Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a lethal malig nant neoplasm of unknown etiology. High age-standardized incidence rates of PDAC are present in first world countries, partially attributed to risk factors associated with specific lifestyles, ageing population and environmental conditions1,2. Classical PDAC risk factors are absent in most patients, and modifiable and non-modifiable risk factors for PDAC have an unconvincing molecular association with the disease3. Over the next 5 years PDAC is expected to ascend from the fourth to the second leading cause of cancer-related deaths, due both to the stagnation in outcome improvement for PDAC over the past 20 years and the improved outcome of other malignancies4-6. Additionally, the lack of screening methods for premalignant conditions, and the hitherto undeciphered PDAC carcinogenesis process, hinders progress in PDAC pre vention and treatment.
As in other gastrointestinal tract malignancies, bacteria have been associated with PDAC carcinogenesis, but the strength of this association is weak. The three most frequent bacteria associated with PDAC are Helicobacter pylori, Porphyromo nas gingivalis and Aggregatibacter actinomycetemcomitans, but each has insufficient empirical support for their role in PDAC carcinogenesis and no translation in clinical practice. Science has shown a pivotal role of Helicobacter pylori in intestinal-type gastric adenocarcinoma carcinogenesis7, but in PDAC patients the isolation of Helicobacter pylori has had contradictory results8,9. On the other hand, Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans are well-known oral bacteria associated with periodontal dis ease, but it is improbable that they exert biological activity in PDAC carcinogenesis from their oral location10,11. While the epidemiological association of Helicobacter pylori with intestinal-type gastric cancer has strong support, clarification of bacterial association with PDAC will be needed before any clinical interventions can be proposed.
Bacteria can reach the biliary tract and the gallbladder from the duodenum and by the entero-hepatic circulation12. The biliary tract, including gallbladder and intra-pancreatic bile ducts, is a semi-closed duct system possessing its own mi crobiota13,14, shaped by local conditions including exposure to high bile concentrations. Indeed, it has been shown that these conditions inform, at least at the phylum level, differ ences in gastrointestinal tract microbiota15. Many surveys have tried to characterize the microbiota in PDAC patients, comparing their results with normal or gallstone patients. To date there has been no success in ascribing a bacterial sig nature to the PDAC carcinogenesis process. It is possible that microbiota-modifying factors such as age, gender and diet, among others, representing a major obstacle in experimen tal control, have obscured the detection of such a bacterial signature16. To overcome microbiota-modifying factors, we compare the microbiota in PDAC patients in two different anatomic locations over the biliary system, and GS patients, with the aim to examine any differences. We found no statis tically significant differences either in alpha or beta diversity between groups, or as other surveys have found18, in the rela tive abundances of bacteria. However, the high similarity in PDAC patients between the two sites sampled in the micro biota analysis (gallbladder bile and bile duct brush over the tumor), upon comparison in alpha and beta diversity, is very interesting. This finding shows that microbiota can be the same along the extrahepatic biliary tract despite the pres ence of a PDAC in the head of the pancreas. As a result, a more accessible anatomic structure such as the gallbladder can act as a surrogate anatomic site for evaluation of the bili ary tract microenvironment.
Materials and Methods
Ethics and sample acquisition
The Institutional Human Ethics Committee of CES Uni versity and Clinic approved this study (minute number No 115/2017), and written, informed consent was given by all patients. Samples were de-identified before performing mi crobiota analysis. A surgical pathologist collected a total of 25 samples, a gallbladder bile sample from 3 patients with gallstones (GS), and 22 samples from patients with PDAC in the head of the pancreas (11 gallbladder bile samples: PC gallbladder group, and 11 intrapancreatic bile duct brush samples: PC duct brush group). All patients were Colom bian, and residents of Medellín (Colombia). For GS patients, bile was obtained in the operating room immediately after laparoscopic extraction of the gallbladder, puncturing the gallbladder fundus with a syringe, and aspirating at least 5 mL of bile. For PDAC patients, bile was similarly collected by aspirating bile with a syringe from the gallbladder pancre atoduodenectomy specimen and sent to the pathology lab for a frozen section margin report. The cyto-brush was also obtained from the pancreatoduodenectomy specimen sent to the pathology lab for a frozen section margin report, cut ting with sterile scissors the common bile duct margin and carefully introducing and moving the cyto-brush inside the duct, without reaching the duodenum. Immediately after co llection, bile samples and the brush were transported on ice, aliquoted, and stored at −80 °C until further analysis. Patients with a clinical history of previous malignant neoplasms, che motherapy, prior biliary tract surgery or biliary stent place ment, HIV, pregnancy, chronic pancreatitis, choledocholithia sis, cystic fibrosis, hepatolithiasis, primary biliary cholangitis, liver cirrhosis, primary sclerosing cholangitis, or acute chole cystitis were excluded from this study.
DNA extraction
Total DNA was extracted from gallbladder bile and brush, using the high pure PCR template preparation Kit (Roche Diagnostics), following the manufacturer’s recommenda tions17. Briefly, 200 μL of bile were used, in the case of the cyto-brush, the brush was washed with 1 mL of PBS, vorte xing for bacteria removal, and extracting 200 μL of the solu tion. Then, 200 μL of binding buffer plus 40 μL of proteinase K was added to each sample, regardless of sample origin. The mix was incubated at 70 °C for 10 min. Then, 100 μL of isopropanol was added, mixed and centrifuged for 1 minute at 8.000 × g. After centrifugation, 500 μL of inhibitor removal buffer was added and centrifuged for 1 minute at 8.000 × g. 500 μL of Wash Buffer was added and centrifuged for 1 minute at 8.000 × g. For DNA elution, 200 μL of prewarmed elution buffer was added and centrifuged for 1 minute at 8.000 × g. The DNA was stored at −80 °C until further analy sis. Negative controls using ultra-pure water alongside the kit reagents were performed, without bacterial DNA retrieval, using the amplification primers verified by gel analysis.
16S rRNA Sequencing and Microbiota Analysis
Amplicon libraries for pair-end (2 x 300 bp) sequencing on the Illumina MiSeq platform (Illumina Inc., San Diego CA, USA) were constructed using universal primers targeted across the V3 and V4 hypervariable regions of the 16S rRNA gene. The 16S rRNA gene was amplified using primers 341F 5’ -CC TACGGGNGGCWGCAG-3’ and 805R 5’-GACTACNVGGGTATC TAATCC-3’ which include overhang adapter sequences at the 5’ end to add multiplexing indices. Fastq files were analyzed using Qiime2-2019.418. The analysis pipeline includes Dada2 for sequence quality control19 and an in-house trained clas sifier based on the Greengenes 13_8 database for taxono mic analysis using a Qiime2 feature-classifier20. Thereafter, the feature table [Frequency] was filtered to exclude archaea and bacteria without at least an identified phylum. All the sequences were used to construct a phylogenetic tree with SEPP21. Using Qiime2 diversity core-metrics with and without phylogeny, we analyzed the alpha and beta diversity indices comparing them using Kruskal-Wallis or PERMANOVA (per mutational analysis of variance) test. A significance threshold of < 0.01was used for p and q values.
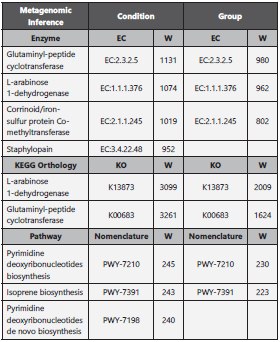
We looked for statistically significant differences in relative abundance between the groups, using the command line ANCOM (Analysis of Composition of Microbiomes) 22, comparing condition, anatomic site and taxonomic level. Additionally, a metagenomic inference analysis was perfor med using Picrust2 (Phylogenetic Investigation of Commu nities by Reconstruction of Unobserved States, v2.1.4 beta) 23,24, and the output tables for KEGG (KO), enzyme nomen clature (EC) and pathways were further analyzed with AN COM to highlight statistically significant frequencies between the groups and among conditions.
Results
A total of 14 patients and 25 samples were included in the study. Samples were divided into three groups; one GS group (N = 3), and two PDAC groups; the PDAC gallbladder group (N = 11) and PDAC brush group (N = 11). In the GS group there were 2 females and 1 male, mean age 47 years, and in the PDAC groups there were 7 males and 4 females, mean age 58 years. We identified a total of 2277953 sequences. The mean sequences per group were 63051 in the GS group, 86972 in the PDAC gallbladder group and 102918 in the PDAC brush group, with a minimum of 48001 (PC brush 8) and a maximum of 194153 (PC brush 11).
After filtering unassigned sequences and archaea, 488 OTUs (operational taxonomic units) were identified; 197 in the GS group and 325 in the PDAC brush group. There were 53 OTUs present in all three groups (shown in Fig. 1). Around 85-95 % of all identified OTUs in each group were comprised of only 3 % of phyla in each group. In the GS group the predomi nant phyla were Proteobacteria (40 %) followed by Bacte roidetes (30 %) and Firmicutes (16 %). Conversely, in both PDAC groups the predominant phylum was Proteobacteria (64-76 %) followed by Firmicutes (14-25%) and Bacteroidetes (5-6 %). In the same way, through different taxonomic levels such as class, order and family, there are differences in bacte rial community structure regarding percentage. At the class taxonomic level, Gammaproteobacteria represents 21 %, 73 % and 64 % in the GS, PDAC brush and PDAC gallbladder groups, respectively. For the order taxonomic level, the per centages of Enterobacteriales in the PDAC brush (71 %) and PDAC gallbladder (57 %) groups were considerably higher than in the GS group (12 %). In the case of the Enterobacte riaceae family, we observed the same trend (12 % in GS, 71 % in PDAC brush and 57% in PDAC gallbladder) (shown in Fig. 2). Nevertheless, the pairwise Kruskal-Wallis analysis for the aforementioned differences compared by group and condi tion, showed no statistically significant results.
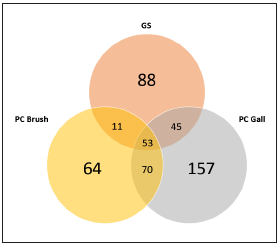
Figura 1 Venn diagram depicting OTUs number per group and shared OTUs. GS: gallstones, PC gall: pancreatic ductal adenocarcinoma gallbladder bile sample group, PC duct: pancreatic ductal adenocarcinoma duct brush sample group
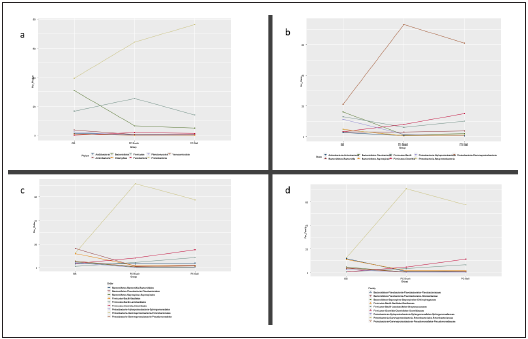
Figura 2 Graphical representation of the 9 % percent higher bacterial community composition for different taxonomic levels (a) phylum, b) class, c) order, d) family). GS: gallstones, PC gall: pancreatic ductal adenocarcinoma gallbladder bile sample group, PD duct: pancreatic ductal adenocarcinoma duct brush sample group
We compared the alpha and beta diversity of the bacterial communities per group (anatomic site) and condition (GS vs PC). Alpha diversity was initially analyzed using Chao1, Shan non and Pielou’s evenness index. There were no statistically significant differences upon comparison by group (GS = 3 vs PDAC gallbladder = 11 vs PDAC brush = 11) or condition, (GS = 3 vs PDAC = 22) in Kruskal-Wallis pairwise analysis. Although the Kruskal-Wallis pairwise analysis was not statis tically significant, the GS group had higher values than the PDAC groups (shown in Fig. 3), and the PDAC groups have very similar values. Regarding beta diversity, we obtained statistically significant results in the PERMANOVA pairwise analysis (shown in Table 1) for Bray-Curtis and Jaccard indices upon comparison by condition (p and q < 0.01). The remai ning pairwise analysis by group and condition for Bray-Cur tis, Jaccard, unweighted and weighted unifrac indices were not statistically significant (shown in Fig. 4).
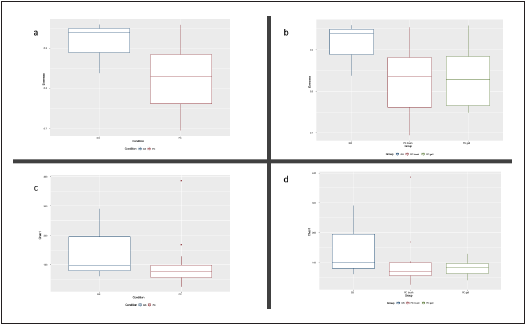
Figura 3 Boxplot of evenness (a) and b)) and chao1 (c) and d)) alpha diversity indices for group and condition. GS: gallstones, PC gall: pancreatic ductal adenocarcinoma gallbladder bile sample group, PD duct: pancreatic ductal adenocarcinoma duct brush sample group
Table 1 Beta diversity indices comparison by group and condition
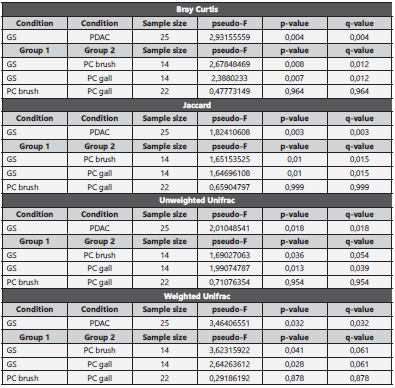
GS: gallstones, PC brush: pancreatic ductal adenocarcinoma patients’ brush sample, PC gall: pancreatic ductal adenocarcinoma patients’ bile sample.
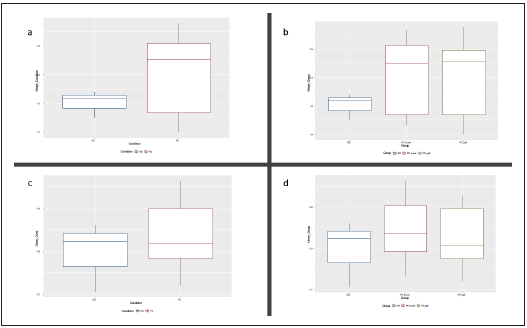
Figura 4 Boxplot of weighted (a) and b)) and unweighted (c) and d)) unifrac beta diversity indices for group and condition. GS: gallstones, PC gall: pancreatic ductal adenocarcinoma gallbladder bile sample group, PD duct: pancreatic ductal adenocarcinoma duct brush sample group
The ANCOM compositional analysis did not reveal any sta tistically significant differences in relative abundances regar ding partially observed species among the groups. Picrust2- ANCOM metagenomic inference pipeline revealed three over-represented enzymes, two over-represented KO and two over-represented pathways (shown in Table 2) that were statistically significant, coinciding in condition and group comparison. All over-represented features were over-repre sented in the GS group regardless of the comparison made by group or condition.
Discussion
Alpha and beta diversity analysis suggest a competitive mi croenvironment and phylogenetic similarities. The analyzed alpha diversity indices, while not statistically significant, point out a possible, more equitable distribution of species regar ding richness and relative abundance in the GS group, and similarities in the PDAC groups. The more equitable distri bution in the GS vs PDAC groups can be interpreted as an indirect sign of competence in the PC groups where just a few species dominate the bacterial community composition at the different taxonomic levels25. The beta diversity results for Bray-Curtis and Jaccard indices were statistically signifi cant upon comparison by condition, but these results can not be extended to other indices such as unweighted and weighted unifrac. However, PERMANOVA pairwise results for beta diversity comparing PDAC groups were almost identical, highlighting that perhaps the biliary tract, a semi-closed sys tem, has similar beta diversity distributions among different anatomic locations. Interestingly, this beta diversity similarity remains unchanged along the biliary tract despite PDAC in the head of the pancreas.
There are just a few studies comparing the biliary tract mi crobiota in PDAC patients. Thomas et al., investigated the impact of microbiota in pancreatic carcinogenesis using tis sue samples, amplifying and sequencing the 16S rRNA V1- V3 regions. The study enrolled 26 patients with pancreatic disease, six normal (no disease), four with pancreatitis and sixteen with PDAC. There were no significant differences in bacterial associations between pathological stage, beta di versity and bacterial taxa. Differences in Chao1 and Shan non indices among normal and pancreatic cancer, after false discovery rate correction26 gave similar results. Del Castillo and colleagues characterized microbiota in PDAC patients using normal surrounding fresh or neoplastic frozen tissue. They obtained swabs and tissue samples of normal pancreas, pancreatic tumor, normal bile duct, stomach, duodenum, jejunum, ileum and stools. Microbiome analysis was carried out, amplifying and sequencing the 16S rRNA V3-V4 gene regions. They found that bacterial profiles in duodenal and pancreatic tissue were very similar in the same patient, and that upon comparison of PDAC vs non-PDAC patients, the relative abundance of genus Lactobacillus was significantly increased in non PDAC patients compared to Fusobacterium spp, which was significantly increased in PDAC patients27. Fi nally, Riquelme et al., designed a multicenter approach that focused on describing PC microbiota in long-term PDAC sur vivors (> 5 years, N = 22, MD Anderson patients), regular PDAC survivors (< 5 years, N = 21 MD Anderson patients, N = 10 Johns Hopkins patients) and very long-term PDAC survivors (> 10 years, N = 15 Johns Hopkins patients), mat ched by age, gender and prior neo or adjuvant therapies. They used formalin-fixed paraffin-embedded tissue (FFPE), amplifying the V4 region. They found that alpha diversity measured by Shannon and Simpson indices in long-term and very long-term PDAC survivor groups was higher than the regular PDAC survivor groups. Additionally, long-term PDAC survivors showed a differential abundance of Proteobacteria (Pseudoxanthomonas) and Actinobacteria (Saccharopolyspora and Streptomyces), proposing this microbiota profile as a sig nature for better PDAC outcomes28.
To the best of our knowledge this is the first study comparing microbiota using 16S rRNA in two anatomic locations of the biliary tract in PDAC patients (bile and biliary tract brush over pancreatic tumor). Like the research of Thomas et al, differen ces in alpha diversity indices were not statistically significant, but in the present study it was considered that the lower va lues of the alpha diversity indices in the PDAC groups may be related to competitive bacterial exclusion. In the relative abundance analysis using ANCOM pipeline in Qiime2, a spe cific bacterial signature associated with PDAC was not identi fied. There are many studies with relevant findings of speci fic bacterial signatures in PDAC patients, both locally and in different anatomic locations. Despite this, specific bacterial signatures cannot be translated to clinical practice, cannot be included in a carcinogenesis model or even be considered a risk factor amenable to detection and control. For these rea sons, a paradigm shift approach in PDAC research is required. The results of the metagenomic inference analysis, while statistically significant, are very complex for inferring meaningful biological information. Further studies are needed from an integrated, complementary analysis standpoint, such as me taproteomics, to correlate the microbiota profile, diversities, metagenomic inference, and differential relative abundance with human and bacterial protein profiles. We hypothesize that through this integrative model, we may find the elusive biological information that will allow the natural history of PDAC to be improved.