Introduction
Access to safe drinking water and sanitization is still a public health problem in South America and the Caribbean. Accor ding to the Pan American Health Organization (PAHO), 28 million people lack access to an improved water source and 83 million people lack access to improved sanitation facili ties1. Low coverage and quality services are more common and evident in low-income, vulnerable groups, and rural po pulations2. Pathogenic microorganisms are the most critical risk factor related to drinking water3. The main bacterial ge nera involved in waterborne diseases are enteric pathogens including Vibrio, Salmonella, Shigella, Escherichia, Campylo bacter, among others4.
Most of the studies related to the drink water quality as sessment have been performed using culture-dependent methods to identify indicator pathogens controlled in the drinking water regulation laws5. The advent of high through put DNA sequencing techniques provides a powerful broad-spectrum and high-resolution tool for microbiota as sessment in different types of environmental samples6. In this way, using metagenomic analysis tools, Kaestli et al. studied Australian water samples from drinking water distribution systems of three indigenous communities and successfully reported bacterial genera associated with fecal coliforms and other intestinal pathogens such as Clostridium, Cam pylobacter, Corynebacterium, Escherichia coli, Mycobacterium, Legionella, Burkholderia, and Leptospira7. In another report, 24 pathogenic and opportunistic bacterial species were de tected in water samples using a metataxonomic approach in a rural area from Haiti in which Klebsiella spp was the most frequent pathogen8. In general, the microbial diversity of drinking water in Latin America has been poorly characteri zed. Using novel NGS/metataxonomic technologies it will be possible to observe both environmental microbiota as well common coliform pathogens and other bacterial pathogens not included in the actual water regulation laws8.
In Colombia, 78% of the population has access to drinking water. However, the rural areas are at high risk since the 45,2% of the rural water purification plants show quality fai lures10. The Colombian Ministry of Health reports that about 1,300 children die each year from diarrheal diseases caused by drinking unsafe quality water9. In this regard, the govern ment objectives include the universal and equitable access to safe drinking water. Nonetheless, the monitoring of indicator pathogens (E. coli and fecal coliforms) is mainly realized in urban areas10. A report of the Colombian Ministry of Health informed that E. coli and fecal coliforms were detected in 23,8% and 32,9% of water samples collected in rural and ur ban areas, respectively.
In the current study, we explored the bacterial diversity of the river intake water and tap water collected from three villages in the Andean region of Colombia, using 16S rDNA sequen cing. These communities obtain water by collecting it from surface water of nearby rivers and storing it in improvised tanks, and, in most cases, the water is not treated with any chemical or physical methods before it gets to its final con sumers. Bacterial pathogens were detected in the tap water, this could affect the health of the communities.
Materials and Methods
Study area and sampling
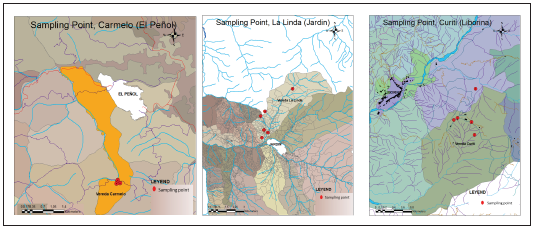
This study was conducted in the rural area from three muni cipalities of the Antioquia Department within the Andean re gion of Colombia: Village El Carmelo (El Peñol municipality), village Curiti (Liborina municipality) and village La Linda (Jar din) (Table 1). All the villages are located in the department of Antioquia (Fig.1).
The municipality of Liborina is located in the middle west of the department of Antioquia (6 ° 40 ‘59’ ‘North, 75 ° 48’ 0 ‘’ West), the average temperature is 26 ° C, with an area of 217 km2, of which 99% corresponds to the rural area11, a total population of 10,028 people, of which 2,296 are located in the urban area and 7,732 in the rural area12. The drinking water coverage is 100% in the urban area and 35% in the rural area13. The rural community of Curiti is located in the southwest of the municipality of Liborina. This rural community has a water supply system without purification treatment, and they take their water from two rivers.
The municipality of El Peñol is located in the eastern sub-region of the department of Antioquia (6 ° 13 ‘08’ ‘North, 75 ° 14’ 31 ‘’ West). El Peñol has a total area of 148 km2, an average annual temperature of 19 ° C14. The economy is based on agriculture with plantations of tomatoes, ba nanas, peas, beans, coffee, blackberries, cabbage, carrots, paprika, among others, as well as extensive livestock. The total population of El Peñol is 21,049 inhabitants, of which 11,022 and 10,027 make up its urban and rural population, respectively12. The potable water coverage is 100% in the urban areas, while in rural areas, it is 73%15. The village of El Carmelo is located to the south of the municipality, the inhabitants of the village do not have access to potable water.
The municipality of Jardin is located in the southwest region of the department of Antioquia (5 ° 36 ′ 0 ″ North, 75 ° 49 ′ 1 ″ West), the average temperatura is 19 ° Celsius. Its ex tension is 224 km2. Its total population is 14,518 inhabitants, distributed in 7,659, in the urban area and 6,859 in the rural area12. Its mountainous topography characterizes Jardin. Its economy’s base is a tourism and the cultivation of beans, sugar cane, cassava, potatoes, avocado, corn, and coffee. Its coverage in terms of drinking water is 99% in urban and rural areas, only in some rural communities there are water treatments by filtration and chlorination16. The rural com munity of La Linda is located 3 km from the municipality.
The water for human consumption in these communities is pumped from surface sources (rivers) into storage tanks without any treatment, except for Jardin in which there are improvised filters (without routine maintenance) for treating the water before the storage. In this study the selected sam pling points were the water intake, the storage tank, tap wa ter in the houses, and the town’s downstream river. A total of 18 water samples were collected on January 2020. Ten liters of water were collected from each sampling site using clean, non-sterilized containers with a screw cap and transported on ice to the laboratory. The intake and downstream water sam ples were taken in the middle part and at a depth between 5 and 20 cm, the tank and tap water were taken through PVC pipes. The specifications of the Institute of Meteorology, Hy drology and Environmental Studies (IDEAM) and the British standard were followed for the sampling: Part 2: Guidance on sampling techniques and ISO 5667-3 Preservation and handling of simple water. (BRITISH STANDARD. Water qua lity -Sampling Part 2: Guidance on sampling techniques. BS EN 25667-2:1993 BS 6068-6.2:1991 ISO 5667-2: 1991 Incor porating Amendment No. 1). The water was filtered through membrane disc filters with a pore size of 0.22mm to ensures the biomass quantity for DNA recovery (Membrane MCE 0.20um 47mm. Advantech MFS).
DNA extraction and Illumina sequencing
According to the manufacturer’s instructions, genomic DNA was extracted directly from membrane filters within the first 24 h of sampling using DNAeasy PowerSoil Kit (Qiagen, Ger many). DNA was quantified using the fluorescent probe Pico Green (Invitrogen P11496) and DNA quality was assessed by gel electrophoresis (1% agarose).
Samples were normalized to a final concentration of 10 ng/μL. Illumina libraries were prepared and sequenced using 300 bp paired-end MiSeq protocol at Macrogen Inc. (Seoul, Republic of Korea) following the service provider recommendations. The V3-V4 hypervariable regions of bacterial and archaeal 16S rDNA gene were amplified with the primers Bakt_341F (5′- CCTACGGGNGGCWGCAG-3′) and Bakt_805R (5′- GACTA CHVGGGTATCTAATCC-3′). Forward and reverse primers con tained the Illumina adapter, pad and linker sequences.
Bioinformatics análisis
The Sequences were analyzed using Mothur’s pipeline ac cording to Miseq standard operating procedure (SOP) 17. Briefly, Paired-end (PE) reads were assembled using Mothur’s tool “make.contigs” and then aligned to the SILVA 16S rDNA reference database. Sequences with ambiguous bases or ho mopolymers longer than 6 bases were removed. VSEARCH algorithm was used to filtering chimeric sequences. Nonbac terial lineages (chloroplasts, mitochondria, archaea and eu karyotes) were removed. Mothur’s subroutine “dist.seqs” was used for clustering reads into operational taxonomic unit (OTU) at a nucleotide identity limit of 0.03. Data were norma lized with the “totalgroup” method and rare OTUs with less than 3 sequences were removed. The phylogenetic classifi cation was obtained with the Classifier Ribosomal Database Project (RDP) tool (80 bootstrap threshold) 18. The micro bial diversity was calculated with the R package Phyloseq and Vegan. The Alpha diversity measurements including Chao1, observed species, Simpson, and Shannon index, were cal culated for each sample. Beta diversity was calculated using non-metric multidimensional scaling (NMDS), the distance method was bray. The Core community was calculated with the R package microbiome according to the core microbiota analysis using amplicon data.
Statistical Analysis
Statistical Analysis was made through R v3.6.2 in RStudio and Phyloseq package. Statistical significance was considered when the P-value was less than 0.05 with the non-parametric tests Kruskal-Wallis, pairwise Wilcoxon and Analysis of va riance using distance matrices (Adonis).
Accession number of sequences
Raw sequences were deposited in the SRA NCBI database under the bio project access number PRJNA662790.
Results
Alpha and Beta diversity analysis
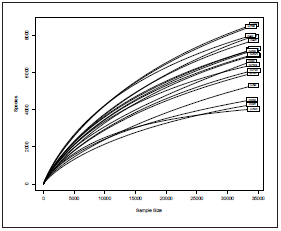
An average of 33,904 sequences per sample was obtained af ter the quality filtering (low quality, chimeras, and nonbacte rial origin), ranging from 32,813 to 34,607. Good’s coverage estimator was between 88% and 99% for all the samples in dicating an appropriate sampling effort for capturing most of the bacterial diversity. The rarefaction curves showed a good depth of sequencing (Fig. 2) The sequences were clustered into 35,236 Operational Taxonomic Units (OTUs) with 97% identity. The richness and diversity index were performed ac cording to the village (Fig. 3) and sample origin (Fig. 4). These results showed a higher diversity in the intake water, but this observation was significant only for the observed index com paring the Carmelo (7333 OTUs observed) and Curiti (5856 OTUs observed) villages (p=0.045).
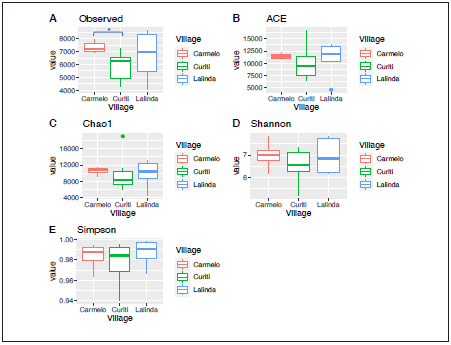
Figure 3 Alpha diversity indices in the water samples collected in three rural villages from Colombia. ACE (abundance-based coverage estimator). The OTUs observed index showed statistically significant differences between the villages Curiti and Carmelo, panel A (p<0.05).
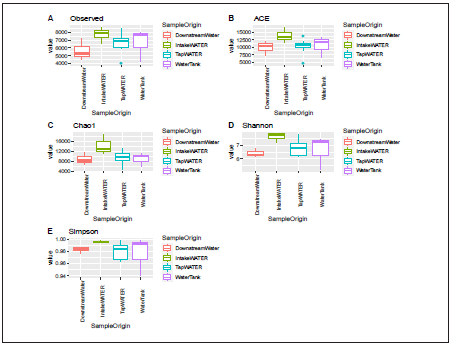
Figure 4 Alpha diversity in the water samples collected at four distribution points in the distribution network. ACE (abundance-based coverage estimator)
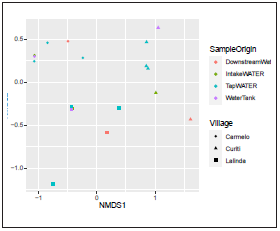
The non-metric multidimensional scaling (NMDS) analysis based on Bray-Curtis distance was applied for inferring the variations within the bacterial community structure grouping according to village or sample origin (Fig. 5). The stress value was 0.079. The obtained results showed a clustering of the bacterial community according to the village in the NMDS ordination space. Also, we detected a separation according to sample origin. This observation was supported by the Adonis test (p=0.001).
Taxonomic composition in water samples
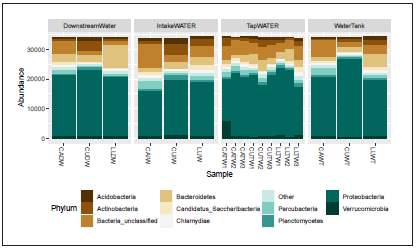
The Bacterial community structure was classified into 33 phyla dominated by Proteobacteria (42.5 to 67.32%) Bacteroidetes (1.88 to 23.68%), Actinobacteria (1.15 to 12.08%), and Aci dobacteria (0.67 to 5.06%). A large proportion of sequences could not be effectively classified (3.84 to 25.17%) by the RDP classifier at 0.8 bootstrap threshold. We observed diffe rences in the abundances of phyla according to the village and sample origin (Fig. 6). The sample collected in the intake water from Liborina village (CUIW) showed higher frequen cy in Actinobacteria and Acidobacteria phyla compared with the other samples. The core community was represented by 19 phyla detected in all villages included in the study. The frequency of Fusobacteria was higher in downstream water after the domestic activity from La Linda village (0.93%). Besi des, this village showed a higher frequency of the candidate phylum WPS-2 (1.12%).
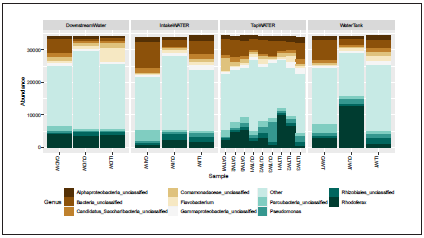
At the genus level, 619 genera were effectively classified in all samples. The dominant genera detected, belonging to Proteobacteria, were Rhodoferax and Pseudomonas (Fig. 7). Flavobacterium belonging to the Bacteroidetes phylum was the most frequent genus classified in the downstream water from La Linda village. Bacterial genera residing in the human intestinal tract were detected in fewer frequencies. However, we observed an enrichment of Prevotella and Paludibacter in the downstream water from La Linda Village.
Microbial pathogen analyses
Potentially pathogenic genera were identified in all the samples collected at different distribution points in the three villages, with differences in their frequencies (Fig. 8). Legionella, Myco bacterium, Yersinia, Burkholderia and Rickettsia were the domi nant pathogenic genera reported in the surface water and water tank from La Linda village. These genera persisted in the tap wa ter of two houses. Notably, the downstream water in this village was dominated by Aeromonas that was less frequent in the in take water. A higher frequency of some potentially pathogenic bacteria such as Streptococcus, Staphylococcus, Corynebacterium and Treponema was detected in the tap water from Carmelo village compared with the intake water and the water tank; this might suggest contamination in the pipeline. The water tank from Curiti village showed a higher frequency of Leptospira, Es cherichia/Shigella, Bordetella, and Serratia. However, we obser ved a reduction in the frequencies of these bacterial genera in the houses’ tap water with no apparent explanation.
Discussion
This study analyzed the bacterial microbiota from source, storage, tap water, and downstream water from three An dean rural communities in Colombia. The variations in the bacterial diversity within different rivers related to the geo graphical localization have been reported previously (figure 2) (19. This observation confirmed our results since the beta-diversity Analysis showed a separation in the bacterial popu lations according to the village. Our results also demonstrate the tremendous bacterial diversity present in the tropical An dean region’s freshwater sources and the lack of knowledge of these bacterial communities due to the high frequency of unclassified bacteria detected in this study20.
The dominant phylum classified in all the studied samples was Proteobacteria (figure 5); this phylum was previously re ported as dominant in several types of freshwater ecosys tems and groundwater21. As in this study, Alpha, Beta, and Gammaproteobacteria were detected in the tap water produ ced from freshwater in Portugal and France22,23. The persis tence of Alphaproteobacteria in drinking water is related to their capability to survive in low levels of nutrients and the chlorine tolerance23. Gammaproteobacteria comprises known pathogenic species belonging to the families Enterobacteria ceae, Pseudomonadaceae, Legionellaceae and Aeromonada ceae. Members of Burkholderiaceae and Comamonadaceae belonging to Betaproteobacteria have been reported in drin king water, thanks to their capacity to their efficient stress response and biofilm formation24.
Safe water supply improves the quality of life in the com munities; however, in the villages included in this study, the water is carried to the houses from a river without any appro priate chemical or physical treatment. The impact of anthro pogenic activities on water quality in these communities is related mainly to agriculture and domestic wastes. For this reason, microbiological contamination of the water sources is a relevant issue for these communities. Pathogens were detected in all phases of the improvised water distribution systems of these villages (figure 7). Our results are in concor dance with the observations of20, that reported dominance of Pseudomonas, Mycobacterium and Aeromonas in the surface water from several districts in Beijing.
Aeromonas presence in the intake water and downstream water in La Linda village in high frequency is a reason for public health concern because this bacterium is considered an emerging pathogen, especially in emerging economies25. Members of the genus have been isolated from aquatic ha bitats or inside various vertebrate hosts, including humans, fish, frogs, and crustaceans26. In humans, Aeromonas is res ponsible for gastrointestinal disturbances, septicemia, endo carditis, pneumonia, and conjunctivitis27. The high reports of Aeromonas in drinking water supplies are related to its capa city to colonize biofilms and resist the chlorine treatment28. Aeromonas has been isolated from surface water, ground water, chlorinated drinking water, and bottled mineral water in several countries such as Brazil29. Despite of the health sig nificance of Aeromonas infections, no routine monitoring of water supplies searching this bacterium is performed.
Storage tanks are essential components of the water distri bution systems and, if not properly maintained and sanitized, it can increase the risk to human health since the long stag nation times of drinking water could foster the risk of infec tion with pathogenic bacteria30. Previous studies have repor ted the presence of Pseudomonas, Legionella, and coliforms in storage tanks30. These observations are consistent with our results in which Escherichia/Shigella and Yersinia were detec ted in higher proportion in water storage tanks. Leptospira also was detected in a storage tank from Curiti village; this bacterium has been isolated from drinking water mainly in areas where sanitization is insufficient31.
In this study, the tap water showed a high frequency of Legione lla, this genus comprises 59 species, most of them are of aquatic origin32. Potable water is the most important source of pathoge nic species of Legionella related with lung infections33. This bac terium was detected in drinking water from Germany at tem peratures above 50oC and have been associated with biofilm formed in water piping. Legionnaire’s disease outbreaks have been reported around the world. In these cases, the primary in fection route is the inhalation of contaminated aerosols from drinking water, whirlpool spas, and cooling towers34.
Thirty potentially pathogenic bacterial genera were detected in the tap water of the three studied villages, some of them at significant abundance. This observation might help us un derstand the hazardous situation in which these people lives, and that this lack of safe drinking water could explain, at least in part, the burden of the enteric diseases of these popula tions. Despite in this study we do not perform viability test, this result could be an indicator of contamination because the DNA of dead cells is degraded in the environment and is less probable to detected through the sequencing platforms. To assess the real risk viability test should be performed. Pathogens such as Escherichia/Shigella, Yersinia and Leptospi ra pose a great threat to human health, especially to the chil dren in these villages, and are consumed on a regular basis in the drinking water by these communities. The pathogens reported in the tap water from La Linda village could indicate that the improvised water filtration systems and without rou tine maintenance offer no improvement in the water quality. This situation causes the final consumer to be exposed to the intake of water contaminated with pathogenic microor ganisms such as E. Coli, total and fecal coliforms, Aeromonas, and others, which generate adverse effects on human health.