35.75%) specimens. Cholangitic abscess was the main clinical presentation. Bacteroides fragilis group was the predominant anaerobe in all 5 specimens. Monomicrobial anaerobic growth was seen in 4 specimens and the polymicrobial anaerobic growth was observed in one specimen, consisting of Peptoniphilus asacharolyticus and Veillonella parvula along with B. fragilis subsp. fragilis. All the anaerobic isolates were found to be susceptible to Metronidazole.
Discussion
Biliary tract infections with bacteria and Candida species are emerging and data regarding the spectrum of microbiota of the biliary tract infections and their antimicrobial susceptibility pattern is warranted for therapeutic treatment decisions12. The current study evaluated the spectrum of microbial aetiology and their antimicrobial susceptibility profile from bile samples. In the present study, the monomicrobial cultures were found to be 80.7%, with Escherichia coli and Klebsiella species being the predominant pathogens which is similar to studies done elsewhere13-15. Monomicrobial infections pose a challenge for the choice of antimicrobial drug because many antibiotics cover predominantly either gram-positive or gram-negative bacteria but not both15. Gram positive bacteria - Enterococcus species and Candida species were predominantly seen among the 36 polymicrobial infections. These findings are similar to studies reported elsewhere16. Aeromonas hydrophila (n=2) was isolated from bile as monomicrobial pathogen. Studies have reported association of Aeromonas to hepatobiliary infections and the frequency is quite low17,18. Stenotrophomonas maltophilia an unusual pathogen was isolated as a polymicrobial pathogen along with Pseudomonas aeruginosa. Stenotrophomonas maltophilia was also reported for its case of rarity from a case of cholangitis by Papakdikas et al19.
In the present study, non albicans Candida species (73%) were found to be having a predominant role in polymicrobial aetiology (53.3%) along with bacterial isolates and to a lesser extent in having monomicrobial role (46.6%). The greater level of concordance between Candida species identified in the gut and bile points up the possibility of an infectious pathway from the intestine. Risk factors for the presence of Candida in bile are biliary stenting, malignant strictures, and repeated interventions20. Mycotic infection of the biliary tract is difficult to diagnose, because the detection of fungi may represent Figure 1. only a colonization due to selection or contamination. The presence of fungal aetiology in the bile samples cannot be ruled out as just contamination but must be taken into consideration while administering anti-infectious treatment of recurrent cholangitis or even cholangiosepsis20.
The antimicrobial susceptibility profile showed predominance of PDR/MDR isolates. This may be due to the empirical prophylaxis. Microbial aetiology and their resistance pattern vary geographically. It is always necessary that narrowerspectrum agents be used as an empirical therapy to avoid superinfection and emergence of antimicrobial resistance due to treatment failure. Thus, it is very essential to know the common aetiology of biliary tract infections and their antimicrobial susceptibility profile to ensure the appropriate choice and timely administration of empiric antimicrobial therapy15. The drug resistant pathogens were susceptible to amikacin and meropenem similar to previous study by Ballal et al13,15,21. Screening and microbiological analysis for biliarytract candidiasis usually in patients receiving long-term antibiotic therapy or with immunosuppressive conditions is essential as this factor may be significant in choosing the appropriate antimycotic treatment. Echinocandins have finest activity against most Candida species and are the treatment of choice if C. krusei or C. glabrata are diagnosed or suspected20,22.
Importance of isolation of aerobic/ facultative anaerobic bacteria from biliary tract disease is known since decades. Polymicrobial anaerobic growth means growth of ≥2 anaerobes per specimen, which excludes facultative anaerobes. However, a very little is known about the significance of role of anaerobes in causing biliary tract diseases. In a study conducted by Brook I on biliary tract diseases, anaerobic growth was found in 49% of the specimens with predominant isolate being Clostridium spp. followed by Bacteroides spp23. However, in our study Bacteroides fragilis group was the most frequent anaerobe to be isolated from biliary tract diseases. B. fragilis is the most prevalent bacterium in the human faecal microbiota and is isolated frequently from pyogenic liver abscess and other intraabdominal abscess which grows well in presence of bile. Our study results were mirroring the findings stated by other workers13,24-26. The cholephilic organisms like Bacteroides fragilis plays an important role in causation of biliary tract infections and should never be overlooked in routine anaerobic cultures.
Even though there are major advancement in the surgical and nonsurgical therapy, biliary tract infections remain a significant cause of morbidity and mortality. Acute cholecystitis and acute cholangitis are the two definitive biliary tract infections most commonly encountered27. Patients with acute and chronic cholecystitis can yield organisms from gall bladder bile in 30-50% of patients. Positive culture is more common in acute compared to chronic disease and in those with an obstructed as opposed to patent cystic duct. When biliary obstruction is caused by a calculus or stenosis of a surgical anastomosis the incidence of positive culture from bile duct bile is highest i.e. around 75-100%28.
The predominant aerobic enteric organisms are Aeromonas spp, Klebsiella spp, and Streptococcus faecalis. Pseudomonas aeruginosa is rarely present and can be recovered after invasive non-surgical biliary procedures28,29. Anaerobes, especially Bacteroides spp. and Clostridia spp., may be recovered from almost 40% of infected bile samples and are cultured more frequently from patients who have had multiple biliary operations or bile duct/bowel anastomoses. Culturing anaerobes is difficult than aerobes and adequate transport and culture techniques are essential. Pure anaerobic infection is rare, mixed infection with aerobes being the rule30.
The exact source of infection is not known. Ascending infection from the duodenum may be the reason responsible for these infections29. Gram negative septicaemia may be a serious clinical complication following biliary infection at any time but occurs exceptionally after surgery or non-surgical invasive procedures such as cholangiography. Surgical risks such as endotoxic shock, acute renal failure, wound sepsis and sub-phrenic abscess in the presence of biliary infection are increased and can deteriorate the patients conditions31. Long-established infection of the gall bladder with Salmonella spp. has been indicated in the pathogenesis of carcinoma of the gall bladder32. Antibiotic prophylaxis is essential and ought to be considered before biliary surgery, endoscopic retrograde cholangiopancreatography (ERCP), percutaneous transhepatic cholangiography (PTC) and non-surgical bile drainage procedures28,29,33. The major intention is to lessen the systemic, intraperitoneal and wound sepsis caused by microorganisms released from the biliary tract.
When acute cholecystitis case is suspected, bile samples will be taken for microbiology culture and sensitivity, antibiotics are initiated once the diagnosis is confirmed. The antibiotic therapy of choice ranges from parenteral cephalosporin or ampicillin and aminoglycoside29,34. The antimicrobial regime selected is governed and depends on the severity of the clinical picture. Acute suppurative cholangitis with biliary obstruction has a high pre and postoperative mortality, thus comprehensive antimicrobial therapy is very much necessary following biliary decompression. Initial treatment is usually started without knowledge of the organism responsible and the antimicrobials given must have a broad spectrum to cover all possible pathogenic organisms. Metronidazole may be included to mask the possibility of anaerobic infection29.
Microbiological analysis of bile is a valuable diagnostic tool which aids to more adequate therapy and helps to establish local antibiotic guidelines for the management of biliary tract infections