Introduction
In December 2019, an outbreak of a novel coronavirus pneumonia called COVID-19 was first identified in Wuhan, Hubei province, China1-3. As of April 9th, 2020, there have been 1’436.198 cases and 85.522 deaths reported in at least 178 countries4. The coronavirus called SARS-CoV-2 has an incubation period of 0-14 days with a median of 5.2 days5. The clinical spectrum of the infection can vary from asymptomatic or mild clinical symptoms (81% of the cases), and pneumonia of different degrees of severity (14%) to acute respiratory distress syndrome (ARDS) with a high risk of death (5%)5,6.
The most common symptoms are fever, cough, myalgia and fatigue, and the most severe include dyspnea and lethargy5,7. The reason why some people develop severe illness while others do not remains unclear. It is possible that the severity of the disease may be associated with the ability of the immune system to control the virus, however the immune response to SARS-CoV-2 has not been fully elucidated. Here, a scoping review focusing on the characteristics of the human immune response to SARS-CoV-2 is carried out.
Methods
Protocol and registration:The protocol was written using the elements established in the PRISMA ScR extension document8. The research was reviewed by the team members of the Immunology and Translational Medicine group of the National University of Colombia, registered in Colciencias. The protocol was prospectively registered in the Open Science Framework on 13 April, 2020 https://osf.io/t4vw7/.
Eligibility criteria:To be included in the review, papers needed to measure or focus on specific dimensions of the human immune response to SARS-CoV-2. Papers in English involving human research, quantitative, qualitative, and mixed design, qualitative review articles, systematic reviews, letter to editors and case reports published from December 2019 to 9 of April 2020 were included in this review. When letters to the editor referred articles of interest that were not found in the initial search, the new articles were also included.
Information sources:To identify potentially relevant documents, the following bibliographic databases were searched: MEDLINE/PUBMED and EMBASE. The search strategies were drafted by the team member discussion and included MESH, emtree and free language terminology. The final search was exported into Mendeley and duplicates were removed.
Search:The final search strategy for PUBMED/MEDLINE and EMBASE can be found in Appendix.
Selection of sources of evidence:The inclusion criteria of the articles were reviewed by title and abstract. To increase consistency among the reviewers, teams of four researchers were generated to evaluate the results of the excluded articles. Disagreement on study selection and data extraction were resolved by consensus and discussion with other reviewers if needed.
Data charting process:A data-charting form was jointly developed by the team to determine which variables to extract. The data was charted independently by each reviewer, the results were discussed by the entire group and the data charting form was continuously updated in an iterative process. Data from eligible studies were charted using a tool designed for this study. The tool captured the relevant information on key study characteristics and detailed information on all metrics used to describe the human immune response to SARSCoV-2. The data was extracted from the main publications and supplemental materials.
Data items: Article characteristics were extracted (e.g., authors, origin, objectives, purpose), as well as the data related to immune clinical parameters, innate and adaptive immune response, cytokine storm, T cell responses, B cell responses and antibodies. The final version of the charting form can be found in the Additional file Table S1.
Synthesis of results:The studies were grouped by researched items. The results were presented in a narrative format, tables, and figures.
Results
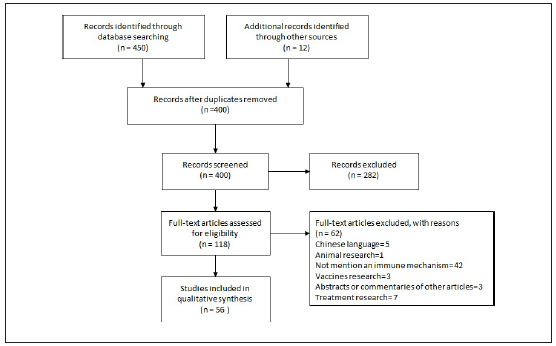
Selection of sources of evidence: The search yielded 240 articles from PUBMED / MEDLINE and 210 articles from EMBASE. After duplicates were removed, a total of 388 citations were identified and 12 indirect articles of comments or letters to editor were added (figure 1). Based on the title and the abstract, 282 were excluded, with 118 full text articles to be retrieved and assessed for eligibility. Of these, 62 were excluded for the following reasons: 5 were in Chinese, 1 was research in animals, 42 not considered the immune response, 3 were vaccine research, 3 were comments or abstracts of original papers and 7 were about possible treatments. Description of the excluded articles can be found in Additional file, table S2. The remaining 56 articles were included in this review.
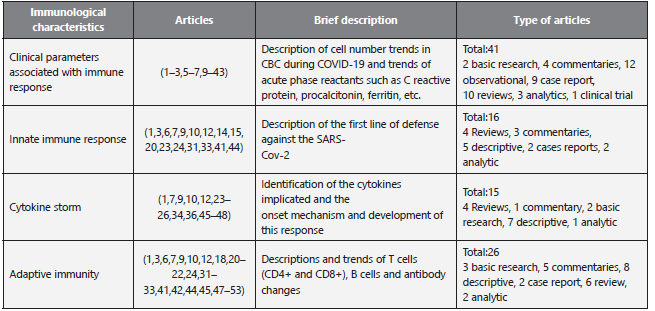
Characteristics of sources of evidence: The selected articles were classified by the topics shown in Table 1.
Results of individual sources of evidence: The type of publication, journal and aims of the search are presented in additional file:Characteristics of included studies (supplementary table S3). 1 clinical trial, 2 analytic studies, 14 descriptive studies, 9 case reports, 15 reviews, 4 basic research studies and 10 commentaries were included.
Results: For the purpose of this article and due to heterogeneity among articles, the stage of clinical compromise was classified as Severe Patient (SP) if patients were admitted to ICU, progressed to ARDS, died or were referred as severe. If that was not the case, they were considered as Non-Severe Patients (N-SP .
Discussion
Clinical Parameters associated to Immune Response
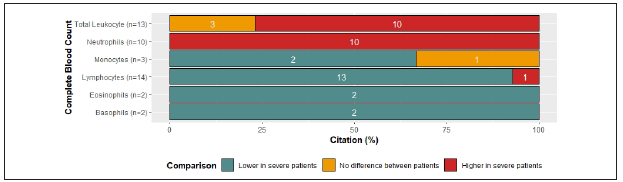
Usually upon hospital admission in the context of COVID-19, infected patients had abnormal laboratory results. In the case of complete blood count (CBC), the majority of articles that reported lymphocytes and eosinophils counts showed numbers below normal values1,3,5-7,9-29, and basophils were either normal or decreased7,9,15,28. On the other hand, total leukocytes, neutrophils, and monocytes counts displayed a tendency towards normal values. (Figure 2A ) (1,2,5,9-12,14-16,18,21,25,28,30-40.
Among cited references, levels of change in CBC varied according to severity of the disease. The majority of references that compared CBC between SP and N-SP reported increased values of leukocytes and neutrophils in SP1,5,7,9-12,18,24,25,33,34,36,41. Moreover, concurrent with clinical worsening, more than half of the references, reported decreased levels of monocytes, lymphocytes, eosinophils, and basophils in SP5,7,9,10,12,18,24,25,33-36,41) (Figure 2 B ). Additionally, it has been proposed that higher neutrophil-lymphocyte-ratio and higher platelet-lymphocyte ratio are associated with poor prognosis9,17,24.
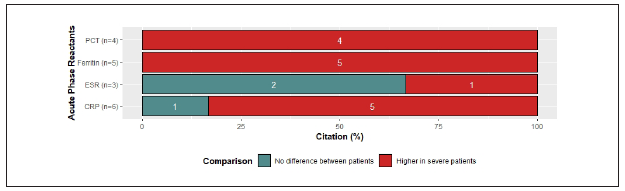
Most cases of COVID-19 have generally increased acute-phase reactants22. Here, we focus on canonical and direct mediators of the systemic immune response secondary to an infection. All of the articles that reported C-reactive protein (CRP) showed increased levels 2,3,7,9-12,14-16,19,21-24,27-29,31,34-37,41-43 except for one, whose cases were children and reported normal values40. Additionally, serum ferritin and erythrocyte sedimentation rate (ESR) were above the normal reference values9,12,19,22,23,25,27,34,37.
Unfortunately, procalcitonin levels reported among the reviewed articles did not allow us to establish a tendency since half of the articles reported normal levels1,2,5,22,36, and the other half increased levels12,15,25,28,40) (Figure 2C ).
Comparing SP with N-SP, increased levels of CRP in SP9,10,12,34,35) were reported in 80% of the references, while 100% reported increased levels of procalcitonin and ferritin in SP1,9,10,12,25,34,36. In addition, 66% of the references reported normal levels of ESR independently of severity status10,11) (Figure 2D). Particularly, abnormally high procalcitonin levels in severe patients have been associated with the development of secondary infections1,27. Although D-Dimer is not produced directly by the activation of the immune response, high levels of this marker in COVID-19 patients are strongly correlated with poor prognosis and, therefore, it has significant clinical value1,7,10,12,15,25,27,33.
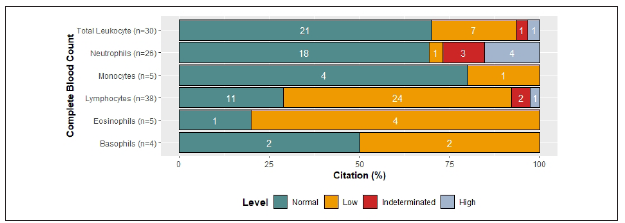
Figure 2 Clinical Parameters associated to Immune Response across references 2A Changes in CBC by citation percent: 79% reported leukocyte counts and 70% of them showed normal values, 23% reduced values, 3.3% increased values and 3.3% did not allow to establish a tendency. 100% reported lymphocyte counts and 63% of them showed reduced values, 29% normal values, 2.6% increased values and 5.3% did not allow to establish a tendency. 68.4% reported Neutrophil counts and 70% of them showed normal values, 13.8% increased values, 3.8% reduced values and 11.5% did not allow to establish a tendency. 13.1% reported Eosinophil counts, 80% of them showed decreased values and 20% normal values. 13.1% reported monocyte counts, 80% showed normal values and 20% decreased values. 10.5% reported basophil counts, 50% of them showed normal values and 50% increased values.
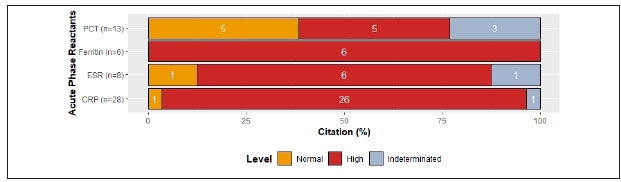
Figure 2C Levels of acute phase reactants by citation percent: 87.7% reported C- Reactive protein and 93% of them reported increased levels, 3.5% normal levels and 3.5% did not allow to establish a tendency. 40.6% reported procalcitonin levels and 38.5% of them showed normal values, 38.5% increased levels and 23% did not allow to establish a tendency. 18.7% reported ferritin levels and 100% of them showed increased levels. 25% reported ESR levels and 75% of them showed increased levels, 12.5% normal levels and 12.5% did not allow to establish a tendency.
Innate immune response
Zhou et al. performed the identification and genomic characterization of SARS-Cov-2 and found that the virus uses angiotensin converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) as cell entry receptors44,45. The ACE2 receptor is expressed on type II pneumocytes and immune cells such as monocytes, macrophages, and dendritic cells20,24,46. In general, after a virus invades the host, it is initially recognized by the innate immune system through pattern recognition receptors (PRRs) (23. The TLR3 and TLR7 recognize viral RNA, this event leads to recruitment of TRIF and MyD88 adapter molecules that activate NF-kB pathway, inducing the expression of type I interferons and the secretion of other proinflammatory cytokines for the activation of T cells, which limits the spreading of the virus6,23,41,47. A well-coordinated innate and adaptive immune response may rapidly control the virus, while a failed immune response leads to viral spreading, cytokine storm, cytokine release syndrome (CRS), ARDS and death6,7,9) (Figure 3).
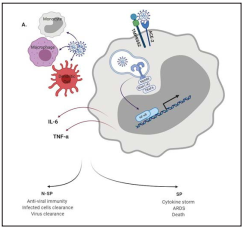
Figure 3 innate immune response against SARS-CoV-2. Innate immune cells are the first line of host defense against SARS-CoV2. Once the virus infects the cells and begins to replicate, the viral RNA copies can be recognized by TLR3 and TLR7. Signaling pathways activated by TLRs induces the phosphorylation of transcription factor (TF) such as NF-KB. TF are then translocated to the nucleus where promote the transcription of IFN type I and other pro-inflammatory cytokines. An effective IFN-I type response favors the clearance of the virus, but a decreased IFN-I levels and the high levels of other pro and anti-inflammatory cytokines related with CSS correlates with disease severity.
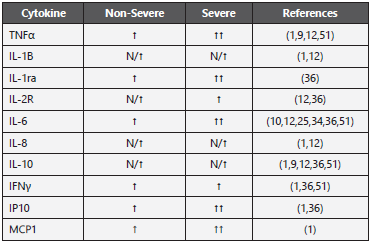
There is not enough knowledge of immunological indicators to understand the underlying mechanisms involved in COVID-193,12. Retrospective studies of SARS-CoV-2 infection have shown that there is limited information available on the host immune status, especially of the innate immune response. Besides the aforementioned parameters, SP compared to N-SP shared common features: 1. lower levels of natural killer (NK) cells and eosinophils in peripheral blood9,33; 2. Increased serum levels of IL-6 and TNFα1,6,9,10,12) and, 3. highly inflammatory macrophages with an enhanced chemokine production in the lung microenvironment48.
In a cohort of 68 COVID-19 patients, the total number of NK cells decreased and had a reduced ability to produce CD107a, IFNγ, IL-2, GzmB and TNFα, and an upregulated expresión of NKG2A, an inhibitor receptor characteristic of functional exhaustion33. The decrease in NK cell count was even more pronounced in SP than in the N-SP group, but there was no significant difference in NK cell function9. Moreover, SP had lower proportions of T and NK cells in bronchoalveolar lavage fluid (BALF) samples48. Two groups have proposed different causes of low eosinophil count: Liu et al. proposed that lung damage due to SARS-CoV-2 may result in upregulation of glucocorticoid secretion, which in turn affects the production of eosinophils in the bone marrow14; Du et al. Argued that eosinophil depletion was related to lymphopenia since T cells produce IL-5, a cytokine that promotes eosinophil proliferation and activation in peripheral blood15.
Monocyte accumulation resulted in elevated lung cytokine/chemokine levels, vascular leakage, and suboptimal T cell responses10,12. A case report showed a lower frequency of CD16+CD14+ monocytes in peripheral blood of a positive case compared to a healthy donor (HD) (31. Through gene expression analysis, Liao et al., identified monocyte-derived FCN1+ macrophages replacing the FABP4+ alveolar macrophages in the lung immune microenvironment of severe patients. These macrophages are potent chemokine producers as well as highly inflammatory48.
Cytokine storm
Cytokine storm syndrome (CSS) is a form of systemic inflammatory response characterized by the excessive and uncontrolled release of a series of cytokines. CSS can be caused by a variety of disorders including infections and rheumatic diseases, and tumor immunotherapy10,36,49) Clinically, it is commonly presented as systemic inflammation, ARDS, multiple organ failure and high inflammatory parameters.
Comparable to similar viral diseases such as SARS and MERS, most COVID-19 patients exhibit substantially elevated serum levels of both pro and anti-inflammatory cytokines. Some studies describe that IL-2, IL-6, IL-7, IL-10, G-CSF, IP-10, MCP- 1, MIP-1A, and TNFα were higher and correlated with worse clinical progression and death1,9,12,24,49. Levels of IL-6, IL-10, and TNFα correlated with T cell depletion51. Other studies reported that additional cytokines and chemokines including IL-1B, sIL-1RA, CXCL8, IL-9, FGF, GM-CSF, IFNγ, G-CSF, PDGF, VEGF, IL-18, HGF, MCP-3, MIG, M-CSF, MIG-1a, CCL27 were increased as well24,36).
In some studies, IL-6 was elevated in SP compared with N-SP throughout the clinical course and there was a correlation with disease severity1,9,10,25,34. However, this was not consistent with the results obtained by other researchers, who did not find significant difference in serum levels of IL-6 between these groups12,24. Interestingly, since IL-6 serum levels did not come from PBMCs, this cytokine might be produced by lung epithelial cells in COVID-19 patients52.
Xiong et al. found that the expression of pro-inflammatory cytokines CXCL1, CXCL2, CXCL6, CXCL8, CXCL10/IP-10, CCL2/MCP-1, CCL3/MIP-1A, and CCL4/MIP1B was significantly upregulated in BALF samples of COVID-19 patients. Furthermore, increased transcription of respective chemokines receptors such as CCR2 (CCL2/MCP-1 receptor) and CCR5 (CCL3/MIP-1A receptor) was also observed, indicating the activation of these inflammatory signaling pathways. Additionally, elevated levels of macrophage chemoattractant CXCL10/IP-10, CCL2/MCP-1, neutrophil chemoattractant CXCL2, and CXCL8 facilitate the migration of these immune cells to the site of infection, which is consistent with mononuclear cell infiltrates observed in injured lung tissue52.
Yang et al. compared the plasma cytokine/chemokine levels collected from SP and N-SP at different days after illness onset (d.a.o). The results showed that most of the cytokines were comparable between the two groups and maintained high levels, even at the later stage of the disease (≥ 15 d.a.o); however, IP-10, MCP-3, IL-1ra, and MIG were significantly higher in samples from SP. Further analysis showed that IP-10, MCP-3 and IL-1Ra expression levels were considerably high upon admission and were maintained during disease severity progression and correlated with altered PaO2/FiO2 values and Murray scores. These results indicate that these three cytokines could be considered as predictive biomarkers of COVID-19 outcome36. SARS-CoV-2 patients not only have upregulated inflammatory cytokines and chemokines that may lead to activated Th1 cell response, but also secreted excessive anti-inflammatory cytokines such as IL-10, IL-4, and TGF-β that may suppress inflammation by Th2 polarization26. TGF-β signaling can be modulated by virus infection to prevent apoptosis and to promote fibroblast proliferation and myofibroblast differentiation, thus playing a critical role in the development of pulmonary fibrosis52. These cytokines (pro and anti-inflammatory) mediate T cell depletion and extensive pulmonary pathology, leading to massive infiltration of neutrophils and macrophages, diffuse alveolar damage with the formation of hyaline membranas and diffuse thickening of the alveolar wall7.
Elevated serum cytokine and chemokine levels in infected patients are correlated with disease severity and adverse outcome, suggesting a possible role for hyper-inflammatory responses in COVID-19 pathogenesis9,52. All these data suggest that widespread lung damage associated with SARSCOV 2 may be caused more by an exaggerated innate immune response than the virus itself. In table 2, we resume the principal findings.
Adaptive immunity
T cell responses. As mentioned above, several articles included in this review showed reports of lymphopenia in peripheral blood of COVID-19 patients10, which correlated with disease severity9,10,12,18,51. Additionally, this reduction seems to be agedependent since elderly patients had lower counts than their young counterparts, which could be partially explaining the high morbidity and mortality rates within this population21,51.
Surprisingly, autopsies of COVID-19 patients revealed destruction of secondary lymphoid organs and atrophy of the spleen9. The immune infiltrate in the lungs of SP corresponded mainly to monocytes and macrophages, with a low presence of lymphocytes, unlike N-SP, whose BALF transcriptome showed high proportions of tissue-resident CD8 T cells (CD8 T cell) (48. These pathological findings suggest a “primary” cytokine storm, which is mainly produced from macrophages and epithelial cells, as opposed to a “secondary” storm that is produced by activated T cells24, and are consistent with an upregulation of genes associated with an acute inflammatory response observed in epithelial cells from severe patients48.
Within the cytokine storm caused by SARS-CoV-2 infection, high concentrations of IL-6, TNFα, and IL-10 have been reported, which could be causing the destruction of lymphatic nodes and depletion of tissue-resident and circulating lymphocytes1,9,12,24,49,51. Both IL-6 and IL-10 can inhibit IFNγ production and upregulate PD-1 expression in lymphocytes through SOCS-3 signaling6, whilst TNFα can be recognized by TNFR1 and induce apoptosis by activating the p53 pathway, whose gene transcription profile was enriched in PBMCs from COVID-19 patients52.
Supporting this theory, Diao et al. found that these three cytokines were negatively correlated with total lymphocyte counts and when serum levels of these cytokines subsided in the late stages of the disease, the number of lymphocytes increased. Moreover, high levels of IL-6 and increased expression of TNFR1 have been associated with aging, making elderly patients more susceptible to developing lymphopenia and therefore greater severity of the disease51.
Another possible explanation for the lymphopenia observed in COVID-19 patients could be selective pyroptosis of these cells through the NLRP3 inflammasome activation induced by viral infection20,22. Few studies concluded that the virus was not capable of infecting lymphocytes due to their low levels of ACE2 expression and because no viral RNA was detected in PBMCs52. However, by using human T lymphocyte cell lines, researchers found that, unlike SARS-CoV, SARS-CoV-2 is capable of infecting these cells.This infection was receptor-dependent and mediated by the S protein of the virus, suggesting that ACE2 is not the only receptor that can interact with the virus53. Other studies have proposed the possible action of CD147, CD163 and DCSIGN, present in other leukocytes, as viral entry receptors12. Fortunately, similar to MERS-CoV, SARS-CoV-2 infection appears to be abortive in lymphocytes, as the virus could not replicate after infection53. Further studies are required to confirm whether this infection activates apoptotic pathways (i.e. pyroptosis) in lymphocytes, contributing to lower counts of immune cells.
Finally, reduction of circulating lymphocytes could also be associated with an impaired or limited lymphopoiesis caused by viral infection54 or redistribution of immune cells via attachment to the vascular endothelium or lung infiltration52. Both of these hypotheses rely on the role of platelets in viral infection, as platelet-derived CXCL4 prevents Agglutinin-A from inhibiting lymphocyte generation in bone marrow, and thus when activated, they can enhance lymphocyte adhesión to the endothelium, thereby promoting homing to endothelial veins and migration to lungs41.
To have an effective antiviral response, adequate activation of helper and cytotoxic lymphocytes is necessary. Hence, researchers have also characterized the frequencies, phenotypes, and functionality of lymphocytes subsets. CD4 T cell counts had the same behavior as total lymphocytes, with SP having the lowest numbers9,10,12,51. Interestingly, a multiple linear regression model revealed that CD4 T cell counts could predict the duration of viral RNA in swabs and stool samples from patients42.
The percentages of different CD4 T cell subsets also appear to influence the severity of the disease. A high proportion of naive CD4 T cell compartment is usually associated with a better response to infectious diseases. However, several authors reported a significant increase of this population in detriment of the CD45RO+ memory subset in SP3,7,9,48. Isolated CD4 T cells from patients had a higher expression of TIGIT, an inhibitory receptor, and produced less IFNγ, TNFα, and IL-2, cytokines characteristic of a Th1 response, when stimulated in vitro; this phenomenon was even more noticeable in SP32. In addition, the number of Tregs also seems to be lower in infected patients, especially in SP9,12. In sum, a reduction of memory and regulatory subsets may contribute to the maintenance of a proinflammatory environment, which leads to exhaustion of T cells and an inadequate helper response. CD8 T cell counts showed contradictory behaviors; Qin et al. found no significant difference in the number of circulating CD8 between SP and N-SP. Nevertheless, they demonstrated that the number of CD8 T cells in COVID-19 patients was lower than in HD9,33. In contrast, other researchers reported a significant decrease in CD8 T cells in SP compared to N-SP12,21,51.
In the lungs of N-SP, there was an increase of CD8 T cells, which expressed high levels of effector molecules including GzmA, GzmK, FAS-L, CCL5, and tissue residence markers such as ITGA1, CXCR6, and JAML48. This cytotoxic CD8 infiltration may have an important role in virus elimination since these cells had an effector phenotype and a higher level of proliferation in N-SP that in SP. However, some authors found that the CD8 T cell population was also increased in SP lungs, which could be explained as a side effect of hyper-inflammation or late lung infiltrate48.
In a case report of a COVID-19 patient, a peak of CD8+ CD38+ HLA-DR+ cells, known for their role in antiviral response, was reported from day 7 to day 9, to finally fall on day 2031. This subset was much higher than in HD, Zheng et al. demonstrated that CD8 T cells from SP upregulate TIGIT and produce significantly lower amounts of GzmB and Perforin B than N-SP32. Another study reported that CD8 T cells from COVID-19 patients expressed higher levels of PD-1 and that among them, SP had the highest percentages of CD8+PD1+ cells51. This exhaustion phenotype in CD8 T cells was accompanied by a higher expression of NKG2A, with a lower expression of CD107a and lower production of IFNγ, IL-2, and GzmB33. In addition, SP had lower percentages of CD8+CD28+ cells, which could mean a decrease in the activation of these cells. However, Chen and Qin found no difference in the number of IFNγ producing cells between patients and HD3. Taken together, an improper T helper response accompanied by a strong inflammatory environment could result in the functional exhaustion of the cytotoxic compartment and therefore a progression of the disease, resulting in ARDS and subsequent death (Figure 4).
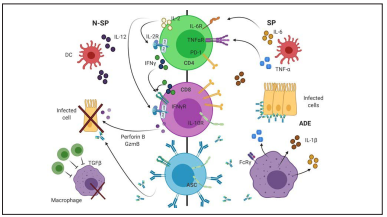
Figure 4 Adaptive Immune Response against SARS-CoV-2. Overview of the adaptive immune changes between severe (SP) and non-severe patients (N-SP). Elevated levels of IL-6 and TNFα in SP drive LT-CD4 and LT-CD8 exhaustion, therefore reducing their ability to secrete effector cytokines such as IL-2, IFNγ, Perforin B and GZMB. In addition, lower levels of Tregs (dark green cells) accompanied by a higher production of specific antibodies against SARS-CoV-2, which activate FcRγ in macrophages, may be maintaining a proinflammatory environment, increasing tissue damage. Our current model of adaptive immune response needs to be confirmed by further studies addressing CD4-CD8-ASC cell interplay as well as the role of ADE in SP. ADE: Antibody Disease Enhancement; ASC: Antibody Secreting Cell; DC: Dendritic Cell.
B cell responses: The production of neutralizing antibodies against SARS-CoV-2 plays an important role in the late stages of infection and might provide long-lasting memory that prevents reinfection41. Qin and Chen found no difference in the total numbers of B cells between SP and N-SP9,12. However, Chen reported differences in LB percentages between these two groups12.
In a case report of one COVID-19 patient, the kinetics of antibody-secreting cells (ASCs) was described. ASCs appeared at the time of viral clearance (day 7), increasing significantly on day 9. The expansion of these cells was accompanied by an increase in circulating T follicular helper cells, which have an important role in isotype switch and affinity maturation of ASCs31. Furthermore, when evaluating the transcriptional profile in PBMCs of patients with COVID-19, the signaling pathways related to humoral immune response mediated by circulating immunoglobulin and B cells immunity were upregulated52.
Antibodies: Antibodies against SARS-CoV-2 were found in all patients samples one month after infection in both N-SP and SP subjects45,55,56. Although no differences were found in either global antibodies or complement proteins between patients and HD9, Zhang et al. proposed that some patients had higher titers of antiphospholipid antibodies, including anticardiolipin and anti-B2-glycoprotein, which has been associated with the development of severe thrombosis and vascular injury24.
The presence of anti-Nucleocapsid (NP) or anti-Receptor Binding Domain (RBD) IgG against SARS-CoV-2 in the serum of patients was correlated with neutralizing titers, therefore it is safe to conclude that neutralizing antibodies naturally develop in response to infection. The seroconversion of IgG and IgM of anti-RBD antibodies developed first than that of anti-NP and, surprisingly, the peak of seroconversion developed faster in SP. Another striking finding was that IgG seroconversion happened at the same time or even earlier than that of IgM, suggesting a dysregulated antibody production18,31,45. IgM and IgG production reached a plateau on day 15 and day 21, respectively56.
Higher production of antibodies is usually associated with a better response to infection, therefore, Kelvin et al assessed antibody titers in COVID-19 patients taking into account their age and comorbidities in order to investigate whether these could be affecting antibody production18. Patients with or without comorbidities had similar levels of specific antibodies, and no significant difference was found between elderly and young adults, although the latter group always had a lower viral load during the disease. They also concluded that serum levels of these antibodies did not correlate with disease severity18, however two other studies found an association between high levels of anti-SARS-CoV-2 antibodies and worse outcome of the disease7,55. These last findings suggest the possibility of development of Antibody Dependent Enhancement during SARS-CoV-2 infection, indicating that SP could be producing non-neutralizing antibodies that overstimulate the innate immune system, increasing production of MCP1, IL-8, and IL-6 and thus aggravating the degree of inflammation and tissue damage, as well as exacerbating the previously mentioned lymphopenia7,53.
Conclusions
Immune response-associated clinical parameters such as lymphopenia, neutrophilia, and elevated levels of CRP and ferritin effectively correlates with disease severity and prognosis in SARS-CoV-2 infection. COVID-19 severe patients display immune dysregulation characterized by a hyperinflammatory response and sustained cytokine production that correlates with high mortality.
Moreover, disease severity in COVID-19 patients strongly correlated with lower T cells numbers accompanied byan overall lower Th1 cytokine production in CD4 cells. Concomitantly, increased expression of exhaustion markers and decreased secretion of effector cytokines in the CD8 compartment revealed an impaired cytotoxic response, especially in severe Patients. Considering that severe patients had a higher and faster rate of specific antibody production, a proper cellular compartment response seems to be more desirable for viral clearance.
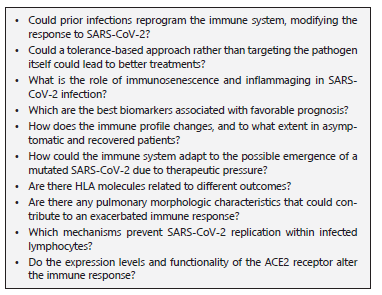
Further research is needed to elucidate the immunological features that allow certain individuals to be better equipped to resist or tolerate the infection, therefore we propose some outstanding questions (Question BOX) to address this issue.