Introduction
Colombia is a middle-income country located in Latin Amer ica, with an estimated 50.7 million inhabitants in 20201. Since the COVID-19 pandemic began, Bogotá -the capital city with a population of 7.4 million inhabitants- has been especially affected by SARS-CoV-2 transmission. Among possible rea sons are high population density, health inequalities, defi ciencies in universal health care access, high percentages of public transport use, and up to 48% of informal employment among adults, impeding compliance with lockdown2.
On March 6th, 2020, the first COVID-19 case in the country was confirmed in Bogotá, followed by strict lockdown de clared on March 20th and until April 27th. Multiple transitions to selective isolation and gradual economic reopening were implemented until August 2020, when the first epidemic peak was observed1,3. By December 2020, the city experi enced a second wave, with the second epidemic peak re corded in January 2021, leading again to a city lockdown. In April 2021, despite the containment and mitigation mea sures implemented, SARS-CoV-2 community transmission remained highly active.
The CoVIDA project is an unprecedented public-private collaboration that implemented two Drive/Walk-through testing centers for active epidemiological surveillance. The centers offered RT-PCR SARS-CoV-2 free testing in Bogotá to decrease barriers in access to COVID-19 testing and re duce times for RT-PCR test results. This initiative started in late March 2020 and aimed to test citizens, including asymp tomatic and mild-symptomatic populations conducting high mobility jobs. We aimed to describe the development and performance of the Drive/Walk-through free RT- PCR for SARS-CoV-2 testing strategy implemented by CoVIDA from May 29th, 2020 to March 20th, 2021.
Material and methods
We performed a descriptive analysis of the characteristics and performance of the CoVIDA Drive/Walk-through testing centers. This strategy was applied by the CoVIDA project as an innovative free screening model to identify transmission patterns in asymptomatic and mild-symptomatic selected populations with high mobility throughout the city of Bogo tá, Colombia4,5. The Drive/Walk-through model was based on various international experiences and scientific literature6-9 and adapted to the conditions of the study population. Two screening centers were implemented in shopping centers in the north and south of Bogotá.
The CoVIDA Drive/Walk-through screening centers were de signed based on the identification data and vital sign evalu ation, sample collection, sample transportation, substances and waste manipulation, and management-related guidelines for COVID-19 testing established by the Colombian Minis try of Health10,11. Therefore, the CoVIDA Drive/Walk-through screening centers complied with the human resources train ing, personal protective elements use, testing infrastructure and supplies, and manual of procedures development re quirements. Also, approval from the District’s Health Secre tary was obtained. Samples were processed at the Gencore laboratory of Universidad de los Andes, which is certified by the Colombian Health Ministry for SARS-CoV-2 detection.
The CoVIDA Drive/Walk-through screening centers performed testing in two modalities: motor vehicles and walk-up testing. The process included the following steps 1) patient identifica tion, 2) vital signs measurement (temperature, oxygen satura tion, heart rate, and respiratory rate), 3) nasopharyngeal swab sampling, and 4) reporting of results and recommendations.
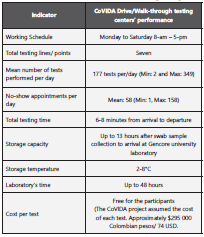
The process indicators to assess the model’s performance included: working schedule, total testing lines/points, the mean number of tests performed per day, no-show appoint ments per day, total testing time, storage temperature, labo ratory time, and the cost per test.
Ethical considerations
The Research Ethics Committee of Universidad de los Andes ap proved the CoVIDA study (Approval Number 1181, 2020). Based on the resolution 008430, 1993, and the resolution 2378, 2008, the study was classified as minimum risk research. Informed consent was obtained from each participant via telephone call.
Results
CoVIDA Drive/Walk-through testing centers performed 36,689 nasopharyngeal RT-PCR tests representing 55.5% of the total RT-PCR tests performed by the CoVIDA project (n=63,775). Participants screened at CoVIDA Drive/Walk-through testing centers had 5.75% cumulative positivity (n=2,109 positive tests). Table 1 presents the Drive/Walk-through process indicators. The test was free to the partic ipant and the CoVIDA project assumed it. Total testing time was six to eight minutes long from arrival to departure. RT-PCR test result was informed to the participants 48 hours on average, based on the laboratory´s processing time.
The most frequent occupations observed in the sample were contact with customers (general services) in 22.2% (n=8,134), essential office workers in 20.4% (n=7,493), and teachers/au xiliaries and students in 13.9% (n=5,120). Table 2 presents the distribution of the participants´ occupations and the positivity rates by each occupational group. Occupations with the hig hest positivity rates were police, military, and firefighter in 9.2%, informal worker in 8.7%, and construction worker in 7.5%.
Table 2 Distribution of participants’ occupations screened at the CoVIDA Drive/Walk-through testing centers and the positivity rates by each group.
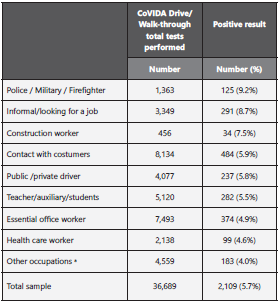
Other occupations:actors, cooks, farmers, musicians, operators, technicians, veterinarians, among others
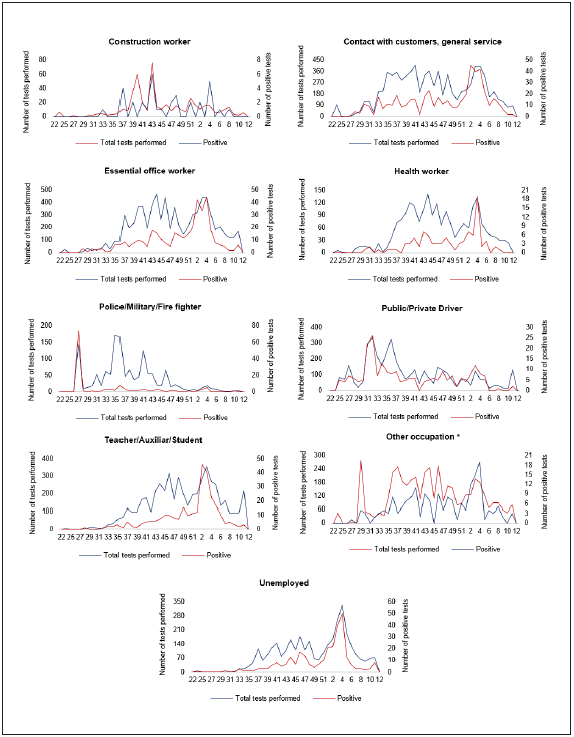
Figure 1 shows the number of RT-PCR tests performed and the number of positive tests observed by epidemiological week. Figure 2 presents the number of tests performed and the positive results by the epidemiological weeks for each occupational group.
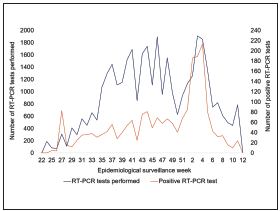
Figure 1 CoVIDA Drive/Walk-through testing center’s epidemiological surveillance in Bogota Colombia: RT-PCR-tests performed and positive RT-PCR tests from week 22 (May 29th, 2020) to week 1)
Discussion
To our knowledge, there were no other free massive testing centers for COVID-19 in Colombia and operating for the most prolonged period in the Americas region. These testing cen ters expanded the capacity of testing of COVID-19 during the first two peaks of the pandemic in asymptomatic and mild-symptomatic populations in Bogotá, which has been the most affected city during the pandemic in Colombia. The CoVIDA Drive/Walk-through model reached high standards of quality in all processes indicators. In addition, the virus transmission pattern with a sample of more than 36 thousand people was consistent with the patterns and pandemic peaks reported by health authorities and, CoVIDA was the only window that re ported on positivity in high-risk occupations.
The Drive/Walk-through testing centers operated for more than ten months of implementation, six days a week and eight hours a day. In Latin America, there is no report of such a prolonged testing strategy. Also, in the region, other expe riences have been carried out for up to ten weeks12,13.
International experiences that have used the Drive/Walk-through screening model have been previously reported in the literature. Evans et al. (2020) reported an average of 217 tests per day in the state of Nevada, US. The Arizona Depart ment of Health Services implemented a drive-through model with 12 sampling lines, 272 to 736 individuals tested per/day, and 184 no-show appointments per/day6. The Kansas Depart ment of Health and Environment reported specimen storage of up to 72 hours7. In Korea, a Drive-through center reported a total testing time of 10 minutes8,9. In contrast, in the US, Evans L. et al. reported a testing time of up to 15 minutes13. CoVIDA Drive/Walk-through testing centers achieved similar perfor mance but handled a greater number of tests per day, less sampling lines and with a scheduling spam of ten minutes.
Similar experiences in lower-middle-income countries such as Nigeria have used a walk-through model in addition to the traditional drive-through. A testing center recorded 1,794 visits with 78% drive-through and 22% walk-through visits. The average visit time for the drive-through was 19.2 ±4.6 minutes and 28 ± 9.2 minutes for the walk-through14. In con trast, the COVIDA testing centers managed to shorten the turn-around time for the participants.
The drive-through systems implemented worldwide have shown an increase in the test-taking capacity up to five times compared to other conventional systems, with the delivery of results within 24 to 48 hours after sample collection9,12,13,15. The CoVIDA Drive/Walk-through testing centers managed to reduce access barriers and expanded the testing capacity in Bogotá. Also, the CoVIDA Drive/Walk-through reduced wait ing times that were already prolonged (4-10 days) in the city due to the sanitary crisis. These actions provided support to the city’s health care system.
The CoVIDA Drive/Walk-through testing centers allowed mass testing of asymptomatic populations with a high risk of exposure to the virus, given their occupations. Likewise, the model design limited the exposure of healthcare workers and saved personal protective equipment (PPE) (16. These achieve ments translated into fewer human resources and lesser ex posure to the virus in health workers17. The use of these mod els reduced the burden of extensive cleaning procedures. A special room for testing in a healthcare facility would require special conditions such as air exchange, increasing turn-around time between people9,16. Therefore, the Drive/Walk-through method for testing could be more effective than an emergency department or a clinic16.
CoVIDA Drive/Walk-through testing centers were the only known free strategies in the region that combined drive and walk-through models. The drive-through model guaranteed the participants isolation within their vehicles, which leads to a reduced risk of infection18,19. Meanwhile, the walk-through model guaranteed a minimum of two meters distance between people. Although some authors have argued that the sched uling of walk-in participants using public transport can be a risk for the transmission of SARS-CoV-218,19, the CoVIDA walk-through testing centers performed symptoms screening before the scheduling of the test. Those with symptoms were tested at home, which could help to prevent the infection transmission. The walk-through model reduced the barriers to testing in a susceptible population that could not be tested at home due to their occupations, type of health insurance, and other social inequities or prejudices among their communities.
Finally, the changes in the overall positivity rate observed in our analyses were consistent with the positivity rate patterns reported in the city20. CoVIDA was the only window informing positivity rates by occupational group, also depicted as the CoVIDA dashboard in the SALUDATA Health Observatory of Bogota21. Police, military, and firefighters showed the high high est positive rate, which could be related to several outbreaks in military settings. The second occupation with the highest positivity rate was among informal workers, which can be ex plained by increased mobility and contacts, which may con fer a greater exposure to the virus.
In conclusion, the CoVIDA Drive/Walk-through testing cen ters increased the screening capacity for SARS-CoV-2 detec tion to support the epidemiological surveillance in Bogotá22. Low and middle-income countries can use the Drive/Walk-through model as a cost-effective and innovative solution strategy to mitigate the pandemic23.