Introduction
Intra-abdominal infections are some of the most common infections in clinical practice. Despite the advancements in physiopathology, the newer and more sensitive diagnostics tests, the progress in noninvasive surgery, the implementation of invasive methods to support the critical patient in the intensive care units and the developments in anti-microbial therapy, mortality is still around 23%1. The mortality rate maybe caused by multiple factors like delays in diagnosis, the presence of patients multiple comorbidities, life expectancy, limited access to health care that leads patients to arrive in advanced infectious stages, and inappropriate initiation of antimicrobials are some of the common factors that account for high mortality rates.
Thus, a consensus on intra-abdominal infection is imperative as a way to discuss the sequential diagnostic and treatment evaluation, including clinical examination, laboratory tests and diagnostic images to be performed, as well as the empirical antimicrobial treatment, and targeted therapy based on the culture results. Experts in microbiology, pediatric and adult infectious diseases, specialists in intensive care, along with general and pediatric surgeons, participated in the development of this guide. With methodological rigor and a search of the scientific literature, these specialists evaluated each of the recommendations described below. The consensus was based in the Colombian context because of cost-effectiveness, accessibility, and priority for implementation. The pillars analyzed were the current diagnostic methods, the ease and cost of their implementation, the increase in bacterial resistance and the impact of antimicrobial choice, the role of the new antibiotics and their cost-effectiveness for the Colombian health system and current evidence for recommendations.
As this consensus covers pediatric and adult population, it is expected that it becomes an integral part of daily clinical decisions in the management of intra-abdominal infection. In accordance with the evolution of bacterial resistance mechanisms and the inclusion of new drugs or diagnostic procedures, periodic updates will be carried out.
Methodology
Composition of the panel
The panel consisted of a multidisciplinary group of 16 specialists from Colombia (internal medicine, general surgery, clinical microbiology, pediatric surgery, pediatrics and adults infectious diseases, mycologists, critical care and epidemiologist), with expertise in patient care of intra-abdominal infection in the adult and pediatric population. All panel members were selected considering their experience in research, diagnosis, treatment, and monitoring of intra-abdominal infection.
General description of the process
The consensus work plan was carried out under the RAND/UCLA method, based on scientific evidence and the collective judgment of a panel of experts2.
A series of questions were developed taking into consideration the critical factors that determine decision-making in patients with intra-abdominal infection.
The panel reviewed and discussed all the recommendations in blocks (diagnosis, empirical and targeted therapy), their strength and the quality of the evidence. Discrepancies associated with the presentation of the evidence were discussed and resolved together, and all final recommendations represent a consensus opinion of the entire panel, based on scientific evidence. For the final version of the consensus, the panel reviewed all the individual sections.
Review of the evidence
The modified GRADE methodology3,4 was used to assess the quality of the evidence and the strength of the recommendations, which assigns each recommendation with a separate rating for the underlying quality of the evidence supporting the recommendation and for the strength with which the recommendation is made. The recommendation includes the following levels of evidence: low (III): the results can definitely change over time; moderate (II): results may change over time, but will not change drastically; high (I): the probability that the results will change is minimal. The strength of the recommendation (strong or weak) was assessed based on the balance between benefits and risks, the quality of the evidence, the values, and preferences of the patients, as well as the cost or use of resources5-9.
With the guidelines and consensus selected after the initial bibliographic search, a document was drafted, and recommendations for the questions asked were made. The panel met once and held a series of videoconferences over a period of four months, during which the recommendations were scored using the modified Delphi methodology10 individually, with two rounds of voting (unknown and open) (11. A consensus was established through an agreement greater than 75% of the expert panel for each recommendation (Annexe 1 and Annexe 2).
Systematic reviews
A bibliographic search for the clinical practice guidelines in intra-abdominal infection was carried out as well as for scientific evidence that supported the recommendations for the different topics of the consensus. For this, sources from compiling bodies (NGC, National Guideline Clearinghouse, Guideline International Network), producers of clinical practice guides (New Zealand Guidelines Group, National Institute for Clinical Excellence, Scottish Intercollegiate Network), clinical practice guides and databases. (PubMed, Medline, EMBASE). The following MESH terms were used: intra-abdominal infection, complicated intra-abdominal infection, appendicitis, diverticulitis, cholecystitis, primary peritonitis, secondary peritonitis, tertiary peritonitis, abdominal surgery, abdominal sepsis, diagnosis intra-abdominal infection, bacterial resistance, adult patient, pediatric patient, rapid diagnosis. Only scientific evidence published from the year 2010 was considered.
Disclosures
The panel of experts complied with the international policy disclosures; these require declaring any financial or other interest that could be interpreted as an actual, potential, or apparent conflict.
All panel members received the Disclosure Statement and were asked to identify ties to companies developing products that may be affected by the consensus. Additionally, information on employment, consulting, stock ownership, fees, research funding, expert testimony, and membership in advisory committees of these companies was requested. Possible conflicts of interest are listed in the annexes section (Annexe 3 and Annexe 4).
Future reviews of the consensus
Each year, panel leaders will be asked for their opinion on the need to update the guide based on a review of the current literature; based on this consideration the need and timing of an update will be determined. If justified, the entire panel of experts or a subset of it will be convened to analyze possible changes.
Glossary
Intra-abdominal infection: multiplication of bacteria in the wall of a hollow viscus and/or an intra-abdominal organ12.
Complicated intra-abdominal infection: infection that extends beyond a hollow viscus to the peritoneal cavity and is associated with abscess or peritonitis12.
Healthcare associated Intra-abdominal infection: patient with an intra-abdominal infection that meets any of the following criteria:
Infection that occurs 48 hours after the initial source control.
Stay in hospital for more than 48 hours or a history of hospitalization in the last 90 days.
Hospitalization in long term care facilities in the previous 30 days.
Home infusion therapy, home wound care or dialysis in the previous 30 days.
Use of broad-spectrum antibiotics for 5 days or more, in the previous 90 days.
Surgical site infection.
History of colonization or infection by multidrug-resistant microorganisms13.
Primary peritonitis: |
monomicrobial infection of the abdominal cavity without rupture of the gastrointestinal tract. Includes spontaneous peritonitis and hemodialysis catheterassociated peritonitis. |
Secondary peritonitis: |
product of the perforation of a hollow viscera. It is usually polymicrobial and includes both aerobic and anaerobic bacteria. |
Tertiary peritonitis: |
persistent (more than 48 hours) or recurrent peritoneal infection, which occurs after management, apparently successful, secondary peritonitis. It is usually associated with intra-hospital microorganisms, including yeast12 |
Diagnosis
Recommendation number 1: How to make the diagnosis of intra-abdominal infections?
The consensus panel recommends performing a sequential diagnostic evaluation, which includes clinical and laboratory examination and diagnostic images, according to institutional resources and availability (Level of evidence I-Strength of the recommendation STRONG).
Evidence
The first approach to patients with a possible intra-abdominal infection is clinical. Symptoms such as pain, anorexia, nausea, vomiting, ileus; and physical examination findings such as fever, tachycardia, tachypnea, abdominal defense, or abdominal rigidity are suggestive of intra-abdominal infection. The clinical evaluation can be complemented with basic paraclinical exams such as hemogram, and in patients who meet sepsis criteria, biochemical analysis for bilirubin, creatinine, lactate, and arterial gases should be requested. In patients with an inconclusive clinical examination, diagnostic images, such as ultrasound or computed tomography (CT), would be indicated according to the available resources14.
Recommendation number 2: When are cultures indicated?
The consensus panel recommends taking cultures of peritoneal fluid, tissue, or both, in the following cases13 (Level of evidence II-Strength of recommendation STRONG):
High-risk patients with community-acquired intra-abdominal infection.
Patients with intra-abdominal health care associated infection to identify multidrug-resistant or opportunistic microorganisms.
Patients with a diagnosis of generalized peritonitis.
Patients in whom there is a delay in the initial control of the focus or an inability to perform control of the focus.
Elevated Mannheim peritonitis index [15].
Patient with ongoing sepsis or septic shock.
If there is a finding of fibrinopurulent fluid.
If resources permit, cultures should be taken to establish local epidemiology and adjust empirical antimicrobial therapy protocols.
The factors that determine a high risk for intra-abdominal infection are (two or more) age over 70 years, malignancy, significant kidney, liver or cardiovascular disease, and hypoalbuminemia. With only two of these criteria, the possibility of infection is significantly increased.
Recommendation number 3: What is the correct way of taking a culture?
The consensus panel recommends taking cultures before initiating empirical antimicrobial therapy from samples like peritoneal fluid, bile, biopsies and tissues, intra-abdominal abscesses, peritoneal dialysis fluid and drainage tubes (Level of evidence II-Strength of recommendation STRONG).
Evidence
It is important to know what specimens should be cultured and the recommendations for correct sampling and transportation, to obtain the best performance that allows issuing an accurate diagnosis:
Optionally, the entire dialysis bag can be send to microbiology laboratory, where it will be treated as previously stated18.
The liquid should be centrifuged at 3,000 rpm for 15 minutes and the pellet resuspended in 3 - 5 mL of supernatant. Perform stains (Gram or Ziehl-Neelsen if applicable)and streak solid culture media (blood agar, chocolate agar and MacConkey agar) and inoculate enrichment broths (brain-heart infusion -BHI- or thioglycolate) (18.
The use of aerobic blood culture bottles is recommended to increase the sensitivity of the diagnosis. These should be inoculated with 10 ml of uncentrifuged dialysis fluid18. The microbiology laboratory should be informed if there is suspicion of slow growing pathogens, such as Nocardia spp., Since the incubation of the primary cultures must be extended or culture media for fungi must be used17.
The use of abdominal wound swabs should be avoided, due to lower sensitivity13,16, (17.
Peritoneal fluid and bile:. (16 obtained by percutaneous aspiration (paracentesis) or by surgical procedures, either by open surgery or laparoscopy. Ideally, a volume of approximately 25 mL should be taken. The sample is sent to microbiology laboratory at room temperature, in a sterile wide-mouth sample container, without additives and hermetically sealed. Transportation times greater than 1 hour; requires refrigeration at 4°C. If cytochemical examination is requested, the peritoneal fluid is send in a sterile tube with anticoagulant such as heparin or ethylenediaminetetraacetic acid (EDTA). For culturing common microbes, sample should be submitted in sterile tube without additives. Blood culture bottles can be used according to the availability of resources and should be inoculated with 10 ml of uncentrifuged peritoneal fluid. Smaller sample volumes must be inoculated into pediatric bottles to keep the sample / anticoagulant ratio17.
Biopsies and tissues:. samples are taken by surgical, percutaneous, or endoscopic procedures. The sample size ranges from 1 to 2 g of tissue. They are placed in sterile wide-mouth, hermetically sealed containers on sterile gauze moistened in saline to avoid desiccation. If a mycobacterial study is requested, the biopsy must be placed in a sterile container without additives. Samples for culturing common microbes must be processed by maceration17.
Intra-abdominal abscesses:. they are taken by puncture and aspiration with a needle and syringe, but their content must be transferred to a sterile tube. It is not recommended to send syringes with needles due to the possibility of occupational accidents17.
Peritoneal dialysis fluid:. it is obtained by puncturing with a needle and syringe the area designed for drug administration from the cloudiest dialysis bag. It is recommended to collect at least 50 mL in a sterile bottle18.
Drainage tubes:. they are not valid for cultures; neither are the samples collected from the drainage bags because they are contaminated. The drained material is collected by aspiration during drainage or directly from the drainage tube after disinfection of the puncture site. If the patient has more than one drainage tube, the location of the tube from which the sample came is indicated both on the application and on the packaging17.
For greater recovery of microorganisms, the use of blood culture bottles is recommended for peritoneal fluids, bile, and dialysis fluid if the availability of resources allows it. Consideration should be given to the need for cytochemical examinations and staining (Gram, Ziehl Neelsen), that should be send in tubes with anticoagulant or without additives respectively, and counter -samples should be refrigerated in case that complementary analyzes are requested or confirmation of result is required13,16.
Blood culture bottles (for aerobes or anaerobes) also serve as transport media and should not be refrigerated. Blood culture bottles also allows a higher percentage of recovery, mainly of anaerobic bacteria. It is important to carry out adequate disinfection of the rubber caps prior to inoculation, to reduce contamination. The recommended seeding volume is 5 - 10 ml of sample (e.g. peritoneal fluid, dialysis fluid, etc.) (16,17,19.
Cultures for anaerobic bacteria are not routinely necessary in patients with community-acquired intra-abdominal infections if empirical therapy is active against the more common anaerobic bacteria. For liquid samples, anaerobic blood culture bottles have an excellent recovery. For solid samples, consider implementing commercial anaerobic transport systems17,19.
Culture and susceptibility testing for anaerobic bacteria can be considered when:
There is persistence of the infection despite adequate antibiotic treatment and source control.
There is evidence of endovascular infection by anaerobic bacteria20.
Recommendation number 4: Should blood cultures be taken in patients with suspected or confirmed peritonitis?
The consensus panel does not recommend routine blood cultures in patients with community-acquired intra-abdominal infections.
Blood cultures in hemodynamically stable and non-immunosuppressed patients do not offer clinically relevant diagnostic information in those with community-acquired intra-abdominal infections and, therefore, are not routinely recommended in this group.
The consensus panel recommends taking blood cultures in patients with health care associated intra-abdominal infections or patients hospitalized in intensive care units (Level of evidence II-Strength of recommendation STRONG).
Evidence
Bacteremia is more common in patients hospitalized in the intensive care unit and / or with health care associated intraabdominal infections.
Taking two sets of blood cultures (2 aerobic bottles and 2 anaerobic bottles) is recommended in adult patients with severe immunosuppression, sepsis, or septic shock21
Good practice recommendation
The abdominal cavity is primarily sterile; therefore, the recovery of microorganisms is considered clinically relevant and the intra-abdominal infection is mostly polymicrobial. It is recommended to perform identification and susceptibility testing to the following groups of microorganisms1,13,17.
Enterobacterales
Gram negative glucose non-fermenting bacilli
Staphylococcus aureus.
Enterococcus spp.
Candida spp
viridans group Streptococci
Isolates of coagulase negative Staphylococcus, aerobic Gram-positive rods, etc., may mean sample contamination. The way the sample is taken and transported should be reviewed and considering, if possible and relevant, taking and sending a new sample for culture13,17.
For the recovery of Candida spp. For abdominal cavity samples, conventional fungal culture media such as Sabouraud agar or Mycosel® agar can be used. Candida spp grows well on Chocolate Agar, Blood Agar, and aerobic blood culture bottles. When fungal infections suspicious to be caused by pathogens other than Candida spp., a note must be included in the laboratory request form to incorporate additional culture media13,17 .
Species identification and antifungal susceptibility testing is recommended on Candida spp. isolates obtained from samples of the abdominal cavity.
Recommendation number 5: Should implementation of rapid diagnostic tests for mechanisms of antimicrobial resistance be recommended?
5.1 What are these tests and what is their diagnostic utility? The consensus panel recommends the implementation of rapid tests for the detection of relevant resistance mechanisms, according to the local epidemiology, the availability of resources and the severity of the patient in the framework of Diagnostic stewardship programs. These tests allow an early adjustment of empirical antimicrobial therapy towards targeted therapy and to apply infection control measures in real time (Level of evidence II-Strength of recommendation STRONG).
Evidence
Test to detect mechanisms of antimicrobial resistance directly on clinical samples available so far are the following: Biofire FilmArray® BCID panel for blood cultures: Detects the mecA gene of S. aureus, vanA/vanB genes of Enterococcus and bla KPC for Gram-negative bacteria. Master Diagnostic Sepsis Flow Chip® for blood cultures: detects the mecA gene from Staphylococcus, vanA and vanB from Enterococcus and the genes responsible for coding the TEM, SHV, CTX-M, KPC, SME, NMC, IMI, VIM, NDM, SPM, SIM, IMP, OXA resistance proteins in Gram-negative bacteria. Direct and rapid commercial methods are not available on samples such as peritoneal fluid or tissue. The use of samples other than blood to perform commercial multiplex PCR methods is considered off-label and is not recommended by device manufacturers. On the other hand, implementation of rapid molecular tests in blood cultures should be considered when detected Gram negative bacilli and Gram-positive cocci in patients with sepsis, septic shock, or severe immunosuppression and if there is blastoconidia growth22.
The implementation of these tests is recommended according to the availability of resources, local epidemiology, and the disease (Figure 1).
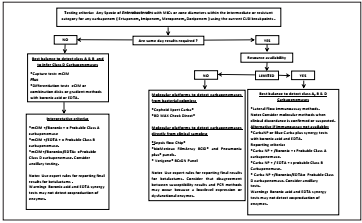
Figure 1. Algorithm for carbapenemase detection in Enterobacterales based on the Diagnostic Stewardship concept
CarbaNP. [23]: allows the detection from bacterial colonies of class A, B and D carbapenemases without differentiating them in Enterobacterales and Pseudomonas aeruginosa isolates. Offers results between 15 minutes to 2 hours with 84% sensitivity and 100% specificity.
Lateral flow immunoassay. 24: using immunochromatography and from bacterial colonies it detects the 5 most frequent families of carbapenemases in Enterobacterales and Pseudomonas aeruginosa (KPC, VIM, NDM, IMP and OXA-48). Its additional advantages are the turnaround time (15 minutes) and the possibility of detecting enzyme co-productions with 80-100 % sensitivity and 95 % specificity.
XpertCarbaR®. 25: using PCR it detects the 5 most frequent families of carbapenemases in Enterobacterales and Pseudomonas aeruginosa (KPC, VIM, NDM, IMP and OXA-48) from rectal swabs and bacterial colonies. Its additional advantages are the turnaround time (1 hour) and the possibility to detect enzyme co-productions with 100 % sensitivity and 98 % specificity.
BD MAX Check Direct®. 26: using PCR it detects 4 families of carbapenemases in Enterobacterales and Pseudomonas aeruginosa (KPC, VIM, NDM, and OXA-48) from rectal swabs and bacterial colonies. Its additional advantages are the turnaround time (1 hour) and the possibility of detecting enzyme co-productions with 93 % sensitivity and 97 % specificity12.
Good practice recommendations
There is no test that detects all carbapenemases with 100% specificity and sensitivity22.
There is no test that detects all allelic variants of the enzymes23.
Commercial carbapenemase detect test do not report the enzymatic level of expression nor predict with total certainty the results of the antibiogram22.
Tests for the detection of resistance mechanisms do not accurately predict the susceptibility of the new carbapenemase inhibitors, therefore it is recommended to screen them individually once they have microbial isolation22.
Recommendation number 6: What is the value of radiological images in the diagnosis of intra-abdominal infection?
The consensus panel recommends the use of computerized tomography, ideally with IV contrast, since is considered the gold standard to identify abdominal or pelvic source of infection (Level of evidence II-Strength of recommendation STRONG)
Evidence
Images can be potentially diagnostic in the evaluation of highrisk patients. Even if the exact etiology of the infection is not determined, they can help to decide treatment strategies27.
Good practice recommendation
Ultrasound28 is the gold standard for the evaluation of cholecystitis and can be useful for the diagnosis of intra hepatic or intra-abdominal abscesses.
Diagnostic imaging is not required in the case of suspected peritonitis due to organ perforation in critically ill patients if it delays surgery27.
Recommendation number 7: What is the role of procalcitonin in the diagnosis of intra-abdominal infection?
The consensus panel does not recommend the routine use of procalcitonin for the diagnosis of intra-abdominal infection (Level of evidence II-Strength of recommendation STRONG).
Evidence
Procalcitonin (PCT) is a specific marker of bacterial infection and does not provide information on the origin of sepsis; therefore, it should not be requested routinely29. It can be useful in cases such as postoperative liver transplantation, possible necrotizing pancreatitis, or suspected postoperative infection29. The negative predictive value (NPV) is important if PCT is < 0.5 ng/mL is interpreted as a low risk of sepsis, but it does not exclude a localized infection29. On the other hand, values > 2.0 ng/mL are predictors of sepsis (OR 2.0) (29.
In pancreatitis, a PCT > 0.5 ng/mL predicts the development of severe acute pancreatitis (sensitivity 72% and specificity 86%) and infected pancreatic necrosis (sensitivity 80% and specificity 91%)29.
PCT values > 3.5 ng/mL for two consecutive days predict infected pancreatic necrosis with multiple organ dysfunction syndrome (MODS) (29.
There is a low risk of infection if the PCT is less than 0.5 ng/mL.
PCT can be a complementary tool for de-escalating antibiotics in conjunction with other diagnostic tests and clinical evolution29.
Empirical management
Recommendation number 8: When should the initiation of an empirical treatment be considered based on the population at risk?
The consensus recommends the initiation of empirical antimicrobial management in patients with appendicitis (Level of evidence I. Strength of recommendation: STRONG).
Evidence
Although the immediate administration of broad-spectrum antibiotics, as soon as the cultures have been collected, can save lives, in intra-abdominal infection empirical therapy should generally be started prior to surgery. The preferred strategy for antimicrobial selection will depend on the characteristics of the patient and the local epidemiology. Rather, targeted therapy should be based on the results of cultures.
Based on sepsis guidelines, it has been recommended that antibiotics should be administered within one hour of identification of the septic shock, and in patients without hemodynamic involvement or organ failure it is suggested that they should be administered within 8 hours after presentation of infection. The Cochrane review by Wong et al. (30 does not establish any specific recommendation for first-line antibiotic treatment in adults with abdominal sepsis, as all regimens showed similar efficacy. Therefore, the decision to choose a specific antimicrobial strategy requires considering other factors, such as local antimicrobial guidelines, patterns of microbial resistance, route of administration, costs and availability31.
A. Appendicitis
Recommendation number 9: In cases of nonperforated appendicitis, is it recommended to initiate antimicrobials empirically?
The consensus recommends always start antibiotics empirically (Level of evidence I. Strength of recommendation: STRONG).
Treatment options based on local epidemiology
Ampicillin/sulbactam 3.0 g every 6 hours (Level of evidence I. Strength of recommendation: STRONG).
Cefuroxime 1.5 g every 8 hours + metronidazole 500 mg every 8 hours or 1-1.5 g every 24 hours (Level of evidence I. Strength of recommendation: STRONG).
Ceftriaxone 1 g every 12 hours or 2g every 24h + metronidazole 500 mg every 8 - 12 hours or 1 - 1.5 g every 24 hours (Level of evidence: II. Strength of recommendation: STRONG).
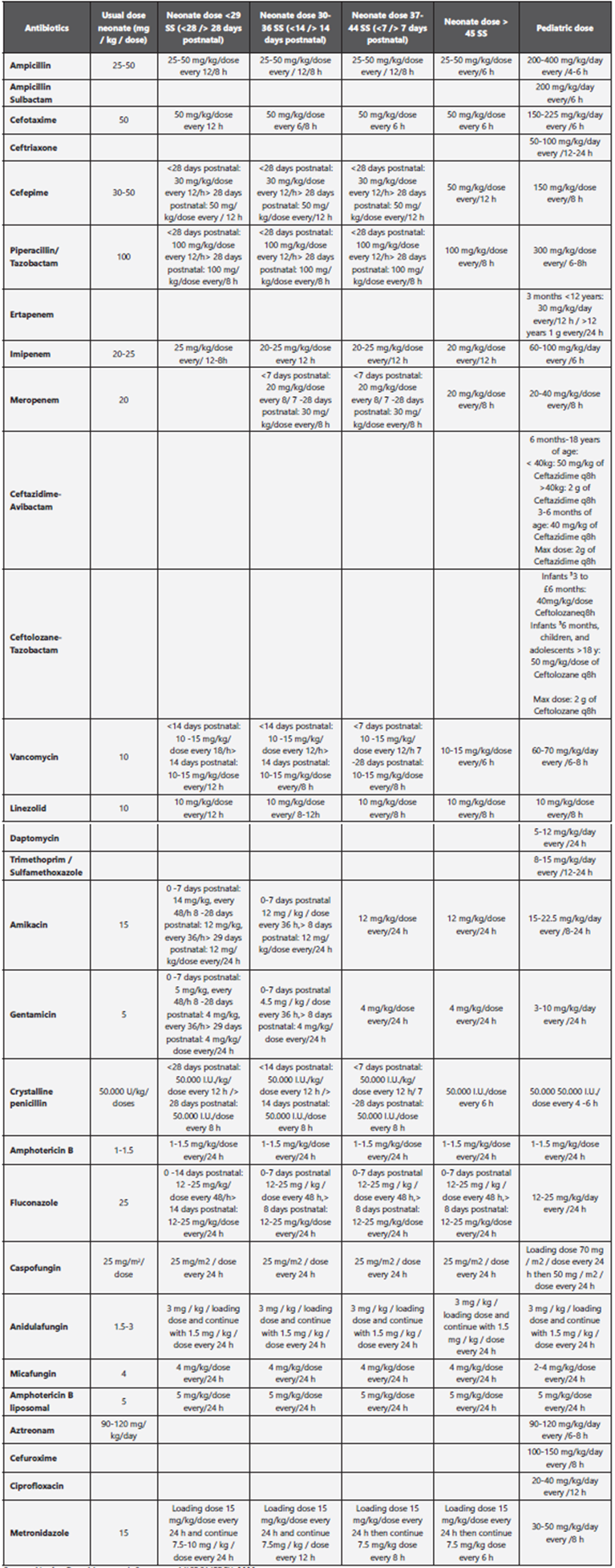
The doses of antibiotics in pediatrics are annexed in Table 1. References: (32,33**,34***,35
Recommendation number 10: Is the initiation of empirical antimicrobials recommended in cases of localized perforated appendicitis?
The consensus recommends always starting antibiotics empirically (Level of evidence I. Strength of recommendation: STRONG).
Treatment options based on local epidemiology
Regardless of whether the patient is taken to surgery:
Ceftriaxone 1 g every 12 hours or 2g every 24h + metronidazole 500 mg every 8 hours or 1-1.5 g every 24 hours or ertapenem 1 g every 24 hours (Level of evidence I. Strength of recommendation: STRONG).
Tigecycline 100 mg loading dose and then 50 mg every 12 hours if epidemiology indicates ESBL in Escherichia coli or Klebsiella pneumoniae or ertapenem 1 g every 24 hours (Level of evidence I. Strength of recommendation: STRONG).
Recommendation number 11: Is the initiation of empirical antibiotics recommended in generalized perforated appendicitis?
The consensus recommends collecting a culture and starting empirical antibiotics always (Level of evidence I. Strength of recommendation: STRONG).
Therapeutic options.
It is recommended to take culture and initiate empirical antibiotics always (Level of evidence I. Recommendation: STRONG).
Therapeutic options based on local epidemiology
Ertapenem 1g/day (Level of evidence I. Strength of recommendation: STRONG).
Tigecycline 100 mg and then 50 mg every 12 hours (Level of evidence I. Strength of recommendation: STRONG).
Ceftazidime / avibactam 2.5 g every 8 hours + metronidazole 500 mg every 8 hours or 1 - 1.5 g every 24 hours if there is a risk of KPC, OXA-48, ESBL and AmpC without being able to differentiate until receiving the culture (Level of evidence I, Strength of recommendation: STRONG).
In severe patients, the use of meropenem, imipenem or piperacillin / tazobactam is considered according to local epidemiology (Level of evidence I, Strength of recommendation: STRONG).
Evidence
The routine use of broad-spectrum antimicrobials or combination therapy for the treatment of Pseudomonas spp., Enterococci spp. and fungi should be avoided30.
Treatment options will be based on local epidemiology and will be the same as for non-perforated appendicitis since surgery eradicates the infection.
Based on intraoperative findings, low-risk patients with community-acquired secondary peritonitis should receive “narrow spectrum” agents if the peritonitis is localized and source control is ensured. Some of the examples mentioned in the literature are ampicillin/sulbactam, second or third generation cephalosporin, fluoroquinolones, and metronidazole for one to two days. However, local epidemiology will define the final choice. In contrast, when peritonitis is generalized, the options will be broader spectrum antibiotics, such as piperacillin/tazobactam, tertiary or fourth generation cephalosporins associated with metronidazole, fluoroquinolones plus metronidazole, a carbapenem, or tigecycline for 5 to 7 days30,31,36,37.
The isolated in secondary and tertiary peritonitis present, in higher frequency, mixed flora of aerobic and anaerobic, grampositive and gram-negative, as well as fungi in certain cases of tertiary peritonitis, or in patients with immunosuppression.
Community-acquired gram-negative and gram-positive aerobic organisms are generally infections originating in the stomach, duodenum, biliary system, and small intestine. Gastric or duodenal ulcer perforations are generally associated with Escherichia coli or Streptococci infections. Commonly, the bacteria associated with secondary peritonitis of the biliary tract are Escherichia coli, Klebsiella spp. and Enterococci, while in infections derived from the small intestine, gram-negative aerobes and anaerobes predominate. For colon infections all types of gram negative and anaerobic aerobes should be considered31. Based on the probable pathogenic flora described. Risk factors for infection by multidrug-resistant microorganisms, in which case antibiotics of a higher spectrum should be started31 include: elevated APACHE score, advanced age, comorbidities with significant impact on any organ (liver disease, cardiovascular disease, kidney disease), malignancy, use of corticosteroid therapy (post-transplant status) and unsuccessful surgery.
B. Cholecystitis
Recommendation number 13: When should the initiation of an empirical treatment be considered according to the population at risk?
The consensus always recommends initiating empirical treatment in patients with cholecystitis (Level of evidence I. Strength of the recommendation: STRONG).
Recommendation number 14: Should empirical antimicrobials be initiated in non-perforated cholecystitis?
The consensus recommends always start antibiotics empirically (Level of evidence I. Strength of recommendation: STRONG). The antibiotic regimens to be used are based on local epidemiology:
Ampicillin/sulbactam 3.0 g every 6 hours (Level of evidence III. Strength of recommendation: STRONG).
Cefuroxime 1.5 g every 8 hours + metronidazole 500 mg every 8 hours or 1-1.5g every 24 hours (Level of evidence II. Strength of recommendation: STRONG).
Ceftriaxone 1g every 12 hours or 2g every 24 hours + metronidazole 500 mg every 8 hours or -1.5 g every 24 hours (Level of evidence II. Strength of recommendation: STRONG).
Recommendation number 15: Should empírical antimicrobials be initiated in localized perforated cholecystitis
The consensus recommends always start antibiotics empirically (Level of evidence I. Strength of recommendation: STRONG). Treatment options based on local epidemiology Regardless of whether the patient is taken to surgery:
Ceftriaxone 1 g every 12 hours or 2g every 24h + metronidazole 500 mg every 8 hours or metronidazole 1-1.5 g every 24 hours or ertapenem 1 g every 24 hours (Level of evidence I. Strength of recommendation: STRONG).
Tigecycline 100 mg loading dose and then 50 mg every 12 hours or ertapenem 1 g every 24 hours if epidemiology indicates ESBL in Escherichia coli or Klebsiella pneumoniae (Level of evidence I. Strength of recommendation: STRONG).
Recommendation number 14: Should empírical antimicrobials be initiated in generalized perforated cholecystitis?
The consensus recommends always initiating antibiotics empirically (Level of evidence I. Strength of recommendation: STRONG). The recommended therapeutic options are the following:
Recommendation number 15: How long is the usual treatment time for perforated and non-perforated cholecystitis?
The consensus recommends extending treatment for up to 48 hours after signs and symptoms resolve; that is, 5 to 7 days on average (Moderate level of evidence. Strength of recommendation: STRONG).
Evidence
The principles of empirical antibiotic treatment should be defined according to the most frequently isolated microorganisms, always considering the local tendency of resistance to antibiotics. The organisms most frequently isolated in biliary infections are the gram-negative aerobes, Escherichia coli and Klebsiella pneumoniae, and the anaerobes, especially Bacteroides fragilis37-40. The pathogenicity of Enterococcus in biliary tract infections remains unclear and is usually not known thus, specific coverage against these microorganisms in case of community-acquired biliary infections is suggested. For selected immunosuppressed patients, that is, those with liver transplantation, there should always be a suspicion of enterococcal infection, and it should be treated41. Patients with acute cholecystitis or cholangitis and suspected infection should receive antimicrobial therapy according to local epidemiology; in general, anaerobic treatment is not necessary unless there is a bilioenteric anastomosis40. In patients undergoing cholecystectomy for acute cholecystitis, antimicrobial treatment should be discontinued within the first 24 hours after the procedure, unless there is evidence of infection outside the gallbladder wall41. For communityacquired biliary infection, activity against Enterococcus is not necessary, since the role and pathogenicity of this germ has not been fully demonstrated in this disease. For immunosuppressed patients, particularly those transplanted, the enterococcal infection can be significant and requires treatment40.
The main mechanism of antimicrobial resistance found in this type of infection is due to the presence of extended spectrum β-lactamases (ESBL) producing Enterobacterales which is frequently found in community-acquired infections in patients with comorbidities that require constant exposure to antibiotic treatments40.
C. Diverticulitis
Recommendation number 16: When should the initiation of an empirical treatment be considered according to the population at risk?
The consensus always recommends the empirical initiation of antimicrobials (Level of evidence I. Strength of the recommendation: STRONG).
Evidence
The traditional Hinchey classification was designed to be used based on laparotomy findings and types of presentation as follows:
I - localized phlegmon or paracolic abscess
II - pelvic abscess
III - purulent peritonitis
IV - fecaloid peritonitis
The preoperative investigations available to the surgeon have changed dramatically since Hinchey’s (1978) classification, so that patients can be stratified preoperatively, and treatment can be tailored accordingly. A useful classification system tries to combine the traditional Hinchey classification and computed tomography findings, with the addition of stage 041. Diverticular disease represents the one that most affects the colon. Most cases remain asymptomatic, but others will have symptoms or complications. The goals of treating uncomplicated symptomatic diverticular disease are to prevent their onset and to reduce the frequency and intensity of symptoms12,13,27,42-46. In uncomplicated diverticulitis, outpatient management is considered the optimal approach for most patients, and oral antibiotics remain the mainstay of treatment. Hospital admission and intravenous antibiotics are recommended only when the patient is unable to take food or medications by mouth, has severe comorbidities, or is not improving. Hospital treatment and intravenous antibiotics are almost always necessary in the management of complicated diverticulitis42,43,47. Oral antibiotics used for outpatient management of diverticulitis should cover gram-negative and anaerobic bacteria, particularly Escherichia coli and Bacteroides fragilis. On the other hand, patients with acute diverticulitis who require hospitalization should be treated with intravenous antibiotics that cover gram-negative enteric bacteria, anaerobic and gram-positive organisms43. The choice of antibiotics is dictated by the severity of the disease (mild to moderate versus severe), the patient’s risk factors (i.e., whether they are at high risk for adverse outcomes or antimicrobial resistance) and whether it is a community-acquired infection vs. a hospital42,43 47. Clindamycin is not considered an acceptable option for intra-abdominal infections involving anaerobes, due to the increased rates of resistance in the B. fragilis group. Empirical coverage of Enterococcus is not necessary for patients with low-risk community-acquired intra-abdominal infection, and should be considered only in patients with high-risk community-acquired infection44,48. In the latter group, and because quinolone-resistant Escherichia coli has become common in some communities, quinolones44 should not be used, unless hospital microbiology indicates susceptibility of 90% or greater44. Aztreonam plus metronidazole is a good alternative, but it also requires the addition of an agent effective against Gram-positive cocci44. New antibiotics, such as ceftolozane/tazobactam or ceftazidime/avibactam, have also emerged as an option for the treatment of resistant bacteria49.
The recommended antibiotic regimens are the following:
Ceftriaxone 1 g every 12 hours or 2g every 24 hours + metronidazole 500 mg every 8 hours or 1 - 1.5 g every 24 hours or ertapenem 1 g every 24 hours (Level of evidence I. Strength of recommendation: STRONG).
Tigecycline 100 mg loading dose and then 50 mg every 12 hours if epidemiology shows ESBL in Escherichia coli or Klebsiella pneumoniae (Level of evidence I. Strength of recommendation: STRONG).
Ertapenem 1 g/day if there are risk factors for ESBL (Level of evidence: moderate. Strength of recommendation: STRONG).
Piperacillin/tazobactam 4.5 g every 6 hours if there is a risk of Pseudomonas aeruginosa (Level of evidence: moderate. Strength of recommendation: STRONG).
Recommendation number 17: What is the recommendation for non-perforated diverticulitis?
The consensus recommends performing percutaneous drainage, when possible, and administering antibiotics (Level of evidence I. Strength of recommendation: STRONG).
Evidence
If an abscess is drained percutaneously, cultures should always be sent, and the antibiotic regimen should be directed at the reported susceptibility results. However, if a patient with a low-risk community-acquired infection shows clinical improvement, subsequent identification of uncovered pathogens probably does not justify altering the antibiotic regimen12. Patients generally improve after two to three days of intravenous antibiotics. The small improvement may mean the development of abscess or other complications, so the need to repeat the imaging should be taken into account12,42,43,47.
Recommendation number 18: What is the recommended treatment for localized and perforated generalized perforated diverticulitis?
The consensus recommends that the treatment choice Will depend on the local epidemiology and will be adjusted with intraoperative culture (Level of evidence I. Strength of recommendation: STRONG).
D. Primary peritonitis
Primary peritonitis is defined as inflammation of the peritoneum without an obvious source of causative organisms, or a localized infection within the abdomen. Culture demonstrates the presence of a single microorganism in more tan 90% of cases50. Organisms commonly isolated from ascitic fluid are Streptococcus pneumoniae, Escherichia coli, and Klebsiella pneumoniae. An unusual cause is Staphylococcus aureus, which accounts for 2-4% of primary peritonitis. Sometimes anaerobes and microaerophilic microorganisms are also reported, according to their availability to be able to perform specific cultures50-52.
E. Secondary peritonitis
Secondary peritonitis is the most common form of peritonitis. It is an acute peritoneal infection that results from the los of integrity of the gastrointestinal tract or infected viscus. It is caused by perforation of the gastrointestinal tract (e.g., perforated duodenal ulcer) or by direct invasion of infected intra-abdominal viscus (egg, gangrenous appendicitis). Anastomotic dehiscences are common causes of peritonitis in the postoperative period12.
F.Tertiary peritonitis
Tertiary peritonitis is a recurrent infection of the peritoneal cavity, after primary or secondary peritonitis12,50. The most common infecting organisms in patients with tertiary peritonitis are Enterococcus spp., Candida spp., Pseudomonas aeruginosa and Enterobacterales. Infectious foci are rarely susceptible to percutaneous drainage. In several studies, they were found to be poorly localized at laparotomy. Recurrent or tertiary peritonitis is a common complication of intra-abdominal infection in patients admitted to an intensive care unit. It differs from secondary peritonitis in the characteristics of its microbial flora and the lack of response to adequate surgical and antibiotic therapy12,14,53.
Recommendation number 24: When should treatment for tertiary peritonitis be initiated?
The consensus always recommends the start of antibiotics (Level of evidence I. Strength of recommendation: STRONG).
Treatment will depend on the bacteria isolated (see, therapeutic options), but multidrug-resistant Gram-negative and Gram-positive bacteria should always be considered.
Recommendation number 25: How long is the treatment time for tertiary peritonitis?
The consensus recommends maintaining antimicrobial therapy until the focus is controlled (Level of evidence I. Strength of recommendation: STRONG).
Recommendation number 26: What is the recommendation for choosing the type of drug, the dose and the duration of empirical treatment according to the population at risk?
26.1 The consensus recommends treating patients with low risk of sepsis with narrower-spectrum antimicrobials, such as antimicrobials with activity against Enterobacterales, aerobic streptococci and anaerobic microorganisms, which are associated with these infections. Level of evidence I. Strength of recommendation: STRONG).
26.2 The consensus does not recommend the routine use of broader spectrum agents or combination therapies with the idea of providing anti-pseudomonas, antienterococcal or antifungal coverage. Level of evidence I. Strength of recommendation: STRONG)
26.3 The consensus recommends using cefotaxime 1 g every 8 h or ceftriaxone 1 g every 12 h or 2 g day plus metronidazole 500 mg every 8 h or 1 - 1.5 g every 24 h or ertapenem 1g day as the preferred agents for the initial empirical treatment for patients with low-risk of sepsis. (Level of evidence I. Strength of the recommendation: STRONG).
26.4 The use of cefuroxime 1,5 g every 8 hours plus metronidazole 500 mg every 8 h or 1 - 1.5 g every 24 h can be considered as an alternative. (Level of evidence II. Strength of recommendation: STRONG).
The consensus recommends in patients at higher risk or with complicated intra-abdominal infection administering antimicrobials that guarantee coverage of gram-negative pathogens that are not common, but potentially involved in these infections. Piperacillin/tazobactam, doripenem, imipenem/cilastatin, meropenem or cefepime plus metronidazole are recommended as the preferred agents for initial empirical therapy in high-risk patients (Level of evidence I. Strength of recommendation: STRONG).
26.5 The consensus recommends empirical management decision making based on local epidemiology or national trends. (Level of evidence I. Strength of recommendation: STRONG).
26.6 The consensus recommends considering the use of ceftazidime/avibactam + metronidazole as an alternative regimen for high-risk patients, as well as the use of aztreonam plus metronidazole plus vancomycin as an option in patients at higher risk, or when they have a severe allergic reaction to β-lactams. (Level of evidence II. Strength of recommendation: WEAK).
26.7 The consensus does not recommend adding an aminoglycoside or fluoroquinolone to a β-lactam for the empirical treatment of patients at higher risk since there is no demonstrated benefit and there is greater toxicity. (Level of evidence I. Strength of recommendation: STRONG).
26.8 The consensus recommends considering the use of ampicillin or vancomycin as empirical anti-enterococcal treatment in patients at higher risk if the patient is not being treated with piperacillin/tazobactam or imipenem/cilastatin (since both options have coverage against Enterococcus). (Level of evidence I. Strength of recommendation: STRONG).
26.9 The consensus does not recommend using antifungal agents routinely for empirical therapy in higher-risk patients, except in empirical therapy for those in critical condition with an upper gastrointestinal source. (Level of evidence I. Strength of recommendation: STRONG).
26.10 The consensus does not recommend the use of ciprofloxacin and levofloxacin as first-line treatment in most geographic regions, due to the high prevalence of resistance to fluoroquinolones. (Antibiotics Level of evidence I. Strength of recommendation: STRONG).
Evidence
Carbapenem antibiotics offer a broad spectrum of antimicrobial activity against aerobic and anaerobic pathogens, both gram-positive and gram-negative (except for resistant grampositive cocci). Group 1 carbapenems, like ertapenem, have activity against ESBL-producing pathogens, but not against Pseudomonas aeruginosa or Enterococcus species. Group 2 includes imipenem/cilastatin, meropenem and doripenem, which share activity against non-fermenting Gram-negative bacilli, such as Pseudomonas aeruginosa, ESBL-producing Enterobacterales, AmpC, and anaerobes. Pseudomonas aeruginosa is isolated in less than 6% of patients with complex acute appendicitis26,44,48. Therefore, its empirical coverage is not considered in most patients with intra-abdominal infections. This microorganism is more common in critically ill patients with in-hospital infections and in immunosuppressed patients26,48,51. Since it has multiple mechanisms that allow it to develop resistance in vivo to all available antibiotics, it is necessary to properly select the antibiotic to be used, optimizing its dose and infusion times in order to achieve adequate pharmacokinetic and pharmacodynamic parameters. (PK/PD). In the case of β-lactams, the use of prolonged infusions for 3 to 4 hours or continuous infusions is preferred54.
Other options include aminoglycosides, particularly when infections by multidrug-resistant gram-negative bacteria are suspected, they are effective against Pseudomonas aeruginosa, but are ineffective against anaerobic bacteria, and require association with metronidazole55. Due to their toxic side effects, aminoglycosides are not recommended for routine empirical treatment of community-acquired intra-abdominal infection, reserving them for patients with allergies to β-lactams, or in combination with β-lactam for the treatment of suspected intra-abdominal infection with multidrug resistant gram-negative bacteria. In case of using quinolones, the result of the culture must be known to guarantee its sensitivity and add metronidazole or use it as an option in patients allergic to β-lactams, who present with a mild intra-abdominal infection12.
Tigecycline is a viable treatment option, especially in empirical therapy, for complicated intra-abdominal infection, due to its favorable in vitro activity against anaerobic organisms, enterococci, various Enterobacterales ESBL and AmpC, or sometimes in association against carbapenemase-producing Enterobacterales, Acinetobacter species, and Stenotrophomonas maltophilia. In the case of patients with suspected or confirmed bacteremia, its use in monotherapy should be reconsidered since the outcomes have not been the best55,56.
Recent challenges in treating multidrug-resistant gram-negative infections, especially in critically ill patients, have renewed interest in the use of “old” antibiotics, such as polymyxins and fosfomycin, which are now commonly used to treat multidrugresistant bacteria in critically ill patients. However, limiting studies in intra-abdominal infection for multidrug-resistant bacteria should be considered before using them51,53,57. On the other hand, ceftolozane/tazobactam and ceftazidime/avibactam49 are new antibiotics approved for the treatment of complicated intra-abdominal infections (in combination with metronidazole), including infection with ESBL producing Enterobacterales and Pseudomonas aeruginosa. These antimicrobials will be of value for the treatment of infections caused by multidrug-resistant gram-negative bacteria, in order to preserve / save carbapenems. Ceftolozane/tazobactam has excellent in vitro activity against multidrug-resistant Pseudomonas aeruginosa58. Ceftazidime/avibactam59-62 has an in vitro activity against KPC-producing Enterobacterales and although less than ceftolozane/tazobactam, it has activity against Pseudomonas aeruginosa. The doses of antibiotics in pediatric population are annexed in Table 1.
Recommendation number 27: What is the treatment of choice in peritonitis caused by ESBL producing Enterobacterales?
The consensus recommends the use of ertapenem 1 g every 24 hours or tigecycline 100 mg loading dose and then 50 mg every 12 hours (Level of evidence: moderate. Strength of recommendation: STRONG).
Evidence
Ertapenem is an effective monotherapy for lower-risk patients with community intra-abdominal infection. Because their spectrum of activity is narrower than that of other carbapenems, the use of broad-spectrum carbapenems will be preferred for the empirical treatment of patients with more severe intra-abdominal infection, and that present with sepsis and comorbidities. Once the patient stabilizes and the culture shows sensitivity to ertapenem, it can be scaled. Ertapenem is also a good option, as an empirical therapy, where there is a high prevalence of ESBL-producers12,63.
Recommendation number 28: What is the treatment of choice in patients with peritonitis and high risk of KPC?
The consensus recommends the initiation of ceftazidime/avibactam 2 - 5 g every 8 hours + metronidazole 500 mg every 8 hours or IV 1 - 1.5 g every 24 hours a day (Level of evidence I. Strength of recommendation: STRONG).
Gram-negative carbapenemase-producing bacteria present an even greater challenge to the clinician. There are limitations of studies for the treatment of patients with hospital acquired intra-abdominal infection (HAAII) infected with multidrug-resistant pathogens. Most of the available literature relates to the treatment of patients with carbapenemase-producing Enterobacterales bacteremia, particularly Klebsiella pneumoniae. For the treatment of KPC-type carbapenemase-producing bacteria, there are studies that use the combination of a carbapenem with an aminoglycoside, polymyxin or tigecycline (200 mg loading and 100 mg q12 h), or both, where the association resulted in lower mortality, especially when the MIC of carbapenem (meropenem) was lower at 16 mg/L64-66.
However, recent studies show a higher mortality with any of these combinations vs. the use of ceftazidime/avibactam. This last association has activity against KPC-producing Enterobacterales and, therefore, may provide an option for the treatment of patients with IIAAH due to this type of microorganism. Treatment of patients with metallo-β-lactamase (MBL)-producing Enterobacterales is more problematic because these bacteria are resistant to almost all β-lactam antibiotics, except aztreonam, which is not hydrolyzed by MBL.
In these circumstances of MBL-producing bacteria, combined therapy, using agents such as polymyxin, tigecycline or fosfomycin among others, and recently the combination of aztreonam with ceftazidime/avibactam, is generally the only therapeutic option available. The use of ceftazidime/avibactam plus metronidazole should be considered as an option for the empirical therapy of adults with intra-abdominal infection, but reserve this regimen in patients at higher risk of being infected by carbapenemase-producing Enterobacterales (type KPC, OXA-48 - for which other agents are not suitable) (59-62.
Some patients with HAAII may be infected with extremely resistant, multidrug-resistant (XDR) Gram-negative bacteria, such as Pseudomonas spp. or Acinetobacter spp. These highly resistant organisms can be found in patients with tertiary peritonitis. The selection of empirical therapy in these patients is customized, based on previous culture and susceptibility data, the history of exposure to antimicrobials, and the type of resistant pathogens found in the local environment. For these multidrug-resistant pathogens and XDR, combination therapy has been used. Depending on the suspected organism, this may or may not include a broad-spectrum β-lactam antibiotic, an aminoglycoside, polymyxin, tigecycline (not applicable to Pseudomonas) or fosfomycin (not applicable to Acinetobacter). The consensus recommends combinations of a β-lactam, including ceftolozane/tazobactam, an aminoglycoside, and/or a polymyxin, for empirical antimicrobial therapy of HAAII in patients considered to be at risk for infection with Pseudomonas spp. multidrug- resistant or XDR67.
Recommendation number 29: What is the treatment of choice in patients with high-risk Pseudomonas aeruginosa peritonitis?
The consensus recommends the use of meropenem 2 g every 8 hours or piperacillin/tazobactam 4.5 g every 6 hours or cefepime 2 g every 8 hours + metronidazole 500 mg every 8 hours or 1 - 1.5 g day or ceftolozane/tazobactam 1.5 g every 8 hours + metronidazole, 500 mg every 8 hours or 1 - 1.5 g based on local epidemiology, always preferring not to use carbapenems if cefepime or piperacillin/tazobactam are susceptible in vitro. (Level of evidence I. Strength of recommendation: STRONG).
Evidence
The ASPECT-cIAI study [58] compared, in a randomized manner, for a population of 806 patients who had intra-hospital intra-abdominal infection, the use of ceftolozane/tazobactam with metronidazole vs. meropenem. Clinical cure was observed in 83% (323 of 389 patients) in the ceftolozane/tazobactam group and 87% (364 of 417) in the meropenem group, while the microbiological cure in the evaluable population of 596 patients was 94.2% in the ceftolozane/tazobactam group and 94.7% in the meropenem group; there were no significant differences in the group of microorganisms.
In those ESBL producing Enterobacterales, the microbiological eradication rate of 95.8% in the ceftolozane/tazobactam group vs. 88.5% in the meropenem group58.
Recommendation number 30: What is the treatment of choice in peritonitis patients with high-risk of Enterococcus spp.?
The consensus recommends adding ampicillin 2 g every 6 hours or ampicillin/sulbactam 1.5 - 3 g every 6 hours, if the initial therapy does not include coverage for Enterococcus (for example, meropenem, cefepime, ertapenem) (Level of evidence I. Strength of recommendation: STRONG).
Recommendation number 31: What is the treatment of choice in patients with high-risk methicillin-resistant Staphylococcus aureus peritonitis?
The consensus does not recommend the empirical initiation of antibiotics with activity against methicillin-resistant Staphylococcus aureus (Level of evidence I. Strength of recommendation: STRONG).
Recommendation number 32: What is the recommendation for the initiation of antifungal therapy according to the population at risk?
The consensus does not recommend initial empirical antifungal therapy (Level of evidence I. Strength of recommendation: STRONG).
The isolation of Candida spp. in community-acquired intraabdominal infection appears not to be significant. Montravers et al. (27 were able to demonstrate that in a nosocomial environment Candida spp. only was an independent risk factor for death, in contrast to patients with peritonitis from the community27,68. Empirical antifungal therapy can be included in the following cases: immunocompromised patients due to cancer, autoimmune disease or recent stem cell transplantation, or patients in the intensive care unit. However, it is still questionable whether proper treatment of Candida spp. Can improve the outcome. The underlying cause and the host’s poor immune response to inflammatory stress are perhaps the biggest contributor to infection and outcome68.
In severe peritonitis, yeasts are a poor prognostic factor. On direct examination of the peritoneal fluid, they indicate a large inoculum, and are associated with excessive mortality. The clinical features that suggest fungal infection are hemodynamic insufficiency, upper gastrointestinal perforation, female sex, and antibiotic therapy during the previous 48 hours. When three of these four criteria are present, the probability of isolating Candida spp. in peritoneal fluid is 71%. No prospective study has formally validated the rationale for antifungal therapy. However, in view of the clinical severity, it seems reasonable to initiate empirical antifungal therapy in this setting68.
G. Abdominal trauma
Recommendation number 33: When should empirical treatment be initiated in patients with abdominal trauma?
The consensus recommends the empirical initiation of antibiotics (Level of evidence I. Strength of recommendation: STRONG).
The abdominal trauma index (ATI) was designed to stratify patients with penetrating injuries and has been used to classify patients with blunt trauma and their relationship to sepsis. This index gives a score according to the organs involved in the trauma as follows: 1 = extrahepatic bile duct, small intestine, bladder, diaphragm, minor vascular system, soft tissues, bone tissues; 2 = kidney and ureter; 3 = spleen and stomach; 4 = liver and duodenum; 5 = pancreas, colon and major vascular system29-31,36,37.
The development of an intra-abdominal infection in patients undergoing surgeries due to abdominal trauma is a complex phenomenon resulting from multiple risk factors during the preoperative, intraoperative, and postoperative periods.
Multivariate logistic regression analysis allowed identifying an abdominal trauma index greater than 24, contamination of the abdominal cavity, and admission to the intensive care unit as independent risk factors for the development of organ-space surgical site infection (SSI) (36.
It is controversial whether prophylactic antibiotics are required in the prevention of infectious complications after penetrating abdominal trauma, as no randomized placebocontrolled trials have been published to date. There is also debate about the precise moment to apply such antibiotic prophylaxis. In 1972, Fullen et al. (69 observed a postoperative infection rate of 7 to 11% when he used preoperative antibiotics, an infection rate of 33% to 57% when he administered the antibiotics intraoperatively, and an infection rate of 30 to 70% when administered antibiotics postoperatively. Current guidelines indicate that there is class I evidence to support the use of a single preoperative dose of broad-spectrum antibiotics, with aerobic and anaerobic coverage, continuing for up to 24 hours postoperatively only in the case of a hollow viscus perforation found at exploratory laparotomy12.
The most important risk factor for the development of infection is the presence of a hollow viscus injury. Colonic wounds carry a higher incidence of infection in relation to the intra-abdominal organs. Pancreatic and liver injuries significantly increase the risk of infection, only when combined with wounds of hollow viscus. The degree of injury measured by the volume of hemorrhage and the presence of shock, as well as the anatomical degree of injury, also correlate with the incidence of sepsis. The use of antibiotics is approached by solving three basic problems: the choice, the duration of administration and the optimal dosage. Treatment options should include anaerobic coverage. In terms of duration, 24 hours of antibiotic administration is sufficient with the currently available agents. In contrast, there are few data on optimal dosing, but higher excretion rates have been shown in trauma patients and large volumes of distribution, so higher than usual doses are suggested. However, studies are required to address the problems of concentration-dependent and time-dependent bacterial death, since these pharmacodynamic considerations are highly variable among the different classes of antibiotics70-72.
Directed treatment Enterobacterales (Escherichia coli and Klebsiella pneumoniae)
Recommendation number 34: What is the recommendation for the treatment of Enterobacterales?
The consensus recommends establishing a phenotypic and/ or defining the mechanisms of resistance as well as the minimum inhibitory concentration (MIC) to target the treatment of Escherichia coli in clinical isolates of peritonitis (Level of evidence I. Strength of recommendation: STRONG).
34.2 The consensus recommends that using phenotypic and/or defining the mechanisms of resistance as well as the minimum inhibitory concentration (MIC) it is possible to start an appropriate treatment as follows:
2a. Resistance to ampicillin/sulbactam: cefuroxime 1.5 g every 8 hours associated with metronidazole 500 mg every 8 hours or 1 - 1.5 g once a day (Level of evidence I. Strength of recommendation: STRONG).
2b. Resistance to second-generation cephalosporins: ceftriaxone 1g every 12 hours or 2 g per day, plus metronidazole 500 mg every 8 hours or 1 -1,5 g once a day (Level of evidence I. Strength of recommendation: STRONG).
2c. Detection of ESBL or AmpC: ertapenem 1 g day without septic shock (Level of evidence I. Strength of recommendation: STRONG).
2d. Detection of ESBL or AmpC: in septic shock, meropenem 1 g every 8 hours (Level of evidence I. Strength of recommendation: STRONG).
2e. If there is coinfection with gram-positive cocci, such as Staphylococcus aureus or Enterococcus spp., tigecycline can be considered with a loading dose of 100 mg followed by 50 mg every 12 hours (Level of evidence I. Strength of recommendation: STRONG).
2f. Isolates resistant to carbapenems including Class A and Class D carbapenemases: if there is in vitro susceptibility, ceftazidime/avibactam 2.5 g IV every 8 hours plus metronidazole 500 mg every 8 hours, or 1- 1.5 g once daily is recommended (Level of evidence I. Strength of recommendation: STRONG) or the combination therapy of carbapenems (meropenem or doripenem or imipenem/cilastatin) ± tigecycline ± polymyxin ± fosfomycin or another antibiotic showing in vitro susceptibility (Level of evidence III. Strength of recommendation: STRONG).
Evidence
Intra-abdominal infections, particularly secondary or tertiary peritonitis, are generally of polymicrobial etiology; in this sense, the use of treatment with a single agent that has activity against isolated microorganisms and that includes a spectrum of antimicrobial activity against aerobic and anaerobic bacteria or agents that are active against coliforms according to their resistance mechanism is recommended, and in these cases metronidazole should be added27.
For isolates of Escherichia coli fully susceptible to β-lactams, the use of ampicillin/sulbactam or cephalosporins with metronidazole is an option. Another alternative is ciprofloxacin with metronidazole or ertapenem is also recommended14,27
The OASIS study63 compared the use of ertapenem vs. piperacillin/tazobactam in community-acquired intra-abdominal infections and no significant differences were found63. In a study of 841 cases of pediatric patients with appendicitis, the use of ceftriaxone once a day, associated with metronidazole vs. ertapenem and cefoxitin, and it was shown that the use of ceftriaxone and metronidazole led to a decrease in febrile time and a significant reduction in costs73. However, the use of any of the antibiotics led to a low rate of complications and fewer abscesses, which suggests that both strategies are effective74.In another study, Hamdy et al. (75 demonstrated that postoperative complications did not differ in children treated with ceftriaxone and metronidazole vs. a broad spectrum antibiotic, and for this reason it is preferred to use the antibiotic according to the mechanism of resistance.
In cases of β-lactam allergy, the use of quinolones with metronidazole or tigecycline monotherapy should be considered. Martínez et al. (55 evaluated the in vitro activity of tigecycline in clinical isolates in hospitalized patients in Colombia and observed an inhibitory activity of 100% in gram-positive cocci, Escherichia coli and Enterobacter cloacae, and 96% in Klebsiella pneumoniae. On the other hand, Osorio et al. (56 evaluated the efficacy of tigecycline reported in the different meta-analysis and clinical studies, and observed that in intra-abdominal infections it is as safe and effective as the comparators.
The use of ampicillin/sulbactam is not recommended in cases of infections caused by ESBL producing Enterobacterales. The use of piperacillin/tazobactam is not recommended in general for the treatment of infections caused by fully susceptible Enterobacterales, to reduce the selective pressure over P. aeruginosa54.
With the emergence of multidrug-resistant bacteria and, in particular, resistant to carbapenems, different schemes of combined antibiotic therapy have been used; In most casecontrol studies, the use of the combination of polymyxin B or colistin (polymyxin E) with carbapenems or other antibiotics, including fosfomycin, tigecycline, amikacin or quinolones, is observed57,64.
The RECLAIM 1 and 261 studies randomly compared the use of ceftazidime/avibactam associated with metronidazole vs. meropenem in hospitalized adult patients with intra-abdominal sepsis. Clinical cure was observed in 81% (337 of 413 patients) in the ceftazidime/avibactam group and in 85% (349 of 410) in the meropenem group, while microbiological cure was observed in the evaluable population of 596, 94.2% of patients in the ceftolozane/tazobactam group and 94.7% in the meropenem group. Stone et al. (61, in a joint análisis of three clinical studies, observed that 78.4 and 57.1% of multidrug-resistant Enterobacterales and multidrug-resistant Pseudomonas aeruginosa had good clinical response when they received ceftazidime/avibactam61. In the CRACKLE62 study it was observed that the differences in mortality between the group that used ceftazidime/avibactam vs. polymyxin, were statistically significant, 9 vs. 32 %. Other antibiotics for the treatment of infections caused by multidrug-resistant bacteria that have been studied are meropenem/vaborbactam (study TANGO I and TANGO II) (76 and imipenem/cilastatin/relebactam (in the RESTORE study, imipenem/cilastatin and colistin were compared, and observed a favorable response in the imipenem/cilastatin/relebactam group) (77.
Aztreonam/avibactam78, cefiderocol79, plazomycin80 and eravacycline81 have also been evaluated, which in the IGNITE-4 study eravacycline was not inferior to meropenem in patients with abdominal sepsis82.
Recommendation number 35: What is the recommendation for the treatment of Pseudomonas aeruginosa?
The consensus recommends establishing mechanisms of phenotypic or molecular resistance and MIC, for the targeted treatment of Pseudomonas aeruginosa in clinical isolates of peritonitis, (Level of evidence I. Strength of recommendation: STRONG).
The consensus recommends the use of the following antimicrobials according to Antimicrobial Stewardship Program and the susceptibility profile: piperacillin/tazobactam or cefepime or ceftazidime or aztreonam or ceftolozane/ tazobactam or ciprofloxacin (Level of evidence I. Strength of recommendation: STRONG).
The consensus recommends, in isolates without resistance to antipseudomonal penicillins, the use of piperacillin / tazobactam 4.5 g IV every 6 hours, and as an alternative cefepime 2 g IV every 8 hours plus metronidazole 500 mg IV every 8 hours or 1- 1,5 g every 24 hours. (Level of evidence I. Strength of recommendation: STRONG).
The consensus recommends, for isolates with resistance to antipseudomonal penicillins or patients with septic shock, meropenem 2 g every 8 hours, and as an alternative ceftolozane / tazobactam 1.5 g every 8 hours plus metronidazole 500 mg every 8 hours or 1-1,5 g every 24 hours (Level of evidence I. Strength of recommendation: STRONG).
The consensus recommends, in case of resistant Pseudomonas aeruginosa with a mechanism other than carbapenemases, ceftolozane / tazobactam 1.5 g IV every 8 hours plus metronidazole 500 mg every 8 hours or 1-1,5 g once a day (Level of evidence I. Strength of the recommendation: STRONG). The consensus recommends, in the case of Class A/Class D carbapenemase-mediated carbapenem resistance the use of ceftazidime / avibactam 2.5 g IV every 8 hours plus metronidazole 500 mg IV every 8 hours or 1 -1,5 g once daily as the first option. (Level of evidence I. Strength of recommendation: STRONG).
In the case of susceptible isolates of P. aeruginosa to piperacillin/tazobactam 4.5 g IV every 6 hours can be used as monotherapy76,77 or the combination of cefepime 2 g IV every 8 hours plus metronidazole 500 mg IV every 8 hours or 1-1,5 g once a day78,79. In case of resistance to cefepime and antipseudomonal penicillins with susceptibility to carbapenems, the use of meropenem 1-2 g every 8 hours is preferred. In the ASPECT-cIAI58 study, which compared the use of ceftolozane/ tazobactam plus metronidazole vs. meropenem in the management of complicated intra-abdominal infections, did not find significant differences in the outcomes or adverse effects of the two groups. In the case of isolates of Pseudomonas aeruginosa with resistance to β-lactams (including carbapenems) and preserved susceptibility to ceftolozane/tazobactam, this antibiotic should be used at a dose of 1.5 g IV every 8 hours adding metronidazole 500 mg IV every 8 hours or 1- 1,5 mg once daily83. Ceftolozane/ tazobactam is an effective combination against several multidrug-resistant gram-negative rods, especially Pseudomonas aeruginosa MDR / XDR [54,67,83-85]. In the RECLAIM 1 and 261 studies, in which the use of ceftazidime/ avibactam plus metronidazole was compared vs. meropenem in the management of complicated intra-abdominal infections, no significant differences were found in the outcomes or adverse effects of the two groups61. In case of resistance to ceftolozane/tazobactam with preserved susceptibility to ceftazidime/avibactam, the use of the latter is recommended at a dose of 2.5 g IV every 8 hours in addition to metronidazole 500 mg IV every 8 hours or 1,000- 1,500 mg once a day61.
If the strain is resistant to ceftazidime/avibactam, the presence of a metallo-β-lactamase should be suspected and in this sense the use of ceftazidime/avibactam plus aztreonam or the use of a combination of antibiotics between polymyxin (colistin or polymyxin B) plus fosfomycin with or without a carbapenem with antipseudomonal action (meropenem or doripenem) (62,86-88. Other antibiotics, such as meropenem/ varbobactam, imipenem/relebactam, cefiderocol, and plazomycin, are in clinical trials to study their efficacy in these types of infections76,77,79,80.
Recommendation number 36: What is the recommendation for the management of Enterococcus spp.?
36.1 The consensus recommends the use of targeted antibiotic therapy for enterococcal isolates if the patient has risk factors (Level of evidence I. Strength of recommendation: STRONG).
36.2 The consensus recommends the use of ampicillin in the case of susceptible Enterococcus faecium (Level of evidence I. Strength of recommendation: STRONG).
36.3 The consensus recommends the use of linezolid, tigecycline or daptomycin in the case of Enterococcus faecium resistant to vancomycin (Level of evidence I. Strength of recommendation: STRONG).
36.4 The consensus recommends starting treatment in patients in whom there is a persistent isolation of Enterococcus spp. (Level of evidence I. Strength of the recommendation: STRONG).
36.5 The consensus recommends treatment for Enterococcus in immunocompromised patients, especially with liver transplantation and at the end of life, heart disease and intravascular prosthetic material (Level of evidence I. Strength of recommendation: STRONG).
Recommendation number 37: What is the recommendation for the management of Staphylococcus aureus?
37.1 The consensus recommends the use of first-generation cephalosporins for methicillin susceptible Staphylococcus aureus (Level of evidence I. Strength of recommendation: STRONG).
37.2 The consensus recommends the use of vancomycin, tigecycline, linezolid, daptomycin, and ceftaroline for isolates of methicillin-resistant Staphylococcus aureus (Level of evidence I. Strength of recommendation: STRONG).
Evidence
The clinical relevance of Enterococcus spp. intra-abdominal infection has been the subject of debate for several years. By extrapolation of bacteremia studies, they have been considered as indications for targeted treatment for Enterococcus spp. the immunosuppression condition and the presence of a prosthetic valve89-94. Studies have been carried out to evaluate the impact of the isolation of Enterococcus spp. in intraabdominal infection in terms of complications, morbidity and mortality. One of the studies carried out evaluated the impact of the isolation of Enterococcus spp. in 473 patients with perforations of the small and large intestines, with subsequent stratified analysis by immunosuppression. The patients who presented the highest 90-day mortality in the multivariate analysis were the patients who presented treatment directed for Enterococcus spp., age older than 60 years, immunosuppression, and the presence of a fistula94. In 2017, Sanders et al. (89 published a cohort study of patients with abdominal infections with identification of Enterococcus spp., in which they did not find that the isolation of this microorganism was associated with any impact on the development of complications, such as new infections, surgical site infection or death. Studies in special populations, such as liver transplant recipients90,92 have documented a higher frequency of complications, such as prolonged stay in the intensive care unit, longer hospital stay, and higher 90 day mortality in patients with Vancomycin resistant Enterococcus infections.
Two lineages of Enterococcus faecium have been described in humans, the hospital clade and the community clade, which differ in susceptibility to ampicillin. The community clade, for the most part, is susceptible to ampicillin (MIC ≤ 2 µg/ml) since it harbors the pbp5-S allele, unlike the hospital clade, which presents the pbp5-R allele with expression of phenotypic resistance to ampicillin (MIC > 16 µg/ml) [95-97]. Based on the above, the consensus recommends ampicillin in case of in vitro susceptibility.
For the treatment of vancomycin-resistant Enterococcus, tigecycline, daptomycin, and linezolid are recommended as therapeutic options97. Tigecycline is a glycylcycline that also has activity against Gram-negatives (except Proteus spp., Morganella, Providencia and Pseudomonas). Comparative studies with imipenem/cilastatin for the treatment of complicated abdominal infections showed an adequate profile of clinical and microbiological cure results98. Resistance to the antibiotic mediated by mutation in the S10 protein of the 30S ribosomal subunit has been described, but it is not a common problem (less than 1% in resistance surveillance studies in Europe). The dose should be adjusted in overweight and critically ill patients (loading dose 200 mg, maintenance 100 mg every 12 hours) (99-101.
Linezolid is an oxazolidinone active only for Gram-positive cocci102. Several cases of therapeutic success have been described with linezolid for the treatment of vancomycinresistant Enterococcus in peritonitis associated with dialysis catheter. Resistance to linezolid is determined mainly by mutations in the 23S ribosomal subunit by expression of resistance determinants, such as cfr and optrA103. According to the resistance surveillance network, resistance data have been described in vancomycin resistant Enterococcus up to 6% (unpublished data from the Colombian resistance surveillance network by a kind personal communication from MV). .
On the other hand, daptomycin is a polypeptide with bactericidal activity for Gram-positive cocci. Peritonitis model studies have shown its favorable activity in reducing biofilm formation compared to linezolid or vancomycin104. In intra-abdominal infection, cases of therapeutic success have been described in catheter-associated peritonitis, including intraperitoneal administration (300 mg/day) [104-106]. The recommended daptomycin dosage in bacteremia for vancomycin-resistant Enterococcus is 10 - 12 mg/kg day associated with ampicillin or ceftaroline. There are no studies on intra-abdominal infection, thus the extrapolation of this dosage has been considered, given the significant effects on mortality and development of antibiotic resistance with lower doses in scenarios of bacteremia or infections with difficult-to-control foci107.
The first-line treatment for methicillin susceptible Staphylococcus aureus are β-lactams such as cefazolin or oxacillin. In the case of methicillin-resistant Staphylococcus aureus (MRSA) infection, vancomycin, linezolid, daptomycin, ceftaroline or tigecycline are recommended108, (109. Studies for peritonitis with Enterococcus associated with a peritoneal dialysis catheter and bacteremia, adverse outcomes had been reported in those who do not adjust the antibiotic treatment according to susceptibility106.
Intra-abdominal/peritoneal candidiasis
Recommendation number 38: What is the recommendation for the management of Candida spp. In peritonitis? (Taken from the Consensus for the Diagnosis, Treatment and Prevention of Candida spp. Disease in Children and Adults)68
38.1 The consensus does not recommend the initiation of an empirical/directed antifungal treatment in the patient with a diagnosis of community-acquired intraabdominal infection (Level of evidence I. Strength of recommendation: STRONG).
38.2 The consensus does not recommend the initiation of an empirical/direct treatment in patients with isolation of a Candida spp., from an abdominal sample, initiate empirical/directed antifungal treatment. Whether the isolation is contamination, colonization, or infection should be determined based on: (a) the anatomical site and type of injury, (b) history of interventions, (c) previous microbiological isolates, and (d) the clinical context of the patient. Antifungal treatment is not recommended for isolates associated with colonization or contamination (Level of evidence I. Strength of recommendation: STRONG).
38.3 It is recommended that, in patients with a diagnosis of intra-abdominal infection, the initiation of empirical/targeted antifungal treatment be considered in the following clinical situations: (a) high-risk immunocompromised patient, and/or hematopoietic stem cell transplant recipients (HSCT), (b) patient with a diagnosis of autoimmune disease, and (c) patients hospitalized in the ICU (> 7 days) (Level of evidence I. Strength of recommendation: STRONG).
38.4 It is considered in patients with clinical evidence of an intra-abdominal infection, with an isolation of a Candida spp taken from an abdominal sample, (taken intra-operatively or from drains), placed within 24 hours, an empirical/directed antifungal treatment should be started (Level of evidence II. Strength of recommendation: STRONG).
38.5 The consensus recommends in patients with a diagnosis of intra-abdominal infection by Candida spp., as a first line of empirical/targeted antifungal treatment, to include an echinocandin (caspofungin -CAS- [70 mg 1st dose, then 50 mg/d], anidulafungin -ANI- [200 mg 1st dose, then 100 mg/d], micafungin -MIC- [100 mg/d]) (Level of evidence I. Strength of recommendation: STRONG).
38.6 Fluconazole -FCZ- (800 mg 1st dose, then 400 mg/d) by IV, is an appropriate empirical/directed treatment option, in the patient with a clinically stable diagnosis of intra-abdominal infection by Candida spp., without treatment history antifungal with azoles and whose clinical isolates are sensitive to FCZ (Level of evidence II. Strength of recommendation: STRONG).
38.7 It is considered that, in the patient with a diagnosis of intra-abdominal infection by Candida spp., the duration of empirical/directed antifungal treatment will depend on the adequate surgical control of the abdominal infectious focus, and the clinical response of the patient (Level of evidence III. Strength of recommendation: STRONG).
38.8 The consensus recommends in patients diagnosed with Candida spp. peritonitis, at risk of candidemia/IC, initiating empirical/directed antifungal treatment with an echinocandin (CAS [70 mg 1st dose, then 50 mg/d], ANI [200 mg 1st dose, then 100 mg/d], MIC [100 mg/d]) (Level of evidence I. Strength of recommendation: STRONG).
38.9 FCZ (800 mg 1st dose, then 400 mg/d) IV, is an appropriate empirical/directed antifungal treatment option, in patients with a diagnosis of clinically stable Candida spp. peritonitis, without a history of antifungal treatment with azoles and whose clinical isolates are sensitive to FCZ. (Level of evidence II. Strength of recommendation: STRONG).
38.10 VCZ (6 mg/kg, two doses, then 3 - 4 mg/kg/12h) by IV, is an acceptable option for empirical/directed antifungal treatment in patients with a diagnosis of Candida spp. peritonitis, with patients with a history of having received antifungal treatment with azoles and/or with clinical isolates resistant to FCZ (Level of evidence III. Strength of recommendation: STRONG).
38.9 It is recommended that, in patients diagnosed with Candida spp. peritonitis, the duration of empirical/ targeted antifungal treatment is at least 2 weeks (Level of evidence II. Strength of recommendation: STRONG).
Evidence
The presence of yeast in the abdominal cavity is common if there is perforation of the gastrointestinal tract, this is common in the gastro-duodenal and small intestine, and rare in the bile duct and in colon-rectal perforations or of the appendix. The isolation of a Candida spp. in the peritoneum, from surgical samples, is a common finding, both in community patients and in hospitalized patients, where cases of post-perforation peritonitis have been established that do not require routine antifungal treatment13,27,50,68,110-118.
Montravers et al. (113 in a retrospective case-control study, in patients hospitalized in the ICU, demonstrated an increase in the mortality rate, in those patients with a diagnosis of nosocomial peritonitis and a fungal isolation (48% vs. 28% [without diagnostic] p <0,01). The authors determined that upper gastrointestinal perforation (OR, 4.9; 95% CI: 1.6-14.8) and the isolation of a Candida spp. (OR 3.0; 95% CI 95%: 1.3 - 6.7, p < .001), were independent risk factors associated with mortality in these patients68,111.
The initiation of an empirical/directed antifungal treatment in a patient with intra-abdominal infection can be recommended in the following clinical situations: (a) high-risk immunocompromised patient, and/or hematopoietic stem cell transplant recipients (HSCT), (b) patient with a diagnosis of autoimmune disease, and (c) patients hospitalized in the ICU (> 7 days). However, it is still questionable whether the initiation of antifungal treatment will improve the patient’s prognosis with a clinical isolation of Candida spp. (68.
Because the mortality due to candidal peritonitis is remarkably high (20-70%), various studies have shown that early antifungal treatment and adequate control of the infectious focus reduce associated mortality. The role of the isolation of a species of Candida spp., in a patient with a diagnosis of secondary peritonitis, (mainly in the presence of a gastrointestinal perforation), has not yet been defined, however, it is considered that the fungal isolation is an important risk factor for the development of invasive candidiasis in the patient with a prolonged stay in the ICU13,27,50,68,110,112,114,118.
Antifungal treatment in abdominal sepsis due to Candida spp., is the same one as for candidemia in non-neutropenic patients in ICU, it should be considered when choosing an antifungal drug: (1) the local epidemiology of Candida isolates and its sensitivity profile, and (2) the previous use of azoles, which could increase the number of resistant isolates. The use of echinocandins can reduce associated mortality when compared to other antifungal drugs (due to their broad spectrum, fungicidal action, and low toxicity), which has led them to be considered a first-line treatment; there is no difference between echinocandins in their efficacy in the clinical setting of a patient with abdominal sepsis, where the choice will depend on side effects, interactions with other drugs, liver failure, and treatment costs68,111,112.
The optimal duration of antifungal treatment for patients with candidal peritonitis has not been well established, however, due to the fact that high rates of relapse and recurrence have been described, it is recommended that the duration of treatment should be extended by 2 - 3 weeks, associated with surgical control of the infectious focus50,113,118.
Recommendation number 39: What is the recommendation for the choice of complementary measures to the treatment of intra-abdominal infection?
39.1 The consensus recommends laparoscopic surgical management (Level of evidence II. Strength of recommendation: STRONG).
Evidence
A meta-analysis published in Cochrane indicated that the main advantages of laparoscopic appendectomy compared to open appendectomy were the reduction of postoperative pain, as well as the risk of wound infection and length of stay, and faster return to activities normal in adults119. In contrast, laparoscopic appendectomy showed advantages over open appendectomy in wound infections and in reducing hospital stay in children.
Two studies reported that adults who received laparoscopic appendectomy had a better quality of life two weeks, six weeks, and six months after surgery. There were no data available for children. Regarding the drawbacks of laparoscopic appendectomy, a higher rate of intra-abdominal abscesses was identified in adults, but not in children With the exception of a downward trend in intra-abdominal abscesses after laparoscopic appendectomy, the results in children were similar to those observed in adults119.
39.2 The consensus recommends the laparoscopic approach among the complementary measures to the treatment of intra-abdominal infection and this will be directly dependent on the surgical team, the skills of the surgeon and the availability of the hospital center. The open approach is a safe strategy with an increase in the complications already explained.
39.3 The consensus recommends irrigation with SSN in case of surgical scrub (Level of evidence III. Strength of recommendation: LOW).
39.4 The consensus does not recommend lavage with SSN in open appendectomy, cleaning of the cavity is selected. In laparoscopic appendectomy or formal laparotomy, irrigation and surgical lavage are recommended (Level of evidence III. Strength of recommendation: LOW).
39.5 The consensus recommends evaluating the risk/benefit of a surgical procedure in case of emplastronate appendicitis (Level of evidence II. Strength of recommendation: MODERATE).
39.6 There is no consensus on the definitive treatment for the appendicular plastron, with the possibility of deciding between immediate surgical management vs. initial medical management and possible subsequent delayed appendectomy (Level of evidence III. Strength of recommendation: LOW).
39.7 The consensus does not recommend the use of a hyperbaric chamber or immunoglobulins for the treatment of abdominal sepsis (Level of evidence III. Strength of recommendation: LOW).
Evidence
The choice for nonsurgical management of patients, will be governed by clinical findings (no inflammatory response, no tachycardia, tolerance of the oral route, no abdominal pain, no leukocytosis) and by radiological findings without perforation or complication. Based on proper patient selection and clinical, imaging, and laboratory criteria, initial nonsurgical management of patients with appendicular plastron can be considered. Delayed appendectomy or interval appendectomy should be considered as part of the subsequent management eight weeks after the end of non-surgical management120,121.
Recommendation number 40: What is the optimal time for surgery?
The consensus recommends that in all cases of appendicitis, the ideal is to operate within the first 12 hours after the diagnosis is established (Level of evidence I. Strength of recommendation: HIGH).
Evidence
In all cases of appendicitis, the ideal is to operate between 6 and 12 hours after the diagnosis is established. Some series, such as the one published by Teixeira et al. (122, have not shown an increase of perforation in patients taken to surgery in the first six hours; in contrast they have shown a higher rate of SSI after six hours after the diagnosis was established122. Other retrospective series, such as that of Gurien et al. (125 in children, found no differences in rates of perforation or SSI between operating before or after six hours after diagnosis 125. A systematic review of the literature by Cameron et al. (123 in pediatric appendicitis concluded that appendectomy performed in the first 24 hours after admission of the patient is not associated with an increased risk of perforation or adverse outcomes123. In elderly patients and according to the resources of the hospital or clinic, it is reasonable to try surgical treatment as soon as possible124.
A meta-analysis of eleven studies showed that short delays, 12 to 24 hours after the onset of the clinical symptoms in stable, uncomplicated patients, are not associated with a high risk of perforation. On the contrary, delaying the procedure in patients with unclear pictures increases the diagnostic precision, without increasing the risk of perforation with the use of diagnostic aids such as abdominal scans126-128.
Recommendation number 41: What is preferable, open surgery or laparoscopy?
The consensus recommends open or laparoscopic surgery; both approaches are appropriate (Level of evidence I. Strength of recommendation: STRONG).
Evidence
The incidence of surgical site infection with laparoscopic procedure is less than 50% compared to open surgery, and the hospital stay is 1.1 days shorter. In addition, the laparoscopic approach is associated with less postoperative pain126-128. The consensus recommends that the approach to patients with acute appendicitis should be laparoscopic since it has a lower rate of complications than open surgery (infection of the surgical site, hospital stay, ventral hernias, postoperative pain, reoperation rates). It is reiterated that both approaches are safe but depend on the skills of the surgeon and the availability of equipment according to the hospital care center119,120.
Recommendation number 42: What is the best way to treat the appendicular stump?
42.1 The consensus recommends knots with traditional suture material (pre-made sliding knots, such as the endoloop), GIA-type cutting linear mechanical sutures, titanium clips, and polymeric clips. Its use will depend on the criteria, the experience of each surgeon and the degree of inflammation of the cecum when choosing the preferred method of ligation of the appendicular stump (Level of evidence I. Strength of recommendation: STRONG).
42.2 The consensus recommends that the closure of the appendicular stump can be done with manual sutures or knots, absorbable or non-absorbable material, or clips (Level of evidence I. Strength of recommendation: STRONG).
42.3 The consensus does not recommend the use of mechanical sutures as routine; these should be limited for when there is compromise of the apendicular base as an intraoperative finding (Level of evidence I. Strength of the recommendation: STRONG).
42.4 The consensus recommends the use of a mechanical suture in cases with perforation or inflammation of the appendix near the base (Level of evidence I. Strength of recommendation: STRONG).
42.5 The consensus recommends the use of mechanical staplers in open or laparoscopic surgery in patients with acute appendicitis in which there is an involvement of the base or in very thickened appendix at the base. Level of evidence I. Strength of recommendation: STRONG).
42.6 The consensus recommends abundant irrigation of the abdominal cavity in cases of appendicitis complicated by peritonitis (Level of evidence I. Strength of recommendation: STRONG).
Evidence
The management of the appendicular base will depend on several factors: its status according to the degree of inflammation or necrosis, its diameter, the technical skills and preferences of the surgical team, or both, as well as the technical means available123,126,129,130. At present, different technical options are available. The most traditional methods have involved making knots and ligatures (intracorporeal or extracorporeal), while in recent decades different automated mechanical devices have begun to be used, such as staplers or endoscopic clips. Despite having various technical options, today it is unknown which is the most appropriate method to use in each case, which is why the limited scientific evidence published on the management of the appendicular stump is striking. Mannu et al. (126 carried out a systematic review of the different methods of closure of the appendicular stump in uncomplicated acute appendicitis. This systematic review included eight randomized controlled trials, with a total of 850 participants. All trials compared mechanical vs. ligatures: five of the eight trials compared the use of endoclips vs. ligation, two trials compared endostapler vs. ligation, and one trial compared the three methods. No differences were found in total complications (OR 0.97, 95% CI: 0.27 to 3.50; eight RCTs in intraoperative (OR 0.93, 95% CI 0.34 to 2.55; eight RCTs or postoperative (OR 0.80, 95% CI: 0.21 to 3.13; eight RCTs comparing the use of ligation and mechanical devices. However, secondary outcome analyzes showed that the use of mechanical devices decreases operative time (mean difference (MD) -9.04 minutes, 95% CI: -12.97 to -5.11 minutes; eight, without a statistically or clinically significant reduction in hospital stay (MD 0.02 days, 95% CI: -0.12 a 0.17 days; eight RCTs.
Regarding total hospital costs and postoperative pain and quality of life, the information provided was not sufficient to make a reliable comparison between both strategies. Prospective studies determine that the selected stump closure method has no significant effect on the presence of infection at the operative site, so that complicated apendicitis is the only independent risk factor for the appearance of intra-abdominal abscess127. Given the low quality based on the available evidence and the limitations of all the studies in terms of bias, it is not possible to clearly determine the superiority of some methods of closure of the appendix stump over others. Currently that closure of the appendix stump can be done with manual sutures or knots, absorbable or nonabsorbable material, or clips. The use of mechanical sutures as a routine is not recommended; these should be limited for when there is compromise of the appendicular base as an intraoperative finding127,128. Currently, the use of endostaplers is recommended in cases of cecal appendix with a very high caliber, in complicated appendicitis with involvement of the base (gangrene, perforation or both), with which it is posible to minimize the risk of intestinal fistula, or when suspected appendicular tumor requiring extended appendicular resection. Today there is not enough evidence to argue for the omission of the closure of the appendicular stump based on conventional ligation in favor of a specific mechanical device over another in uncomplicated appendicitis128.
Recommendation number 43: When should an abdominal drain be left?
The consensus does not recommend the use of drains in complicated and uncomplicated acute appendicitis (Level of evidence I. Strength of recommendation: STRONG).
Evidence
The use of drains does not reduce the incidence of intra-abdominal abscesses and there is an increased risk of complications. There is insufficient evidence to support the use of drains. In the most recent review of the collaborative group of colorectal surgery in 2018, published in Cochrane131, it was not possible to show a difference in the number of patients with intraperitoneal abscess or wound infection when the use was compared vs. no use of drains. The mortality rate was higher in the drain group than in the no drain group. The hospital stay was longer (about two days - a 43.5% increase in an “average” stay -) in the drain group than in the no drain group. None of the studies reported on costs, pain, or quality of life. In general, there is no evidence of clinical improvement with the use of an abdominal drain in patients undergoing open appendectomy for complicated appendicitis131.
Recommendation number 44: What should be done in cases of appendicitis in patients with ventriculoperitoneal shunt?
The consensus recommends leaving the catheter in situ in patients with appendicitis and ventriculoperitoneal shunt; the risk of infection in non-perforated appendicitis is low (Level of evidence II Strength of recommendation: STRONG).
The consensus recommends evaluating catheter removal in perforated appendicitis (Level of evidence II Strength of recommendation: STRONG).
Evidence
The decision to keep the ventriculoperitoneal bypass catheter is highly debatable. Few studies have shown that in cases of non-perforated appendicitis, the catheter can be left without observing infection of the catheter [126-128,131-133]. In a perforated appendicitis, the situation is more complex, and although there is no absolute indication for externalization of the catheter, it is recommended to take peritoneal cultures, blood cultures and cerebrospinal fluid, and to evaluate the best antibiotic treatment in conjunction with infectious diseases, taking into account adequate penetration of the drug to the central nervous system. The decision to externalize the catheter is left to the discretion of the surgeon according to the intraoperative findings and the patient’s condition [133].