Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive illness, characterized by episodes of acute deterioration in respiratory symptoms and lung function and usually requiring hospitalization1. The epidemiology of chronic obstructive pulmonary disease (COPD) in Colombia was described by the PREPOCOL study2 in which they showed an overall prevalence of 8.9% and it was higher in subjects of more than 60 years old. The main risk factor was wood smoke, which had a prevalence of 39.3% (use wood for cooking for >10 years) and smoking 18.3%2.
Exacerbations are a frequent complication of COPD. It is known that an average patient will suffer 2 or 3 episodes per year3, and it can be either infectious or not. The most important cause are respiratory infections, bacterial being the one that accounts for at least 70% of them in subjects who require hospitalization4. Virus are another important cause and they account for at least 18-60% exacerbations either as a sole pathogen or as a co infection, that can perpetuate bacterial infection, being more common in winter months5-7. In one third of patients that present a severe exacerbation the cause is unknown8. The consequences in clinical status of the patient are variable, but is it accepted that in 14% of the events they will not return to baseline symptoms in 35 days and some of them never will9. The in hospital mortality reaches approximately 10% and is up to 40% in the next year in subjects who need mechanical ventilation8. Moreover, when acute exacerbation of COPD is secondary to pneumonia, at 30 days after discharge, mortality in these patients have been estimated to be of 14,6 % compared with patients only with pneumonia, 12,4% and 6,7% of patients with solely acute exacerbation of COPD. The overall mortality of patients admitted for exacerbation of COPD and pneumonia has been found to be of 66,2%1.
On the other hand, the epidemiology of COPD and pneumonia has been evaluated in a study published some years ago10. The COPD cohort consisted of 40.414 adults. During the observation period, (1996-2005) 3.149 patients (8%) experienced pneumonia with an incidence rate of 22,4 per 1.000-person years. Risk factors for pneumonia include age over 65 years, heart failure, dementia and prior COPD exacerbations10.
COPD exacerbations have also a great impact in health costs. There are studies that show an average cost of hospitalization of U$7.000 and more in the patients that need admission to the ICU5 being the exacerbations the most common cause for hospitalization in these patients it accounts for almost 4060% of the annual treatment cost5.
The goal of this study is to compare the microbiology of severe exacerbations of COPD that require admission to the intensive care unit, between the ones that have alveolar infiltrates or not in chest X-Rays. We believe this is important, because we do not have knowledge of the epidemiology of such COPD exacerbations in our community. Therefore, we do not know if they have differences in microbiology, and if they need a different antimicrobial approach depending if they have alveolar infiltrates or not.
Material and Methods
This is a retrospective cross sectional study that included patients from January to december/2012 with increased dyspnea, increased sputum purulence and increased sputum in the 7 days prior to hospitalization and requiring invasive and / or non-invasive mechanical ventilation. All patients were diagnosed as severe COPD exacerbation that needed admission to the Intensive Care Unit (ICU), 40 years or older, with known exposure to wood smoke (>10 years) or cigarette smoking (>12 packs). The clinical chart was reviewed in the electronic clinical history, there was no need of spirometric confirmation. Patients with a pulmonary illness aside from COPD (TB sequelae, bronchiectasis, silicosis or interstitial pulmonary disease), that were submitted from another healthcare center, that were readmitted in less than 6 weeks or that no sample for culture, gram, viral panel and BK could be obtained were excluded: when the patients with these criteria were admitted, a blood sample was taken to do a complete blood count, C reactive protein and arterial blood gases and a chest X ray in the first 24 hours or before the third empiric antibiotic dose. If a patient was first admitted in the general floor it was taken for the study the first culture sample that was obtained, and the one obtained as the patient was admitted in the ICU was analyzed as a co infection. For the pathogen to be considered the cause of the infection it had to be representative in the sputum culture or had to have a growth of more than 10x6 CFU (Colony Formation Units), taking into account current guidelines11.
For the analysis we compared patients that presented with alveolar infiltrates in the chest X-Ray when they were admitted to the ICU, with the ones that did not have alveolar infiltrates. To define if there was or not alveolar infiltrates in the chest X ray we took the concept from the radiologist and from the pulmonologist. They both reviewed the images after the data collection. If they differed in the concept, we stayed with the radiologists opinion on whether the patients had were alveolar infiltrates or not.
We followed the patients for as long as they were admitted in the ICU and we evaluated the outcome of the hospitalization. The information-collecting tool was available in the ICU and was only filled out by the investigators of the study with the data from the medical history. Data were registered and confronted directly by the investigators. The study was approved ethics committee from the Fundación Neumológica Colombiana, the act of the institutional review board waiver the study was 201112-17403 (9/12/2011). The necessity for informed consent was waived by the institutional review board due to the retrospective nature of the study.
Statistical analysis was performed using SPSS Version 21 (IBM Corporation, Armonk NY, USA). The baseline characteristics were summarized using descriptive Statistics. Categorical variables were compared using Fisher’s exact test or the chisquared test, as appropriate. Spearman’s correlation coefficient was derived for numeric nominal parameters to evaluate alveolar infiltrates in the chest ray between radiologist and pulmonologist. A P-value less than 0,05 was considered as statistically significant.
Results
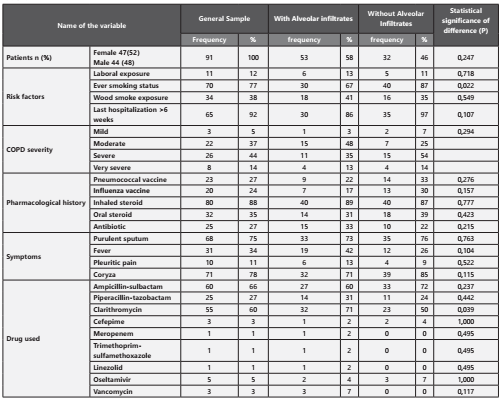
All patients who met the inclusion criteria were included, the characteristics of the 91 patients are shown in Table 1. There was a high concordance in the diagnosis of alveolar infiltrates between the radiologist and the pulmonologist (p= 0.00, Kappa= 0,978). 53/91 (58%) had pneumonia. The major differences that were statistically significant between the two groups, were a more frequent history of smoking (87,0% vs. 66,7%, p= 0,022) and use of clarithromycin (71,1% vs. 50,0%, p= 0,039) in those patients who had pneumonia. It is also of notice that patients who had pneumonia were older than those who didn’t (76,6 vs. 72,6 years old, p= 0,037), had a higher tobacco index (55.1.6 vs. 36.3 packs, p= 0,021) and higher forced expired volume in the first second (1043 vs. 765 mL/sec, p= 0,028). Patients who had pneumonia also had a higher APACHE index (19,7 vs. 16,6, p= 0,023).
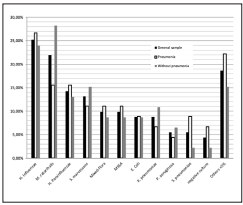
The most prevalent bacteria isolated in severe COPD exacerbations are shown in Figure 1. When we analyzed the percentage of presentation from the bacteria between the two groups (alveolar infiltrate or not), there could not be demonstrated a difference that was statistically significant. We detected a 24,2% bacterial resistance in both groups, being the most frequent AMPc (13 cases) without any difference in the patients who had pneumonia and the ones who didn’t (p= 0,357). Other resistances found were 2 cases of BLEE and one case carbapenemase resistance (KPC) in the group who did not have pneumonia, and two of methicilin resistance in the group who had pneumonia. There were no specific risk factors found to have any of these resistances.
In 71,6% of the cases the antibiotic that was empirically started was right and there were no tuberculosis cases detected. In 9/91 (9,8%) of the cases we detected a viral agent, from those cases 2 were AH1N1, one patient had Influenza A, Parainfluenza I and II (1 patient each) and respiratory syncytial virus 4 patients.1. It was necessary to intubate the patients for mechanical ventilation in 36.3% of the cases and it was most frequent in patients who had pneumonia (48,9 vs. 23,9, p= 0,013). Moreover, 9.8% of the samples taken were considered as mixed-flora and 4,4% of the cultures were negative. The majority of the patients needed noni-nvasive mechanical ventilation at any point of the hospitalization (86,8%). The need of reintubation and the infectious relapse was not frequent. (1,1 and 9,9% respectively). Global mortality was 16.7% and there was no difference statistically significant between patients who had or not pneumonia. (20,5 vs. 13,0, p= 0,346).
Discussion
In our study group of 91 patients admitted to the ICU with a COPD exacerbation, 53/91 (58%) had pneumonia, a higher prevalence than that reported in the literature10,12. Moreover, there are no differences in the microbiology in a severe COPD exacerbation between the patients who have an associated pneumonia and those who did not. In contrast with the literature, in a Spanish study published by Boixeda et al13. they actually found microbiological differences, with S. pneumoniae predominating in the pneumonia group, and P. aeruginosa in the exacerbation group with no differences in hospital stay, need for admission to ICU or in-hospital mortality13. In comparison with the literature14 we found a similar prevalence of microorganisms in order of frequency: H. influenzae (25,7%), Moraxella catarrhalis (21,98%) and H. parainfluenzae (14,29%)., without any statistically differences between the two groups. Moreover, in the pneumonia group 53/91 (58%), the microbiological trend was the same for both groups. There is also a higher frequency of methicillin susceptible Staphylococcus aureus (MSSA) in the pneumonia group; 11,7% vs 8,7%. It is noteworthy that even though there is no statistically significant difference between the two groups, there is a high prevalence of resistance (24%) and Serratia marcescens is the third one in prevalence in the without pneumonia group and the fourth in the group of patients with associated pneumonia. This finding could be due to the antibiotic pressure that has been stablished during the last years, and we must also take into account that this bacteria has the ability to form a biofilm15 and our patients might be colonized.
In our cohort, past smoking history was associated with pneumonia in the bivariate analysis (P=0.022). When reviewing literature in a Chinese cohort of 164 patients12. Multivariable logistic regression analysis showed that smoking history (OR=2.64, 95%CI 1,15-6,07, P=0,022), and other factors such as the use of COPD drugs, D-dimer levels, percentage of neutrophils in sputum were also associated with pneumonia12.
COPD exacerbation with an associated pneumonia has been linked to like chronic use of inhaled steroids and more advanced disease as it was described by File et al16. Although the mechanism by which this happens is not completely comprehended Drummond et al17, propose that this might be because of a local airway immunosuppression associated with a posterior diminished response of the innate immune system. We however, did not find any difference between the two groups.
When we evaluated the severity of the disease in the group who had associated pneumonia we found a higher APACHE score (19,76 vs 16,62) and a lower PaO2 (60,15 mmHg vs 73,41 mmHg). This finding could be used as an initial tool in the moment of the diagnosis of pneumonia to have a stricter monitoring of the ventilation function in these patients. This is important because they have a higher necessity of endotraqueal intubation that is statistically significant (48,9 vs. 23,9, P=0,013) as is also reported in the study by Daubin et al18. Overall mortality was 10,9% (10/91), with no difference in the mortality between the two groups.
Our study has limitations that need to be addressed. First, the analysis was carried out retrospectively and our data stem from a limited number of patients treated at a single center, so that not all potential confounders (eg, frequency of previous antibiotic treatment) could be systematically assessed, and our results may thus not be uncritically generalized to all patients. Second, obtaining bronchial aspirates are more prone to contamination by upper airway flora than, eg, samples stemming from bronchoalveolar lavages or protected specimen brushes. Third, there was no need of spirometric confirmation.
In our cohort of 91 patients, 94.5% had a microbiological identification. This is probable due to the fact that when as soon as a patient is admitted to the ICU, sputum cultures or endotracheal cultures are taken. However, it is important to note that the study was done in an observation cross-sectional way and in order to really affirm that most of the patients admitted to the ICU have an etiology it will be necessary to do a prospective clinical study. On the other hand, some of the patients were not able to have an etiology. It can be due probably to the fact that at that time we did not have molecular techniques such as real-time PCR with sensitivity between 57 and 100%, and an excellent specificity between 98.2 and 100% and a higher capacity of identifying other viruses and bacteria such as B. pertussis, M. pneumoniae and C. pneunomiae19.
In conclusion, in this study group of 91 patients with severe COPD exacerbation admitted to our ICU, most of them had bacterial pneumonia, higher than that reported in the literature. Pathogens between the groups were not significantly different, and patients with pneumonia were more likely to have an ever-smoking status and had higher APACHE scores. Attention should be focused on the management of underlying conditions.