Introduction
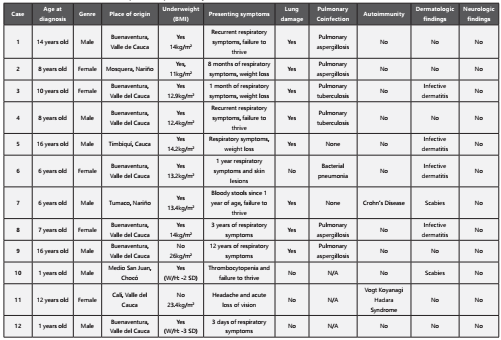
Human T-lymphotropic virus type 1 (HTLV-1) is member of the Retroviridae family and it is classified as an oncovirus1. It was isolated in 1980 making it the first human retrovirus to be identified2. HTLV-1 infection has been mainly studied in the adult population as the causative agent of T-cell leukemia/lymphoma (ALT) (3,4 and HTLV-1-associated myelopathy/ tropical spastic paraparesis (HAM/TSP) (5. The prevalence of HTLV-1 infection varies broadly depending on the demographic region, affecting predominately intertropical regions in Africa, Japan, Melanesia, Australia, and South America (Chile, Perú, Brazil, Ecuador and Colombia) (6-8. The Pacific coast of South America is known to have the highest prevalence of HTLV-1 infection, as described in demographic distribution studies in Perú9 and Colombia10, and it further affects afro-descendant and indigenous communities11,12. It is estimated that 10 to 20 million people worldwide are infected5,6, although the majority remain asymptomatic. Similar to other retroviruses, screening for the infection can be done with enzyme immunoassay or by a particle agglutination test. Confirmation tests include indirect immunofluorescence assays and Western Blot13. The modes of transmission for HTLV-1 include mother-to-child transmission, primarily through prolonged breast-feeding; sexual transmission; and transmisión with contaminated blood products, which underlines the importance to test blood products for this particular virus14,15.
Traditionally, HTLV-1 infection has been associated with low morbidity, since only 5% of HTLV-1 infected individuals develop ALT or HAM/TSP5. However, studies have shown that HTLV-1 seropositive individuals may present with a broad range of clinical manifestations, including inflammatory syndromes (uveitis, thyroiditis, and alveolitis), neurological symptoms not explained by myelopathy (polyneuropathy, myositis, dysautonomia), and opportunistic co-infections (strongyloidiasis, tuberculosis, and aspergillosis) (7. Some studies have even shown that HTLV-1 infection may be associated with clinical depression and nutritional deficiencies16,17. However, the vast majority of patients that are HTLV-1 positive remain asymptomatic, and the precise mechanisms underlying the development of symptoms in a subgroup of patients remain to be determined14.
Compared to the adult population, studies focused on pediatric/juvenile HLTV-1 infection and its clinical manifestations are scarce. Most reports describe the development of neurological symptoms directly associated with HAM/TSP in children18-20. Another common finding is infective dermatitis, which serves as a marker for the development of neurological manifestations in the pediatric population5. Pulmonary symptoms and radiological findings associated with HTLV-1 infection have been reported in adult cohorts, mainly parenchymal abnormalities, including thickening of bronchovascular bundles, ground-glass opacity and bronchiectasis21-23. However, these pulmonary findings have not been extensively studied in the pediatric population. Here, we aim to clinically describe a cohort of juvenile patients with confirmed HTLV-1 infection and no neurologic symptoms, that presented with a high rate of pulmonary disease and opportunistic co-infections. It is important for clinicians to consider the wide variety of clinical manifestations in pediatric HTLV-1 seropositive patients and their potential pulmonary compromise.
Methods
Patients and diagnosis
A descriptive, retrospective cohort study was conducted in our referral pediatric hospital in Cali, Colombia. It included all pediatric patients (1 to 18 years of age) diagnosed with HTLV-1 infection, between January 2017 to February 2020.
Clinical suspicion of HTLV-1 infection was raised based on a high-risk demographic background (African-Colombian and indigenous patients), along with clinical manifestations suggesting immunocompromise, nutritional deficiencies, inflammatory disorders or chronic pulmonary diseases. Screening for HTLV infection was performed with ELISA serological testing and later confirmed with a Western Blot exam.
Data and análisis
The patients demographic and clinical data were collected from medical records and institutional databases between January 2017 to February 2020.
Patient Evaluation
The clinical history and laboratory tests from patients with confirmed HTLV-1 infection by Western Blot were examined. Age of diagnosis and region of origin was assessed. Nutritional status was determined according to the Centers for Disease Control and Prevention (CDC) classification24. All patients had a complete blood count, peripheral blood smear and clinical assessment of lymph nodes to screen for ALT. The clinical history, including anamnesis and physical exam, were reviewed to assess potential neurological manifestations compatible with HAM/TSP. Associated diseases and potential opportunistic infections were also studied. Three consecutive stool tests were performed to rule out Strongyloides coinfection. An HIV ELISA test was performed to test coinfection with this virus. Patients presenting with respiratory symptoms and/or sepsis were initially studied with a conventional chest radiography and/or chest computed tomography scan. If parenchymal damage was observed in imaging studies, a fibrobronchoscopy with bronchoalveolar lavage was performed. Several studies were carried out in the bronchoalveolar fluid, including a galactomannan test, tuberculosis PCR, film array, bacterial and fungal cultures. Those presenting with manifestations of inflammatory diseases were assessed according to their clinical findings. Inflammatory Bowel Disease (IBD) was confirmed with colonoscopy and intestinal biopsy. The diagnosis of autoimmune thrombocytopenic purpura with persistent thrombocytopenia rulling out malignancy by flow cytometry. The diagnosis of Vogt-Koyanagi-Harada syndrome was made by a retinologist after ocular angioresonance evaluation.
Results
Demographic and Clinical Features
Twelve patients with confirmed HTLV-1 infection were included in this study, seven males and five females (Table 1). Eleven patients were originally from and resided in the central and southern regions of the Colombian Pacific coast. Ten patients had underweight per BMI calculation according to the CDC index.
Importantly, none of the patients showed clinical or laboratory signs of ALT. Moreover, none of the individuals presented with neurological symptoms and their physical exam did not exhibit abnormalities suggestive of HAM/TSP. Regarding dermatological symptoms, six out of the twelve patients presented findings (four with infective dermatitis and two with scabies).
In terms of HTLV-1- associated diseases and opportunistic infections, none of the patients had a positive HIV ELISA test, and stool tests were all negative for Strongiloydes. Most patients (eight) presented with respiratory symptoms such as coughing with sputum, dyspnea and respiratory distress. Initially, chest X-rays were performed, showing signs of chronic pulmonary disease such as bronchiectasis, pneumatocele, reticulonodular opacities. Posteriorly, chest CT scans were performed to further characterize these lesions, showing chronic inflammation, bronchiectasis, and subpleural bullae as the major findings (Figure 1). Given the degree of pulmonary compromise, additional testing was carried out to rule out potential pulmonary co-infections. Four out of eight patients with structural lung disease and respiratory symptoms had positive galactomannan test in (cut off > 0.5) suggesting pulmonary aspergillosis; interestingly, none of them had a positive serologic test to diagnose systemic aspergillosis. Additionally, two patients exhibited positive gene PCR testing for Mycobacterium tuberculosis in bronchoalveolar fluid. All fungal cultures were negative. One of the patients died from pulmonary sepsis.
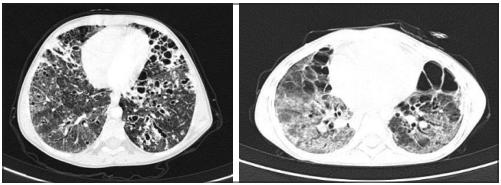
Figure 1 Chest CT scan with chronic pulmonary damage of a patients coinfected with aspergillosis. Image A. Radiological findings described as ground-glass opacity diffusely distributed in both lungs with multiple bronchiectasis involving predominantly lung bases. Cystic images diffusely distributed in both lungs, some subpleural and other centrilobular. Image B. Radiological findings described as ground-glass opacity diffusely distributed in both lungs, septal enlargement, cystic bronchiectasias predominantly in the upper lobe, subpleural bullae mainly in the apex.
Regarding other clinical manifestations and inflammatory diseases, one patient presented with weight loss, bloody stools, intermittent diarrhea and fever, ruling out an infectious etiology by repeated stool examination and stool culture. A colonoscopy and intestinal biopsy finally revealed histologic findings compatible with IBD, specifically Crohn’s disease.
Other patient presented with acute fever and general symptoms that progressed to acute visual loss with a neurological clinical exam revealing II and III cranial nerves compromised.
Infectious causes were ruled out by CSF culture and meningeal PCR (film array), then, assessed with ocular angioresonance with a later evaluation by a retinology specialist concluding severe non granulomatous bilateral panuveitis, optic nerve edema, multifocal retinal detachment.
Finally, another patient presented with a history of long-lasting hospitalization in the pediatric intensive care unit due to extreme thrombocytopenia without adequate response to corticosteroids, also ruling out other autoimmune diseases, malignancy and other causes of acquired immunodeficiency.
Discussion
In the present study, we describe the clinical presentation and diagnostic testing of twelve pediatric patients with confirmed infection by HTLV-1. Our main observation was that none of the patients exhibited signs, symptoms or laboratory findings suggesting ALT or HAM/TSP, the two main entities associated with HTLV-1 infection, but rather presented with respiratory symptoms and were found to have an important degree of structural pulmonary disease. Similar to previous reports in the literature, infective dermatitis was a frequent finding in these pediatric patients25. Another important observation was that most individuals in this cohort presented with nutritional deficiencies and underweight, which could be directly associated to chronic HTLV-1 infection or a risk factor for it. It is important to note the origin of these patients and the difficult socioeconomical conditions in the Colombian pacific coast as a bias. For most patients the clinical suspicion for HTLV-1 infection was only raised after a torpid clinical course and recurrent visits to the emergency department in other institutions.
Mechanistic studies suggest HTLV-1 associated inflammatory disease may be due to a genetically determined inefficient Tcell response to the infection21,26. Recently, pulmonary complications associated to HTLV-1 infection, such as alveolitis and bronchiectasis, were reported in an Australian indigenous community and a Japanese adult cohort21,23. However, to the best of our knowledge, there are no published reports of HTLV-1 associated lung disease in children. We found eight out of twelve HTLV-1 seropositive patients presented with respiratory symptoms and had a clear evidence of structural pulmonary lesions in their chest CT scans, consisting of interstitial damage, bronchiectasis and subpleural bullae. Further research and reports are warranted in the pediatric population to alert clinicians of pulmonary complications in these patients. Also, future studies should focus on establishing risk factors that could predispose to lung lesions in the context of pediatric HTLV-1 infection.
The few reports that have studied the association between HLTV-1 and tuberculosis have been carried out in countries where both infections are endemic. A study in Brazil found that in adult population there is a threefold risk of acquiring pulmonary tuberculosis when there is an underlying HTLV- 1 infection27. Consistently, we found that two out of eight patients in our cohort had pulmonary tuberculosis and important pulmonary compromise. Both HTLV-1 and tuberculosis are neglected diseases associated to social vulnerability, however, the mechanism driving the increased susceptibility of developing tuberculosis in the HTLV-1 infected patient is still unknown. The other opportunistic-like agent we found in the bronchoalveolar fluid of our patients was Aspergillus spp. To the best of our knowledge, there are currently no reports of pulmonary aspergillosis in pediatric patients with HTLV-1 infection. Pulmonary aspergillosis can definitely accentuate the pulmonary inflammation and scarring, worsening the child’s prognosis. We suggest that these two opportunistic infections (tuberculosis and aspergillosis) should be considered when evaluating pulmonary symptoms in a HTLV-1 seropositive pediatric patient, since they are both susceptible to treatment. An association between HTLV-1 and Strongyloidiasis and/or HIV has been widely described28; however, we did not find co-infection with these agents in our study.
Finally, HTLV-1 has been associated to the development of autoimmune diseases in adults, including Rheumatoid Arthritis, Systemic Lupus Erythematosus, Sjögren’s Syndrome, uveitis, and thyroiditis7. The biological mechanism behind this correlation is still being studied; current reports suggest a loss of tolerance to self-antigens and cell function modification mediated by the HTLV-1 infection29. Interestingly, we found three patients expressing different types of autoimmune disease, one presented with Crohn’s Diseases, another with autoimmune thrombocytopenic purpura, and the other was diagnosed with Vogt-Koyanagi-Harada syndrome (granulomatous uveitis).
In conclusion, most of the published literature regarding HTLV-1 focuses on the adult population, and the studies carried out in the pediatric population mostly concentrate on neurological or dermatological manifestations of this infection. It is important for the clinician to keep an open mind and consider alternative manifestations of HTLV-1 infection, including pulmonary disease, opportunistic co-infections, and inflammatory disorders. Furthermore, HTLV-1 infection has not been yet included in the list of neglected diseases, and we believe this should be addressed in order to improve detection and treatment. An early diagnosis can lead to better care and quality of life for pediatric patients. It is also crucial to diagnose this disease in childhood and potentially identify the mode of transmission, which is in many cases mother-to-child. This could lead to better control of this neglected infection that affects predominantly vulnerable population in low-income countries.