Introduction
Seborrheic dermatitis (SD) is a chronic and recurrent inflam matory skin condition with a preference for areas rich in se baceous glands1. The disease is characterized by poorly de fined scales and erythematous plaques, with high variability in amplitude and morphology, depending on the location of the lesions2. Areas as the scalp, facial region, especially nasolabial folds and lines and the supraciliary region, exter nal auditory canals, anterior auricular areas, and genitalia are affected. Itching is a symptom referred by 80% of patients, especially those whose scalp is affected3. Dandruff is refered to a mild condition of SD, characterized by itchy, flanking skin without inflammation1.
According to the age group, four principal variants of SD are recognized: SD in adults, infants, SD associated with HIV (Human Immunodeficiency Virus) and seborrhea type der matitis associated with medications4. On the other hand, the spectrum of the clinical variants in adults include blepharitis, exfoliative dermatitis, pityriasiform SD, flexural SD, folliculitis and pityriasis capitis. In the infant, the variants include SD of the scalp, Leiner’s disease and pityriasis amiantacea. Even though, Malassezia spp. role in the pathogenesis of sebo rrheic dermatitis remains controversial, fungal eradication showed a strong association with the remission of the cli nical presentation5. Therefor host and yeast factors involved in the transition of Malassezia from commensal to pathogen are matter of studies. Besides, consequences of removing the yeast population from the skin, the resistance profiles of Ma lassezia spp. and the side effects on different groups of me dications are little known. Here, we are presenting a revision of the pathophysiology of SD, the role of Malassezia spp. and alternatives for both topical and oral antifungal treatment in the management of the disease.
Materials and methods
A bibliographic search of review articles or management guidelines was carried out between December 1, 2018, and May 31, 2019, in the databases: MEDLINE-PUBMED, EMBASE, and SCOPUS, as follows: Title or type of publication: Review OR update. Title/Abstract: «dermatitis, seborrheic» [MeSH Terms] AND treatment [MeSH Term] AND guidelines [MeSH Terms]. We only included papers in English. The articles found were evaluated in terms of their content, feasibility, comple teness, consistency, and the quality of the evidence.
The disease and Malassezia
The etiology of the disease is multifactorial and it is associa ted with the presence of Malassezia yeast, hormonal factors, levels of sebum secretion, immune response, neurogenic fac tors, and external factors. The three main etiological factors correlated to incidence of SD are: presence of Malassezia spp., sebaceous secretion and the individual susceptibility to develop the pathology6. Immune system plays an essential role in develop of symptoms, being asymptomatic in immu nocompetent individuals7.
Malassezia species appear to contribute by triggering the skin innate immunity through complex interactions between fungal cells and virulence factors. This ends up in increasing the production of lipases, inducing an inflammatory respon se by releasing oleic acid and arachidonic acid, and produ cing bioactive components from sebum lipids8. Released un saturated fatty acids and metabolites have direct irritant and scaling effects on keratinocytes, while triggering an increased inflammatory response secondary to loss of the epidermal barrier function. Also, the arachidonic acid, metabolized by cyclooxygenase, serves as a source of pro-inflammatory ei cosanoids, as well as prostaglandins, which contribute to the damage in the corneum stratum. Simultaneously, the kerati nocytes produce pro-inflammatory cytokines, IL-1α, IL-6, IL- 8, and TNF-α, which increase and maintain the inflammatory response, thus establishing the chronicity of the infection 9.
Epidemiology
Prevalence of SD is highly variable, between 3 to 10% of the general population, and it is more frequent in men than in women(5). Some studies reported a high incidence in adoles cents, reaching up to 11%10. Other studies estimate that this condition affects between 2 and 5% of the population, with no racial bias9. Regarding the age of presentation, a higher frequency of cases has been demonstrated in children, es pecially among those younger than three months, with the cradle cap presentation, affecting from 11.6% to 70.0% in some cohorts, and also in adults between 30 and 60 years old, with an epidemiological peak between the third and fourth decade of life11.
The main host’s risk factors described are the genetic predis position, hyperhidrosis, malnutrition, immune deficiencies, using systemic corticosteroids and contraceptives, as well as environmental conditions such as high temperature, humi dity, and the ocurrence of dysbiosis processes of the micro biota, especially of the genera Cutibacterium (Propionibacte rium)12, Staphylococcus and Malassezia13.
SD is one of the most common dermatoses in individuals infected with HIV, especially those with a CD4 T lymphocy te count below 400 cells/mm3,14. In Colombia, Rincón et al. reported the isolation of Malassezia spp. from lesions of pa tients with different dermatological entities, M. globosa was the predominant species in HIV-positive SD patients15. In subsequent studies on HIV-positive SD patients, the predo minant species were M. restricta and M. furfur, whereas in patients HIV-negative was M. sympodialis16.
Other medical conditions associated with an increased inci dence of SD are parkinsonism, neuroleptic-induced Parkin son-plus, familial amyloidosis, Down’s syndrome, and pa tients with psychiatric conditions being treated with halope ridol, lithium, buspirone, and chlorpromazine (9.
Malassezia and its pathogenicity
The yeast of the Malassezia genus (formerly called Pityros porum) belongs to the phylum Basidiomycota, sub-phylum Ustilaginomycotina, class Malasseziomycetes17. It is characte rized to be lipid-dependent and lipophilic and for being ca pable of metabolizing the fatty components of sebum18. This last ability influences its preferencial distribution on body areas rich in sebaceous glands. Malassezia has been recogni zed, for more than a century, as a normal resident of the hu mans skin microbiota but, in recent times, also as a potential pathogen, because it is isolated from the skin in individuals affected by SD, as well as for the therapeutic response to the use of antifungals19.
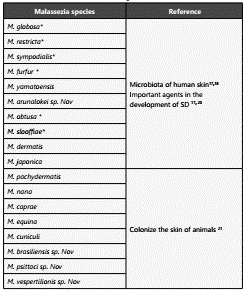
The Malassezia genus has been subject to multiple taxonomic reviews. However, in recent decades, the introduction of mo lecular techniques has allowed to describe the presence of at least 18 species17,18(Table 1). Two especies, Malassezia restricta and M. globosa, are considered the most important species in the development of SD. However, some studies have also in volved M. furfur, M. sympodalis, M. obtusa and M. slooffiae20,21.
Due to the lipid requirements of this yeast, their identifi cation and characterization can be performed using phe notypic characteristics such as the production of catalase and β-glucosidase, as well as with lipid assimilation profiles (Tween)22. Genotypic characteristics based on the sequencing of ribosomal RNA subunits (rRNA) is the gold standard for their identification17. However, these techniques are not con ducted in the routine diagnosis.
There are different hypotheses regarding the pathophysio logical mechanisms of Malassezia causing disease 22. Malas sezia yeast is found mainly in the infundibulum of the se baceous glands, where the freely available lipids constitute their primary source of energy. On the skin, Malassezia can establish two kind of relationships with its host. The first is a commensalism, where the yeasts and the skin are in a state of equilibrium, and the yeast is managing to evade the lo cal immune response. The second is a pathogenic relations hip, where Malassezia proliferate without producing inflam mation, as happens in pityriasis versicolor, or it can increase widely and produce swelling, as in the cases of SD, atopic dermatitis, and psoriasis 23.
In an inflammation scenario, Malassezia can generate the process associated with its overgrowth by three main me chanisms. First, causing evident damage to the epidermal barrier through the production of lipases and phospholipa ses. On a healthy skin, this yeast uses the essential nutrients for its growth without causing disease. However, when the epidermal barrier is disturbed, the yeast adapts and modi fies the expression of enzymes involved in the acquisition of energy, such as lipases and phospholipases. At the same time, they synthesize bioactive indols (ligands) that act on the aryl-hydrocarbon (AhR) receptor, expressed in most of the epidermic cells24.
Lipases hydrolyze triglycerides present in human sebum, pro ducing the release of free unsaturated fatty acids, such as oleic acid and arachidonic acid, which are capable of crossing the epidermal barrier. These metabolites play a crucial role in the initiation of the inflammatory response, causing hyperprolife ration and aberrant differentiation of keratinocytes, resulting in abnormalities in the corneum stratum such as parakeratosis, intracellular lipid droplets and abnormal development of stra tum corneum cells32. Susceptibility to toxic metabolites, and therefore the development or not of SD, depends on innate differences in individuals. In turn, this innate susceptibility is given depending on the barrier of the corneum stratum, the permeability of the skin, as well as the immune response of the individual. Variations in these characteristics make an indi vidual more susceptible or not to the epithelial barrier disrup tion induced by unsaturated fatty acids (Figure 1)4,6,9.
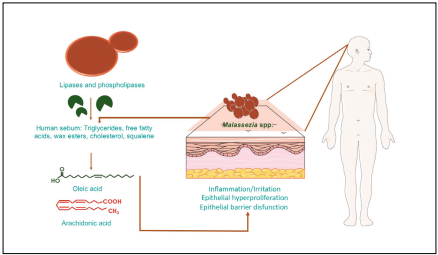
Figure 1 Role of Malassezia spp. lipid metabolism and their relation with the physiology of seborrhoeic dermatitis (DS). This figure was created using images from Servier Medical Art Commons Attribution 3.0 Unported License (http://smart.servier.com).
At the cellular level, inflammatory activity is evidenced by the infiltration of leukocytes in the affected skin, including lym phocytes, NK cells, and neutrophils11. Finally, the AhR receptor ligands (malassezina, indirubin, indol [3,2-b] carbazole, for myl-indo [3,2-b] carbazole) produce a down-regulation of the respiratory burst of human neutrophils, as well as a decreased ability of dendritic cells to mature and present antigens upon stimulation of the TLR receptor (toll-like receptor)25.
Secondly, an increase in the local immune response is eviden ced through the production of different pro-inflammatory cytokines by the keratinocytes, such as IL-1α, IL-6, IL-8, and TNF-α, thus prolonging the inflammatory response triggered by these yeasts. Furthermore, arachidonic acid is a source of prostaglandins, which are pro-inflammatory mediators that can cause inflammation through neutrophil recruitment and vasodilation9,11.
Thirdly, a sensitization to cross-reactive allergens produced by Malassezia can be evidenced. According to this model, there is an increased inflammatory response against the ex posure to Malassezia spp. by the action of allergens released by the microorganism (e.g., Mala s1, 7-9, cyclophilin, thio redoxin). M. sympodialis secretes nano-vesicles containing allergens capable of inducing the release of inflammatory cytokines, mainly IL-4, which generate type IV hypersensitivi ty reactions, especially under conditions of a pH more alka line than usual, both in healthy individuals as in individuals with atopic dermatitis and SD26.
Malassezia and the host response
In the process of the interaction between two organisms, there are determinants specific of the immune response type established to the antigenic exposure. In the case of Malas sezia spp., the maturation of dendritic cells derived from the monocytic line is the main cellular component that is res ponsible for the antigenic presentation and induction of a predominantly Th2-type lymphocytic response. An alternati ve mechanism described, which contributes with the increase in the immune response against this microorganism, is the interaction of dendritic cells with NK and NKT cells in the skin for the removal of phagocytic cells with incorporated fungal antigens27. Recognition by PRR (Pattern Recognition Receptors) has also been reported, with the β-glucans being the PAMP (Pathogen-Associated Molecular Patterns) identi fied for Malassezia spp, which are recognized by the human type 1-dectins28. Some studies have also proposed chitin as a probable PAMP in the initial recognition of Malassezia spp29.
Regarding the characteristic itching in SD, the recognition of zymosan by the TLR-2 receptors has been established as the probable mechanism, with activation of second messengers (MyD88) and cross-reactivity with the high-affinity receptor for IgE (FceRI) on mast cells, triggering the release of histami ne, proteases, chemotactic factors, IL-6 and arachidonic acid metabolites, all responsible for the characteristic itching30.
In this scenario, the diversity of the interaction mechanisms still to be characterized represent an arsenal of opportunities as therapeutic targets for the prevention and treatment of dermatological pathologies associated with Malassezia spp.
Treatment
Therapy focuses on the control of acute episodes, as well as long-term maintenance without symptoms. The goals of the treatment are to eliminate lesions, prevent skin infections, as well as reduce the inflammation and associated itching. Treatment includes eliminating the cause of the disease (an tifungal treatment or suppression of sebum secretion) and controlling cell proliferation and inflammation4. The first-line treatment has traditionally been the combination of an anti fungal agent, usually topical, and a corticosteroid31.
Topical use compounds
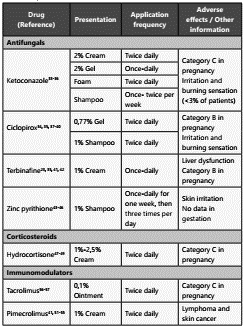
Among the compounds of topical used in the treatment of SD, we find antifungals (azoles, Ciclopirox, Octopirox, Bifona zol, Allylamines [Terbinafine]), coal tar, corticosteroids, Me tronidazole, zinc pyrithione, selenium sulfide and immuno modulators (Pimecrolimus and Tacrolimus) (Table 2).
Antifungals
Ketoconazole is an antifungal compound of the imidazole group. It produces a disruption of the synthesis of ergosterol in fungi through its inhibitory action on one of the microsomal cytochrome P-450 enzymes, 14-α-sterol demethylase, and the inhibition of the synthesis of 5-lipoxygenase, which blocks the synthesis of the B4 leukotriene, thereby conferring its anti-inflammatory properties32. For the treatment of SD, ketocona zole comes in four presentations: cream 2%, gel 2%, foam 2%, and shampoo 1-2%33. Shampoo with 2% ketoconazole is the most widely used form for the initial management of SD.
Seven double-blind, randomized controlled clinical trials using ketoconazole cream and shampoo with populations of up to 1162 individuals, showed a clinical recovery of up to 88% of the treated individuals31. A double-blind clinical trial included 1162 individuals, presenting mild to severe SD com promising multiple body regions. In this study, the treatment produced the complete resolution of symptoms and lesions, within the fourth week of treatment, in 56% of patients re ceiving ketoconazole 2% ointment twice daily, compared to 42% receiving placebo (p<0.001)34. A clinical trial, of 459 indi viduals with a moderate to severe SD and multiregional com promise, compared the ketoconazole 2% gel used once daily versus placebo. This study showed complete remission of le sions in 25% of patients receiving ketoconazole, compared to 14% in the placebo group on day 28 of treatment (p=0.001)35.
Ciclopirox 1%, or cycloxisporamine, is a synthetic antifungal derived from hydroxypyridone and has been shown to have antifungal, antibacterial, and anti-inflammatory properties33. This medication works by inhibiting the absorption of essen tial components and altering cell permeability, disrupting the DNA, RNA, and protein synthesis32. In addition, ciclopirox che lates metal ions, restricting the availability of iron to the fungal cell and, in consequence, inhibiting the iron-dependent enzymes responsible for the degradation of peroxides within the fungal cell. The antifungal properties of ciclopirox, to gether with its tolerability and low rate of toxic effects, make it an ideal alternative for both the treatment and prophylaxis of SD36. A multicenter, randomized, double-blind clinical trial with 1000 patients with scaly erythematous lesions on the scalp found a total remission of the lesions within four weeks of administration in 45% of patients with one weekly applica tion and 58% of patients with two weekly applications, com pared to the placebo group with a 32% reduction (p<0.001)37. Other studies have shown ciclopirox effectiveness against a placebo, specifically in the concentration of 1%38. In the same group, the octopirox (Piroctone olamine), an hydroxamic acid, it’s a topical agent that inhibits ergosterol synthesis. In the presentation of shampoo at 0.5-1%, has demostrared reduc tion in the erythema and pruritus with excellent results39.
Terbinafine interferes with the fungal biosynthesis of sterols by inhibiting the squalene epoxidase enzyme, which leads to the intracellular accumulation of squalene. In addition to its antifungal properties, terbinafine for the topical application also has anti-inflammatory activity40. One study compared the efficiency of terbinafine 1% cream against the efficacy of ketoconazole 2% cream and placebo. A randomized, double-blind study included 90 patients with facial SD, performed a score of perceived symptoms, it found that the mean of total decrease of symptoms (erythema, itching, and exfoliation) went from 5.04 to 1.78 in the group receiving terbinafine, from 5.04 to 1.81 in the group receiving ketoconazole, and from 4.97 to 3.73 in the placebo group (p=0.003). However, when comparing terbinafine with ketoconazole, there is no significant difference (p>0.05) (41. Another study suggested that terbinafine may be a useful alternative in infections with a low response to azoles22.
Zinc pyrithione is an ionophore, which facilitates the trans port of zinc through the membranes. This drug inhibits fungal growth through the increase of the cellular levels of copper, an ion responsible for altering sulfurous iron proteins, essential for fungal growth42. A randomized, double-blind study with 53 patients, presenting mild to moderate scalp SD, demonstrated the effectiveness of the active ingredient when compared to placebo (p<0.05). Furthermore, it was evidenced that the high deposit zinc pyrithione shampoo was significantly superior when compared to the very low deposit shampoo, in terms of both improving the severity of scaling and reducing the levels of M. furfur43. As to the effectiveness of zinc pyrithione com pared to ketoconazole, several trials have shown a decreased effectiveness44. However, a satisfactory effect is obtained when used in combination with ketoconazole45.
Corticosteroids
Topical corticosteroids are used for short periods and on a li mited body area, to control the erythema and itching of acu te episodes. These medications have anti-inflammatory pro perties and are active in rapidly eliminating visible signs and associated symptoms. Their long-term use should be avoi ded due to their well-known side effects such as skin atrophy, telangiectasias, hypertrichosis, and perioral dermatitis46.
Different randomized clinical trials have compared several topical corticosteroids used for short term periods, including hydrocortisone, betamethasone dipropionate, clobetasol 17-butyrate and clobetasol dipropionate with topical anti fungals47. A recent study comparing the effectiveness of ser taconazole 2% cream with hydrocortisone 1% cream repor ted a 90% satisfaction in patients receiving sertaconazole and 83.3% in the group with hydrocortisone, the difference was not statistically significant48. A randomized, controlled clinical trial found that regimens containing clobetasol propionate were significantly more effective in ultimately reducing the severity of the disease than those receiving only ketocona zole (p<0.05)49. Despite this, so far, the evidence is limited to establish whether the combination of topical corticosteroids and topical antifungal agents confers a more significant be nefit than a single-agent therapy1.
Additionally, a significant association has been described in the increase of the values of the minimum inhibitory con centration for fluconazole and terbinafine secondary to the previous use of topical corticosteroids22.
Immunomodulators- Calcineurin inhibitors
Pimecrolimus 1% cream and tacrolimus 0.03 and 0.1% ointment are part of the topical calcineurin inhibitors, which suppress the inflammatory activity associated with SD and prevent the side effects of steroids50. In addition to its tradi tional mechanism of action, both tacrolimus and pimecroli mus have been shown to have fungicidal activity40. Calcineu rin inhibitors selectively inhibit the transcription and release of pro-inflammatory cytokines by T cells, by binding to a cytosolic receptor, the macrophillin-12 immunophilin5151. The pimecrolimus-macrophillin complex inhibits the calcineurin protein, which results in the blocking of signal transduction pathways in T cells and the inhibition of the synthesis of in flammatory cytokines, specifically the Th1 and Th2 type cyto kines, such as IL-2, IL-4, IFN-g, and TNF-α52.
A randomized, double-blind study demonstrated that topical therapy with pimecrolimus 1% cream was effective and well-tolerated in the treatment of facial SD. Another randomized, double-blind clinical trial showed that 83% of the patients achieved complete elimination of symptoms after two weeks of application of pimecrolimus 1% cream53. Pimecrolimus has also been used for the treatment of SD in patients infected with HIV54.
In respect to tacrolimus, a clinical trial reported its effecti veness as the betamethasone 17-valerate lotion or the zinc pyrithione shampoo55. Otherwise, a randomized clinical trial found a higher level of satisfaction in patients who received sertaconazole (90%) compared to the group receiving tacro limus (83.3%); non-statistically significant results56.
Metronidazole
This drug has antibacterial and antiparasitic activity. At the same time, it has a direct anti-inflammatory effect, which could be the key to its efficacy in different dermatological diseases57. A clinical trial found that metronidazole 1% gel was significantly more effective than placebo, with improve ment after two weeks of starting treatment58. Other studies demonstrated the superiority of metronidazole when com pared to placebo59,60.
Fifty-one studies and 9052 participants were included in a systematic review conducted by Cochrane in 2015. It asses sed the effect of different topical agents (ketoconazole, ste roids, pimecrolimus, zinc pyrithione, ciclopirox, climbazole, metronidazole, lithium and herbal medicines) in comparison with placebo for the treatment of SD of the scalp and face in adolescents and adults 61. The failure rate in itch resolution was found to be much lower in patients treated with ketoco nazole 2% than with placebo (31% less risk of failure). Addi tionally, the resolution of erythema and scaling was better in ketoconazole treated versus placebo treated patients. On the other hand, ketoconazole and steroids were found to have similar effects in improving itching, erythema, and scaling, as well as in the rates of remission. However, the occurrence of side effects was 44% lower in the ketoconazole group of patients than in the steroids group. The study suggests that ketoconazole and ciclopirox are more effective than placebo and that, despite limited evidence, these two antifungals are more effective than any other agent in the same class.
Other topical therapies that should be considered are salicylic acid, selenium sulfide, sulfacetamide, glycerin, benzoyl pero xide, aloe vera and phototherapy 62. Another topical alternati ve is the coal tar, compound that has been used traditionally worldwide for the treatment of Dandruff. Nevertheless, there is a concern about it’s potential carcinogenicity, reason why it’s use has decreased. Also some studies have demostrared that non tar shampoos (piroctone olamine, salicylic acid and elubiol), produce significantly better reduction of symptoms compared with de coal tar shampoos63.
Oral use compounds
Oral antifungal therapy is indicated when multiple anatomic sites are involved, for patients who do not respond to tradi tional topical treatments, and patients with a severe develo pment of SD64.
Itraconazole is a member of the group of imidazoles, which shares the mechanism of action discussed for ketoconazo le. Its use has significantly increased in the last decade, been the most studied antifungal in oral therapy. One of the main recommendations for its use is the inadequate response to topical corticosteroid treatments. In a systematic revision 6 clinical trials were found64. The treatment regimen, length, se verity of the infection as well as the clinical and microbiologi cal remission of each of the studies are represented in Table 3.
Table 3 Evidence of oral Itraconazole in the treatment of seborrheic dermatitis (DS)
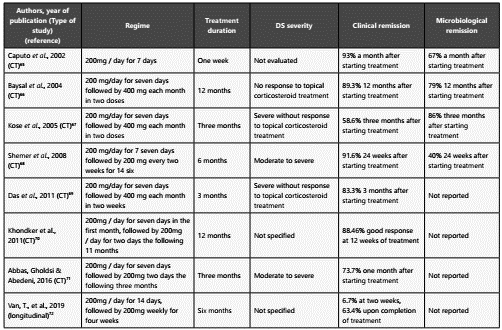
* CT: Clinical Trial Made by the authors
A double-blind, randomized controlled trial was conducted in 2016 on the effects of the itraconazole therapy on the quality of life of patients with a moderate to severe seborrheic der matitis, an aspect that had not been evaluated in previous cli nical trials. The impact of oral therapy with itraconazole versus placebo was assessed at the same time. The Dermatology Life Quality Index was implemented for the evaluation of the qua lity of life before and after treatment. It was found that itra conazole was superior compared to placebo, both in disease resolution and in the quality of life (p=0.001), with a 93.8% clinical improvement after two weeks, 87.5% after one month and 93.1% after four months. At the same time, the relapse rates were significantly lower with itraconazole71. The last stu dy, of longitudinal type and shorter treatment (6 weeks), was carried out in 2019 and showed an acceptable response72.
As for ketoconazole, one randomized controlled clinical trial and six case series support its use in SD. Ford et al. conduc ted a clinical trial with a 200 mg/day regimen of ketocona zole versus placebo. They found a more significant reduction in the severity of symptoms and the number of yeasts per field on direct examination, despite the absence of clinical or microbiological remission73. Nevertheless, in 2013 the FDA issued warnings against it clinical use due to the risk of liver injury, reason why it was withdrawn in Europe and Canada.
On the other hand, fluconazole showed that the decrease in the severity rate had no significant difference with the pla cebo group64. Another study compared the response to the fluconazole 200 mg regimen with placebo once a week and found a clinical and microbiological remission in the four pa tients studied74.
For terbinafine, two single-blind controlled randomized clini cal trials and one open, noncomparative trial have been carried out (Table 4). The in-vitro activity of the terbinafine against Ma lassezia has been estimated in comparison to itraconazole and ketoconazole, showing that it has the least inhibitory effect on growth in contrast to the other two compounds75.
Table 4 Clinical trials with oral terbinafine in the treatment of seborrheic dermatitis (DS)
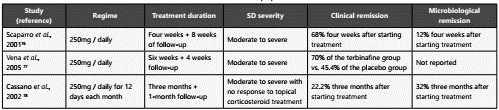
Adapted from: Gupta, A. K., Richardson, M., & Paquet, M. (2014). A systematic review of oral medications for seborrheic dermatitis. Journal of the uropean Academy of Dermatology and Venereology, 28(1), 16-26. https://doi.org/10.1111/jdv.12197
In recent years, the use of low doses of isotretinoin, a widely used drug for the management of acne, has been suggested. Its application has not been approved for seborrheic derma titis. This drug contributes to a decreased sebum secretion by reducing the size of the sebaceous glands, curbing cell proli feration, and stimulating the apoptosis of sebum cells79. Fur thermore, anti-inflammatory properties have been demons trated with the reduction of pro-inflammatory cytokines20. This can be considered as a therapeutic option in patients with a moderate to severe SD, with facial and scalp involve ment, however, more studies are required.
Finally, cases of systemic SD recalcitrant to the above-outli ned management have been described in patients with co morbidities such as HIV and Parkinson’s disease. Apremilast, a 4-phosphodiesterase inhibitor used to treat psoriasis, has been effectively used in 3 case reports of recalcitrant SD80.
Conclusions
Seborrheic dermatitis is a clinical condition that affects a sig nificant group of people, causing a high emotional and eco nomic impact on individuals with this disease. Its etiology is not completely understood and it is considered to have a multifactorial origin. However, the dysbiosis processes seem to have a significant effect on the clinical presentation of the disease. The treatment of these conditions is equally diverse, with the use of antifungals for long periods, both topical and systemic, aspect that can also induce resistance in Malassezia as has been revealed recently. For this reason, it is essential to search for new therapeutic alternatives that favor the homeos tasis of microorganisms in regarding host conditions. Besides, it is crucial to understand what is the role of this yeast in the interaction with other microorganisms, the host, and the SD.