Introduction
Tuberculosis (TB) is the ninth cause of death in the world, especially in a population infected by the Human Immunodeficiency Virus (HIV)1. During 2016, 10.4 million new cases of TB were reported, 1.3 million deaths in the population without HIV and 374,000 deaths in HIV population1.
In Colombia, TB is a notifiable disease and is part of events of interest in public health2. In the country, the incidence of TB of all forms is 24.2 cases per 100,000 inhabitants, with the regions of Antioquia, Valle del Cauca and Bogotá accounting for 42.5% of the cases2, and the success rates of anti-tuberculosis treatment reach 57%1.
Due to biotechnological advances and the appearance of biological agents like tumor necrosis factor-alpha (TNF-α) antagonist drugs, such as Infliximab and Etanercept, which used for the treatment of immuno-rheumatological diseases, cases of associated TB rapidly emerged3. This situation has leadto the development of guidelines for the detection of latent TB infection (LTI). A study conducted in the United Kingdom determined the overall prevalence of 10% for LTI in patients with inflammatory arthritis, who were prescribed biological therapies in an endemic area between 1998 and 2014. The researchers recommended careful evaluation and monitoring of all patients through two phases: first, clinical assessment, a chest X-ray and a BCG scar examination; and the second the Interferon-Gamma Release Assays (IGRAs) test as part of the new detection protocol, in order to reduce the risk of active TB disease4. Another study conducted in the USA reported 70 cases of TB from the information system of adverse events between 1998 and 2001, during or after treatment with Infliximab, and of these, 48 patients developed the disease after three or less infusions5.
In Colombia, it has been reported that approximately 23.5% (12/51) of the patients in management with biological therapies have an LTI, which evidences the need to carefully evaluate the risk of LTI in populations where the prevalence of TB is high6.
Although there is evidence linking TB infection to the use of TNF-α antagonist drugs, in the country there are few studies on the relationship between biological therapies and TB, and there is a gap in the knowledge of epidemiology in these patients. In this study, we aim to determine the epidemiology of ILT among patients with autoimmune disease who receive treatment with Infliximab/Etanercept.
Study population and methods
A retrospective observational study was conducted at the Fundación Valle del Lili (FVL), a non-profit university hospital that serves as a referral health center for the management of highly complex patients in the southwestern region of Colombia.
The protocol for this study was approved by the institutional review board (IRB) - Comité de Ética en Investigación Biomédica of the FVL, and it was conducted following the Declaration of Helsinki. Patient consent was not required because this study was performed retrospectively.
We reviewed the ‘Registry of patients exposed to tumor necrosis factor-alpha (TNF-α) antagonist drugs in Fundación Valle del Lili’, and this information was supplemented by reviewing the medical records. Patients with a diagnosis of chronic inflammatory disease (rheumatoid arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, Crohn’s disease, ulcerative colitis, psoriasis, psoriatic arthritis, among others), who received treatment with Infliximab, Etanercept or both and with a follow-up period of at least two years were included.
Sociodemographic and clinical data were collected directly from the patients’ clinical record. TB close contact was defined as contact with people diagnosed with TB in the last six months before the initial evaluation. Additionally, respiratory symptomatic was defined as the presence of cough for more than 15 days.
For the diagnosis of LTI, the PPD skin test was used by the Mantoux technique, defined as the M. tuberculosis infection manifested with a PPD result greater than 5mm, but without evidence of active TB disease, that is, ruling out symptoms, progressive radiographic changes or microbiological evidence of microorganism replication7.
The data was stored in an electronic database. The continuous data were presented as mean and standard deviation or median and interquartile ranges, according to the normality test. The qualitative data were presented as frequencies and percentages. The prevalence of LTI was calculated as the quotient between the number of cases of LTI between 2011 and 2017 and the population exposed to Infliximab/Etanercept in that same period. Data analysis was carried out through Stata® (StataCorp, 2011, Stata 12 Base Reference Manual, College Station, TX: StataPress).
Results
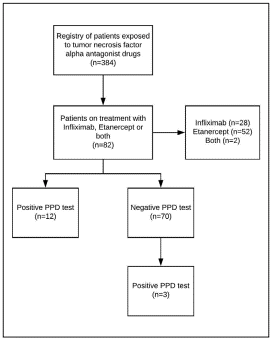
The study included 82 patients who received biologic therapy with Infliximab, Etanercept or both between 2011 and 2017; the selection of patients is represented in figure 1.
The median age was 47.5 years (IQR=28-60 years) and 76% (n=62) were female. Thirty percent were from rural areas, whereas 70% were from Cali (capital city). Clinical characteristics are presented in table 1. Also, 2% (n=2) had TB close contact and 15% (n=12) were older than 65 years.
Table 1 Clinical characteristics of patients with autoimmune diseases treated with Infliximab and Etanercept (n=82).
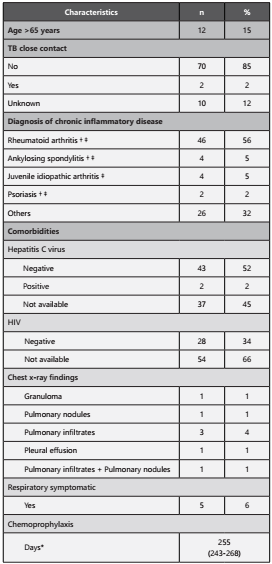
**Median (IQR). † Diseases to which Infliximab is indicated. ‡Diseases to which Etanercept is indicated. TB: Tuberculosis; HIV: Human Immunodeficiency Virus
Fifty-six percent of patients receiving these medications had rheumatoid arthritis, 5% (n=4) ankylosing spondylitis, 5% (n=4) juvenile idiopathic arthritis, and 2% (n=2) psoriasis. The other patients had a diagnosis of Crohn’s disease (n=7), ulcerative colitis (n=5), psoriatic arthritis (n=4), seronegative spondyloarthropathy (n=4), systemic lupus erythematosus (n=1), Behçet (n=1), Henoch-Schönlein purpura (n=1), sarcoidosis (n=1), septal panniculitis (n=1) and recurrent polychondritis (n=1).
Among the comorbidities evaluated, it was found that 2% (n=2) of the patients presented infection with the hepatitis C virus; it was also found that 34% (n = 28) had negative results for the HIV test, although in 66% the result of HIV test is unknown.
During the initial evaluation, before the start of the biological treatment, 15% (n=12) presented a positive PPD test result. The median of PPD skin test was 12 mm (IQR=10-17 mm). The most frequent radiological finding was the presence of pulmonary infiltrates (n=3), and in one case, there was evidence of pulmonary infiltrates and pulmonary nodules.
In the follow-up, three patients presented seroconversion, resulting in a prevalence of 3.8% of LTI. Of the patients included in the study, six had active TB, for a prevalence of 7.3%. Six percent of the cases were identified as respiratory symptomatic, ruling out the presence of active TB. Regarding isoniazid chemoprophylaxis, all patients received a preventive therapy, with the range of treatment days between 243 and 268 days (median, 255), which is equivalent to eight and a half months
Discussion
This study described the population with autoimmune diseases that received treatment with Infliximab/Etanercept and presented LTI, with a prevalence of 3.8%. Although it is an important prevalence, PPD skin test shows that it has not been able to identify well the patients exposed to TB, since studies conducted in Colombia report prevalences of 23.5% in Bogotá and 25.3% in Cali6,8. This shows the need for patients undergoing biological therapies with TNF-α antagonist drugs to undergo a diagnostic test for LTI before and during treatment, to prevent progression to active tuberculosis, since the risk of reactivation of the disease in these patients can be as high as 12 times that of the normal population9, being the IGRAs an alternative test to consider. That is why a call is made to medium and low income countries to establish measures in public health, in order to detect all cases of LTI and substantially reduce the global burden of tuberculosis consistent with Millennium Development Goals and the Global Plan to End TB10,11.
In our series, LTI was diagnosed with a conventional test, PPD; however, currently, there are newer tests for the diagnosis of LTI, such as IGRAs. IGRA has been described as a useful tool in the diagnosis of ILT in immunocompetent people with several advantages over PPD, because its results are numerical (less subject to reader bias), it does not require a follow-up to read the results and it uses TB-specific antigens that are not present in BCG and, therefore, there are not affected by the BCG vaccination status12; it is also an accurate test with a specificity of 98-100% and sensitivity of 70-90%13.
A study conducted in Spain showed that PPD test in patients with inflammatory disorders mediated by the immune system presents higher rates of false-negative results14; however, discordant results between IGRA and PPD in patients with autoimmune diseases are common12. Currently, an optimal strategy of sequential or simultaneous testing by PPD and IGRA is under discussion, which is why more research is needed in this field to improve the diagnostic tests for LTI in patients with these diseases. The new WHO recommendation for the diagnosis of LTI suggests PPD or IGRA as diagnostic tests, without a specific preference15.
Regarding comorbidities, such as HIV and hepatitis C virus infection, it is observed that when reviewing the medical records, a significant number had not been performed the corresponding screening test prior to the start of biological therapy, this is mainly due to the fact that when these therapies were implemented, the patients were not assessed by the infectious diseases service and were not requested routinely. Something similar happened with the control PPD, where it is observed that it was not requested regularly. We consider the indication of PPD test to be a priority before start of biological therapy with Infliximab/Etanercept and carry out routine controls on patients with negative PPD test results because the population receiving treatment with these biological therapies has a considerable prevalence of LTI.
Although there is many international guidelines in sight, suchas the Spanish guidelines (Ministerio de Sanidad, Política Social e Igualdad, Spain), and the American guidelines (US Centers for Disease Control and Prevention-CDC, USA), there is little local evidence in Colombia16,17. The only guide availableis Circular Externa 0007/2015, which updated the guidelinesfor the programmatic management of tuberculosis in Colombia, where it is recommended to perform screening for prioritygroups following the WHO guidelines18, one of these beingthe people who will initiate treatment with biological therapy for autoimmune diseases. Screening, in the first place, is clinical to rule out active tuberculosis based on symptoms (cough,hemoptysis, fever, night sweats, weight loss or poor weightgain in children, dyspnea and fatigue) and secondly, the performance of the PPD or exceptionally, the IGRA, being PPD thetest of choice in the country19. Likewise, adherence to established guidelines must be improved, and research studies must be conducted to evaluate IGRA as a diagnostic test for LTI in Colombia. Therefore, stakeholders and policymakers should focus their efforts on developing evidence-based recommendations for the diagnosis of LTI, which have been insufficient to contribute to the control of tuberculosis in Colombia.
Limitations Although our hospital is a referral health center of high complexity for the southwestern region of the country, insurers in Colombia determine the place of management and medical follow-up of patients with this disease, so a significant number of patients were temporarily treated in our institution and later continued their treatment in another health center of lower level of complexity; given the above, it was posible to introduce a selection bias with respect to the information that was collected in the database.
Conclusions
This study describes the prevalence of LTI in patients with autoimmune diseases treated with Infliximab and Etanercept. Although TB guidelines have been developed, including LTI screening in this population, it has not been adequately implemented in our environment yet. There are alternatives to PPD test such as IGRA, but its performance in population with inflammatory pathologies should be evaluated. It is convenient in our context, to perform a routine follow-up of patients undergoing these therapies to detect cases in time and initiate the corresponding management.