Introduction
Because Enterobacteriaceae are a common cause of community- and hospital-acquired infections, antimicrobial resistance in these bacteria have a significant impact on antibiotic use and patient outcomes. The emergence and spread of Carbapenemase-producing Enterobacteriaceae (CPE) have become a public health problem in recent decades due to their great ability to spread and became endemic in several countries of the world, including Colombia1. Their presence has been associated with a negative impact on health care systems, since they are a frequent cause of nosocomial infections 2-8 with reported mortality rates up to 50% 7-10. The diagnosis of CPE in the clinical laboratory is difficult because they can test susceptible to carbapenems despite the presence of a carbapenemase, according to the minimal inhibitory concentration (MIC) breakpoints defined by the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST), causing a “silent dissemination” in many hospitals11,12. CPE can be resistant to a broad range of antibiotics, leaving only very few therapeutic options. Some “second-line” drugs such as tigecycline, colistin, fosfomycin, aminoglycosides and occasionally quinolones may remain active against CPE. The combination of a carbapenem in high doses and prolonged infusion with one or two of the previously mentioned drugs has been associated in many reports with better outcomes for high-risk patients13. Unfortunately, none of these studies was designed with the purpose of comparing different treatment regimens making it more difficult to choose the right combination14.
In the last years, new β-lactamase combinations such as ceftazidime-avibactam and meropenem-vaborbactam have become available for the treatment of CPE. Early results suggest they are safer and more efficacious for the treatment of CPE infections compared with some of the older agents, particularly polymyxin regimens. Other new drugs active against selected CPE isolates are in different stages of development and/or approval by the Food and Drug Administration agency of the United States (FDA) or the European Medicines Agency (EMA). Among the antibiotics evaluated are imipenem-relebactam, plazomicin, cefiderocol, eravacycline, and aztreonam-avibactam15.
The aim of this review is to focus on the current evidence regarding the diagnosis, prevention, control and treatment of CPE infections, with emphasis on the association between different treatment options and mortality. In addition, ceftazidime/avibactam will be reviewed due to its near launch in Colombia.
Diagnostic challenge
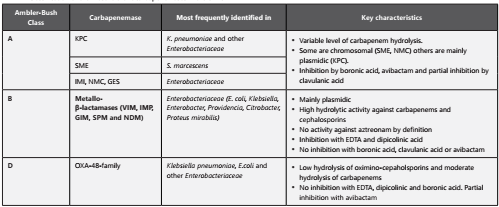
Resistance to carbapenems in Enterobacteriaceae is caused by two principal mechanisms16,17:
Expression of a cephalosporinase (extended spectrum β-lactamase (ESBL) or plasmid or chromosomal AmpC) combined with defects in membrane permeability.
Expression of a carbapenemase combined with permeability defects of the outer membrane.
Clinical and epidemiological importance of carbapenemase detection:
The resistance to carbapenems mediated by the production of carbapenemases is of high clinical, therapeutic and epidemiological relevance because
They cause hospital outbreaks associated with clones and plasmid dissemination
Depending on the molecular class (table 1), they can have a high hydrolytic activity against carbapenems, reducing their efficacy and therefore the combination of antibiotics such as polymyxins, aminoglycosides, fosfomycin and tigecycline might be necessary18.
The simultaneous production of other β-lactamases and activation of other resistance mechanisms leads to multiresistance and pan-resistance, leaving none or very limited therapeutic options.
Infections caused by carbapenemase-producing bacteria are associated with higher mortality rates(19).
Although the CLSI and the EUCAST do not recommend the routine screening for carbapenemases for therapeutic purpose (only to control its dissemination) 20,21, the antimicrobial resistance committee of the Colombian Association of Infectious Diseases (ACIN), recommends the routine testing for the presence of this enzymes based on the following key aspects:
Carbapenemases have special pharmacodynamical implications. For example, KPC enzymes have the highest hydrolytic activity against cephalosporins and carbapenems while OXA-48 enzymes have only weak activity against these antimicrobials(13)
KPC might be associated with higher mortality rates in comparison with other carbapenemase families such as metallo-β-lactamases (MBLs) (22.
Aztreonam is not a substrate of class B carbapenemases, thus classifying these enzymes and testing the susceptibility for aztreonam, allows the use of this antibiotic in combination with a second drug for MBLs.
The use of new inhibitor combinations such as ceftazidime/avibactam and meropenem/vaborbactam requires the identification of the enzyme class since these treatment regimens are inactive against class B carbapenemases23.
Some therapeutic strategies, such as the combination of two carbapenems, have been used successfully only in class A CPE (KPC) (24.
KPC is endemic in Colombia and due to its high capacity of dissemination in the nosocomial environment and between patients, it is key to identify them from the infection control perspective.
In the following section, the most frequently used methods for the detection of these enzymes in clinical isolates and clinical samples will be reviewed. The screening of colonized patients will not be addressed.
Phenotypic tests for the detection of carbapenemases
Production of carbapenemases in Enterobacteriaceae should be suspected and confirmed when intermediate susceptibility or resistance to any carbapenem is observed applying the current CLSI breakpoints25.
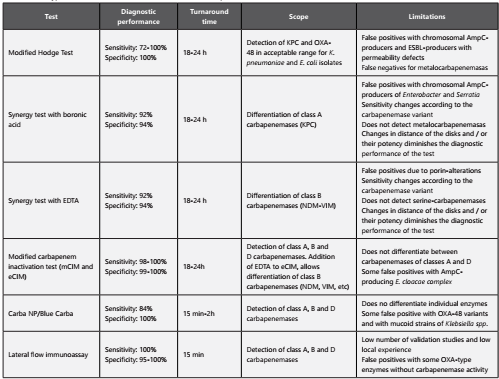
Phenotypic laboratory tests used for the detection of these enzymes can be divided into “capture” tests and “differentiation” tests. It is recommended to apply them together based on the arguments presented above26.
Capture Tests (table 2 ): These tests seek to determine the presence or absence of carbapenemases in a given isolate. According to the technique used, the turnaround time can range from 15 minutes to 24 hours. These methods do not allow differentiating between individual enzymes, or classifying carbapenemases into class A, B or D.
Differentiation and classification tests (table 3): These methods allow to identify the class to which an enzyme belongs to (class A, B or D). It is important to emphasize that due to the diversity and versatility of these enzymes, to date there is not a single phenotypic test capable of detecting and differentiating all classes of enzymes in a single step. The microbiology laboratory in collaboration with the institutional antimicrobial stewardship committee needs to decide which of these tests have higher applicability and represent a better cost-effectiveness for the institution. Colombia, as well as many other countries is frequently reporting isolates that produce simultaneously more than one type of carbapenemase (for example VIM plus KPC or NDM plus KPC) (27) . The co-expression of various enzymes decreases the efficiency of phenotypic tests to differentiate these enzymes correctly.
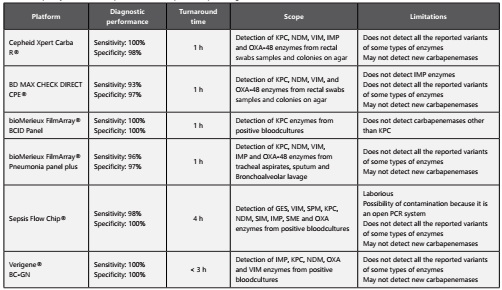
Molecular tests for the detection of carbapenemases
Currently, there are several molecular platforms based on polymerase chain reaction (PCR) for the detection of carbapenemases in Enterobacteriaceae and other gram- negative glucose non-fermenting bacteria. Among the advantages of these techniques are28,29:
Higher sensitivity and specificity compared with conventional phenotypic tests as PCR based techniques are not affected by the co-expression of other enzymes and/or defects of outer membrane permeability.
Less turnaround time: These tests take approximately one hour compared to other methods that require up to 24 hours.
Possibility of detecting enzyme co-expression: These platforms can detect the co-production of class A, B or D carbapenemase enzymes simultaneously.
Expert rules for reporting antimicrobials when CPE are detected:
Class A carbapenemases (detection of KPC or inhibitory enzymes by boronic acid)
Report as resistant aminopenicillins, combinations of β-lactams plus β-lactamase inhibitors and all cephalosporins including ceftolozane/tazobactam.
Report as resistant ertapenem (regardless of the MIC result)
Report meropenem and doripenem with the MIC result and interpretation according to the breakpoints. Include a footnote warning the carbapenemase detection.
Report aminoglycosides, quinolones, tigecycline, colistin and fosfomycin according to the breakpoints.
Test and report ceftazidime/avibactam susceptibility using a MIC method (E-test or broth microdilution). Interpret results using the current EUCAST or CLSI breakpoints.
Class D carbapenemases (detection of OXA-48 or enzymes not inhibited by boronic acid and EDTA/dipicolinic acid)
Report as resistant: aminopenicillins, combinations of β-lactams plus β-lactamase inhibitors as well as first and second-generation cephalosporins and aztreonam.
Report the results of third and fourth generation cephalosporins with the MIC result and interpretation according to the breakpoints. Include also a footnote warning the detection of a CPE.
Report ertapenem as resistant (regardless of the MIC result)
Report meropenem and doripenem with the MIC result and interpretation according to the breakpoints. Include a footnote warning the carbapenemase detection
Report aminoglycosides, quinolones, tigecycline, colistin and fosfomycin according to the breakpoints.
Test and report ceftazidime/avibactam susceptibility using a MIC method (E-test or broth microdilution). Interpret results using the current CLSI breakpoints.
Class B carbapenemases (detection of NDM/VIM/IMP or enzymes inhibited by zinc chelators such as EDTA or dipicolinic acid)
Report as resistant aminopenicillins, combinations of β-lactams plus β-lactamase inhibitors
Report as resistant all cephalosporins, including ceftolozane/tazobactam.
Report ertapenem as resistant (regardless of the MIC result)
Report meropenem and doripenem with the MIC result and interpretation according to the breakpoints. Include a footnote warning the carbapenemase detection
Report aminoglycosides, quinolones, tigecycline, colistin and fosfomycin according to the breakpoints.
Test and report aztreonam susceptibility according to the current CLSI breakpoints.
Quality control: To guarantee reliable results that translate into appropriate therapeutic decisions, internal and external quality control is definitive to ensure clinically relevant reports. Quality control must be carried out in accordance with the volume of work of the microbiology section, the endemicity of carbapenemases in the institution, country and/or region, and the type of methods performed by the laboratory. Table 4 suggests some of the quality control strains that can be used to evaluate phenotypic and molecular tests. Discrepancies should be confirmed using molecular test.
Table 4 Quality control for phenotypic detection of carbapenemases
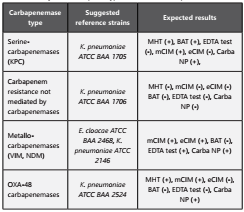
eCIM: EDTA-modified carbapenem inactivation method; mCIM: modified carbapenem inactivation method; MHT: Modified Hodge Test, BAT: Boronic Acid Test, Adapted from CLSI 201856.
Note: The modified Hodge test was removed from the CLSI recommendations for the detection of carbapenemases. However, the ACIN Bacterial Resistance Committee considers that this test is still useful in regions of high endemicity of KPC in K. pneumoniae and E. coli. Due to its limitations, a negative result does not exclude the presence of carbapenemases, which must be screened with an alternative method.
Epidemiological challenge
Due to the fast dissemination of CPE, some countries have implemented national strategies for the containment of these carbapenemases. Israel after the implementation of its national policy in 2007 achieved a decrease in the monthly incidence of nosocomial CPE-infections from 55.5 to 11.7 per 100,000 patient/days. The effective strategy was based on dedicated task force of appointed professionals. The task force paid site visits at acute-care hospitals, evaluated infection-control policies and laboratory methods, supervised adherence to the guidelines via daily census reports on carriers and their conditions of isolation, provided daily feedback on performance to hospital directors, and intervened additionally when necessary30.
In the area of infection control, the lack of randomized studies is one of the limitations when it comes to choose the most effective method in a given hospital because recommendations are not based on a high-level evidence.
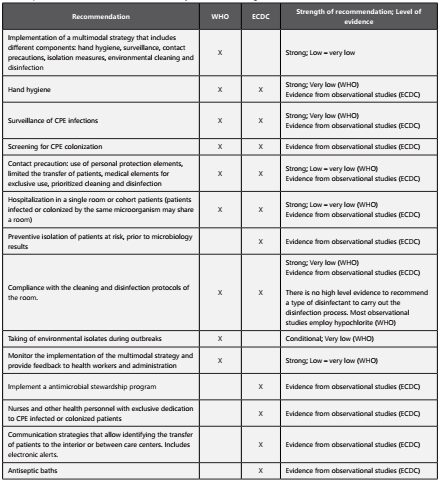
Different agencies, societies and countries have published guidelines and recommendations for the control of multidrug resistant gram-negative bacteria. In the last five years, three guidelines have been published which qualify the level of evidence using the methodology described by the Grading of Recommendations Assessment, Development and Evaluation Working Group (GRADE). The first guideline was published in 2014 by the European Society of Microbiology and Infectious Diseases (ESCMID), the second in 2017 by the World Health Organization (WHO) and the third, also published in 2017 by the European Prevention Center and Disease Control (ECDC). The ESCMID Guidelines includes recommendations for extended-spectrum β-lactamases producing Escherichia coli and multi-drug resistant Klebsiella pneumoniae but not specifically for carbapenemase producers. The WHO guideline is specific for CRE and includes studies published until 2016. Finally, the ECDC guideline reviewed studies until 2013 and focuses not only on prevention measures but also on the identification of patients at risk for CRE infections. Table 5 describes the specific recommendations for CRE control included in each of these guidelines31-33.
Screening for CPE carriers
It is recommended to perform CPE carrier screening during outbreaks or high endemicity within a hospital, in all patients that meet one or more of the following risk factors:
History of infection or colonization by CPE.
History of hospitalization in regions or centers whose epidemiology suggests a high incidence of CPE.
History of hospitalization during the last 12 months.
History of dialysis or chemotherapy during the last 12 months.
Epidemiological link with a patient infected with CPE(32,33).
Microbiological samples can be obtained from stool, rectum or perianal area or any other site where an infectious process is evidenced.
Regarding the duration of contact precautions, the ECDC and SHEA (Society for Healthcare Epidemiology of America) guidelines recommend that contact precautions should be maintained throughout the hospitalization, particularly in cases of XDR and PDR. In contrast, the WHO did not issue any recommendation in its guideline. Banach et al. recently published an article proposing the elimination of contact precautions 6 months after the initial positive CPE culture, when a patient has two negative control rectal or perirectal CPE cultures taken with one-week interval34.
Therapeutic challenge
Due to the reduced number of randomized clinical trials, the therapeutic approaches for CPE-infections are mainly based on accumulating clinical experience. A wide heterogeneity of most of the published studies (different types of infections, different groups of patients, a variety of treatment regimens, different methods and outcome definitions) can be a serious obstacle in comparing data, which precludes the possibility of a rigorous meta-analysis. Therefore, it is difficult to recommend a single therapeutic approach8. It is important to mention that some of the studies included the term CPE, whereas other studies included carbapenem resistant Enterobacteriaceae (CRE). The CDC initially defined CRE as those Enterobacteriaceae, which were non-susceptible to ≥ 1 carbapenem and were resistant to 3rd generation cephalosporins. In their November 2015 update, this definition was revised and CRE are now defined as any Enterobacteriaceae which are resistant (excluding intermediate resistance) to any carbapenem or are documented to produce a carbapenemase. In addition, for those Enterobacteriaceae, which may have intrinsic reduced susceptibility to imipenem such as Proteus mirabilis, resistance to a non-imipenem carbapenem is required. The CDC acknowledges that this definition lacks specificity for CPE, especially in low-prevalence areas35.
In 2017, Trecarichi and Tumbarello performed a literature review of the therapeutic options for CRE and/or CPE infections. The review points out the difficulty of finding comparable studies with a design that allows the identification of the best therapeutic options for patients with these infections36. In this review, the authors analyze 11 available studies. However, there are some fundamental limitations when it comes to compare these studies and draw conclusions that is important to take into account when making a therapeutic decision:
All but one of the studies included K. pneumoniae-infections. All studies were observational cohorts, which implies certain variability in the clinical conditions of patients, as well as differences in the severity of the infection and the type of CPE/CRE. There is also variation in the site of infection in the different studies, with multiple clinical samples (including blood in variable percentages) and a non-systematic use of the MIC since there are different breakpoints of susceptibility or resistance according to the region of the world (CLSI vs. EUCAST). Furthermore, a detailed definition of “monotherapy” and “combination therapy” is not included in most of the articles. In addition, there is a small percentage of reports of Verona integron-encoded metallo-β-lactamases (VIM) alone or in combination with KPC strains making it impossible to extrapolate the clinical or therapeutic impact of each enzyme. To make the comparison even more difficult, in several studies the isolated strains that expressed carbapenemases were carbapenem-susceptible in vitro based on the EUCAST cutoff point. All studies reported different antimicrobial schemes and their association with mortality rates. The different therapeutic approaches were included in the analysis of independent risk factors for mortality in most of the studies; however, there is no adjustment by multivariate analysis for appropriate antibiotic treatment, combined therapy and removal of the source of infection, making the analysis for risk factors and/or mortality associated with CRE/CPE difficult.
Finally, the characteristics of the patient population included in the multivariate models of risk factors for mortality could be divided into two categories: (a) those whose cohort included all patients diagnosed with CPE infections, regardless of whether they received active antimicrobial treatment for a small period of time, and (b) those that included only patients with CPE infections who were treated for at least 48 hours with an effective antibiotic treatment (defined as one or more active drugs due to in vitro sensitivity against CPE/CRE strains). This important difference of cohorts should be considered, especially in the group of patients who did not receive enough treatment time for a definitive adequate therapy for CPE/CRE-infection, as may represent a bias when analyzing the impact of different treatments on mortality rates.
Evidence for combined therapy
Despite the limitations of design in the studies, some studies have shown lower mortality rates using combinations of two or even three antimicrobial agents. In absence of the ideal study, those with the greatest clinical impact will be reviewed.
In a study by Tumbarello et al., the mortality of patients with bacteremia caused by KPC-producing K. pneumoniae who received monotherapy was 54% compared to combined therapy with a mortality rate of 34% at 30 days in 125 patients. Interestingly, patients who received a combination of tigecycline plus colistin and meropenem showed an independent association with higher survival rates at 30 days (Odds Ratio [OR] 0.11) (37. Similarly, Daikos et al. analyzed the clinical outcomes of 205 patients with bacteremia caused by K. pneumoniae producing KPC or VIM, demonstrating an independent association with higher survival rates in patients who received a combined antimicrobial regimen. Combination therapy had the greatest effect on survival in patients with severe sepsis, septic shock, and rapidly fatal underlying diseases38. Qureshi et al. also evaluated the results of 41 patients with bacteremia caused by KPC-producing K. pneumoniae, finding that combined therapy was independently associated with survival (OR 0.07), and a significantly lower 28- day mortality rate (13.3%) in comparison with monotherapy (57.8%)39. In a studied executed by Falcone et al. which involved 141 patients in the ICU with KPC-producing K. pneumoniae infections and septic shock, treatment with colistin (Hazard ratio [HR] 0.21) and the use of ≥ 2 active in vitro antibiotics as definitive therapy, was associated with favorable results (HR 0.08) in terms of mortality rates40. And finally, a study by Trecarichi et al. in 149 patients with hematologic malignancies and K. pneumoniae bacteremia who had received ≥48 hours of adequate antibiotic therapy, demonstrated that combined therapy was independently associated with higher survival (HR 0.32) (41.
Nevertheless, there are several publications, although in a lesser amount, which have not found a survival advantage by giving combination therapy. Some of these published studies are:
Gomez-Simmonds et al. evaluated the clinical outcomes of 141 patients with bacteremia caused by carbapenem resistant K. pneumoniae according to the number of active in vitro agents received and whether an extended-spectrum β-lactam antibiotic had been administered previously. There was no association between the individual treatment characteristics and clinical outcomes, regardless of the use of monotherapy or combined therapy or a β-lactam. Interestingly, different schemes of meropenem were used: 500 mg every 6 hours, 2 g every 8 hours, 2 g in extended infusion over 3 hours every 8 hours, but no stratification was done for each of the treatment schemes42. Likewise, no association was found between combined therapy and survival in their cohort of 118 patients with KPC-producing Enterobacteriaceae infections (of which 78 were bacteremia) by De Oliveira et al. The use of polymyxins in this study was an independent predictor of mortality. However, one of the biases of this study was that during the first 10 days of treatment for KPC-producing Enterobacteriaceae, 47 patients (40%) had 51 infections due to other etiological agents such as other bacteria (77%), fungi (21%), or virus (2%)43.
Regarding therapy and the different antibiotic options, there is more evidence for KPC producing Enterobacteriaceae than for other bacteria, enzyme types or therapies. The antibiotics with the best evidence will be discussed below.
Evidence for carbapenems in CPE infections
Some studies showed a benefit of meropenem in combined therapy by an association of lower mortality rates; therefore, carbapenems have been one of the most important pillars of combination therapy in CPE. In the following section, we will discuss the literature that has evidence in favor of carbapenems and their association with the minimal inhibitory concentration (MIC) of the antibiotic.
In an article published in 2011, Daikos and Markogiannakis reviewed the clinical data of the treatment and the results of patients infected with carbapenemase producing K. pneumoniae, reporting 44 patients who had received carbapenems as monotherapy. The analysis of the clinical result according to the MIC of carbapenem showed a survival rate of 29% with a MIC of > 8 mg/L, 60% for a MIC of 8 mg/L, and 69% for a MIC of 4 mg/L or less. Notably, the mortality rates in the group of a MIC of 4 mg/L or less are similar to those reported for K. pneumoniae infections that do not produce carbapenemase, or even CPE infections that have received appropriate combined therapy with a carbapenem. The authors concluded that carbapenems could be a therapeutic option for CPE when the MIC is ≤ 4 mg/L if it is administered at high doses and prolonged infusion and in combination with another active antibiotic44.
Other studies have also investigated the correlation between treatment with a carbapenem, its MIC and the clinical outcomes. In a study by Tumbarello et al., which included 36 patients with bacteremia caused by carbapenemase-producing K. pneumoniae, treated with a combined therapy with meropenem, the patients were stratified according to the MIC: in K. pneumoniae with a meropenem MIC of ≤ 8 mg/L, all-cause mortality at 30 days was 15.8%, vs 35.2% for patients with strains with a MIC of ≥ 16 mg/L37.
A mortality of 19.3% was observed by Daikos et al. in patients with carbapenem-resistant K. pneumoniae bacteremia treated with a combination that included a carbapenem, when the MIC for carbapenems was ≤ 8 mg/L, in contrast to an increase in mortality of 35.5% in patients who received a carbapenem in combination therapy when the MIC was > 8 mg/L for carbapenems38. A similar effect was observed by Tumbarello et al., describing lower mortality rates at 14 days in patients who received combined therapy that included a carbapenem and its MIC was ≤ 8 mg/L14. In all these studies, carbapenems were administered at high doses14,37,38 and in the studies by Tumbarello et al. prolonged infusion was included14,37.
Evidence for polymyxins in CPE infections
In January 2019, the first International Consensus Guidelines for the Optimal Use of the Polymyxins was published 45. In this guideline, the question about the treatment of CPE infections using polymyxins in monotherapy or combination therapy is addressed. However, no consensus was obtained due to the heterogeneity in the methodology of the published studies. The authors decided to issue recommendations based on voting, therefore, the level of evidence is low. The recommendations based on the previous methodology are:
Polymyxins should be used in combination with a second antibiotic with in vitro activity against the bacteria
A second or third agent (with the lowest MIC) should be added to polymyxins if the bacteria is not susceptible to other antibiotics.
Ceftazidime/avibactam as a new therapeutic option
Ceftazidime/avibactam is the combination of the third-generation cephalosporin ceftazidime with a novel β-lactamase inhibitor called avibactam. The initial studies and the clinical conditions for which the drug was approved by the FDA were urinary tract infections, complicated intra-abdominal infections and ventilator-associated pneumonia. However, due to its potent in vitro activity against CPE, especially KPC producing Enterobacteriaceae, and, partial activity against enzymes of the OXA-48 family, in addition to its high stability against other enzymes such as ESBL and AmpC, this antibiotic presents an excellent profile as a therapeutic option for infections caused by KPC producing Enterobacteriaceae. It is important to highlight that this antimicrobial agent has no activity against metallo-enzymes46.
Shields et al. reported a series of cases, including 37 patients with CRE treated with ceftazidime/avibactam, observing a 76% survival (28 out of 37 of patients) and a clinical cure of 59% (22 out of 37 patients), although this rate did not reach statistical significance in patients who received monotherapy (58% [15/26]) versus combination therapy (64% [7/11]). However, there was a recurrence of CRE-infections in 23% between patients with initial clinical success. Microbiological failure was diagnosed in ten of 37 patients (27%) and in three of the ten patients (30%) resistance to ceftazidime/avibactam (MIC > 8 mg/L) was detected47.
In a study conducted by Temkin et al. that involved 38 patients with infections caused by CRE and in some cases Pseudomonas aeruginosa, 65.8% of the participants received a second antimicrobial agent with in vitro non-resistance of the microorganism. Of these patients, 73.7% had clinical and/or microbiological cure48. Carmeli et al. published a study in 16 countries including patients with complicated urinary tract and intra-abdominal infections caused by Enterobacteriaceae or P. aeruginosa resistant to ceftazidime. Patients randomly received ceftazidime/avibactam or the best available treatment chosen by the physician. The results were comparable, with 91% success in both arms49.
Finally, van Duin et al. compared the efficacy of ceftazidime/avibactam versus colistin against CRE, finding that in the adjusted analysis for greater probability of response, patients treated with ceftazidime/avibactam had a lower mortality rate (9%) and a 64% greater probability of better clinical results than those treated with colistin (with a mortality rate of 32%)50. On the other hand, some of the concerns are the likelihood of selection of resistance with KPC-3 due to lower affinity to avibactam51, which is why, in severe infections, the possibility of combination therapy is raised, which still needs to be studied.
Conclusions
In recent years, carbapenem resistance in Enterobacteriaceae has increased dramatically and represents an important threat to global health. The optimal clinical management of CPE infections has not been established because clinical trials have not been performed with this objective. We aimed to summarize in the present review data provided by previous observational clinical studies that have investigated the impact of different treatment strategies and their outcomes. Most of these studies reported that combination therapy with two or more in vitro active agents is superior to monotherapy providing a survival benefit. The role of carbapenems in treatment of CPE infections is widely debated; however, the use of carbapenems in association with other active drugs is probably more effective for CPE isolates with carbapenem MICs ≤ 8 mg/L. Ceftazidime/avibactam already launched in other countries has shown clinical efficacy against KPC and some OXA-type carbapenemases. However, its wise use may help limit the selection of resistance.