Introduction
More than a half of children who start antiretroviral therapy (ART) in developing countries are underweight and malnu trition has been associated to triplication of mortality during the first month of ART1. In Colombia, proportion of patients with undernutrition during course of pediatric HIV infection has not been recently determined. Latin American sources have reported a prevalence of malnutrition among children living with HIV that varies between 52,4% to 69,3% in coun tries such as Peru and Venezuela2,3. In Colombia, a hospital based study in Cali found that 72% of children infected with HIV by vertical transmission were underweight, 67% stunted and 35% wasted4, which is consistent with a 70,4% of children with underweight that was found in a University Hospital in Medellin5. However, it has been more than a decade since thepublication of those findings and Colombian nutritional pa norama could have changed. The objective of this work is to describe nutritional status of pediatric patients infected with HIV in Bogota, and to explore a possible interaction between malnutrition and ART failure.
Patients and methods
Retrospective longitudinal descriptive study, in a simple of 28 patients belonging to HIV program of the outpatient clinic Asistencia Científica de Alta Complejidad SAS, in Bogota city. Variables were retrieved from clinical records between years 2012 and 2020. Given the exploratory nature of the study, sampling was made by convenience, gathering information of all pediatric patients who met two inclusion criteria: 1) A confirmed diagnosis of HIV, according to local guidelines6 and 2) An age of less than 18 years. In order to calculate Z scores of Weight for height (WHZ), height for age (HAZ), weight for age (WAZ) and BMI for age (BAZ) in children un der 5 years, the software WHO Anthro v3.2.2 (OMS 2011, Geneva, Switzerland) was used. To calculate HAZ, WAZ and BAZ in children from 5 to 19 years of age, the software WHO AnthroPlus v1.0.4 (OMS 2009, Geneva, Switzerland) was used.
For nutritional diagnosis the following definitions were adopted7:
Wasting or acute malnutrition: WHZ < -2.
Stunting or chronic malnutrition: HAZ < -2
Underweight or global malnutrition: WAZ < -2.
Thinness: BAZ < -28.
Different therapeutic failure definitions that were used can be consulted in supplementary material .
Variables were typed in the software Microsoft Excel 2016. Statistics were processed in the software STATA 13 MP-Para llel Edition for Windows. Differences among categorical va riables were explored with Chi squared test or Fisher F test, and relative risks with 95% confidence intervals were estima ted using a generalized lineal model. Shapiro-Wilk normali ty test was applied and then differences were explored with non-paired Student t test, assuming equal variances, or Wil coxon signed rank test. In spite of the assumption of a one tailed p<0,05 as a limit to consider statistical significance, interpretation must be cautious, given the exploratory and hypothesis generating nature of this study, that was concei ved from the moment of sampling calculation. In order to describe the longitudinal behavior of some variables, a com parison was made from the entry to the HIV program to 12 months later, with paired Student t test and McNemar margi nal homogeneity test. Patients with missing data were exclu ded from respective analysis. Figures were generated using the software Prism 7 for Windows (GraphPad Software Inc, San Diego, CA, United States). As this research posed no risk for investigation subjects, no informed consent was obtai ned from recruited patients and data retrieved from clinical records were protected with confidentiality and privacy. An institutional Ethics Review Board superintended this process.
Results
36 clinical records met the inclusion criteria and 8 patients were excluded because of missing longitudinal anthropometric data in 5 of them, erroneous data in 1 patient and missing longitudinal immuno-virological data in another patient. One additional patient was excluded because of pregnancy. A total of 28 patients were included in this work.
In table 1, basal features at the moment of entry to the HIV program are shown. 21 out of 28 patients had vertical transmission of HIV (75%; 95%CI: 59 - 91), 9 (32,1%) had at least one non-HIV associated comorbidity, among which bronchiolitis, acute lymphoid leukemia and resolved B hepatitis were highlighted. On the other hand, 11 (39,3%) patients had opportunistic infections such as chronic diarrhea, recurrent pneumonia, oropharyngeal and esophageal candidiasis, herpes zoster and ganglionic tuberculosis. Only 7 (25%) patients were ART naive and time interval from HIV infection diagnosis to start of ART had a median of 22 days (intercuartil range: 4 - 74).
Table 1 Baseline features of recruited patients
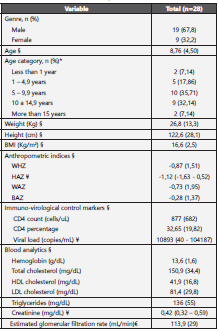
Anthropometric and paraclinical features of patients at the moment of entry to the HIV program are shown. n: number of subjects; BMI: Body mass index; WHZ: Weight for height Z score; HAZ: Height for age Z score; WAZ: Weight for age Z score; BAZ: BMI for age Z score; HDL: High density lipoprotein; LDL: Low density lipoprotein; §: Mean (SD). ¥: Median (ICR). * Frequency (%). €: Determined with Schwartz formula9
After a clinical follow-up interval elapsed, just 1 patient chan ged ART from zidovudine-lamivudine-efavirenz (AZT-3TC-EFV) to raltegravir-lamivudine-lopinavir/ritonavir (RAL-3TC-LPV/r) because of virological failure. 6 out of 28 patients su ffered adverse events (AE) to ART (21,4%; 95%CI: 6,2 - 36,6%), such as LPV/r -associated vomiting in half of all cases and hy pertriglyceridemia in the remaining half of affected patients. No AE led to treatment discontinuation. ART adherence was 82,1% (95%CI: 68 - 96,3). Percentage distribution of different ART is depicted in http://dx.doi.org/10.22354/in.v26i1.1022
Most of patients were classified in clinical and immunological A1 category (http://dx.doi.org/10.22354/in.v26i1.1022). Except for ART naïve pa tients, just 5 out of 21 (23,8%) participants had undetectable viral load at program entry. Proportion of patients with virolo gical, immunological and clinical failure could be determined in all patients except for 7 of them who were ART naïve and 3 participants who had started ART less than 90 days befo re being recruited. After a mean clinical follow-up interval of 424 (SD: 82,5), virological, immunological and clinical failure outcomes were determined again, according to WHO10, the Department of Health and Human Services of the United States11 and the Expert Panel of Spanish Society of Pediatric Infectology - National AIDS program standards12; this time in all patients. The results are shown in http://dx.doi.org/10.22354/in.v26i1.1022; keeping in mind that there is neither a standardized definition of clinical failure in Spanish guidelines, nor a definition of im munological failure in North American guidelines11,12.
After a paraclinical median follow-up period of 378 days (ICR: 310 to 459), changes in count and percentage of CD4 lymphocytes, viral load (figure 1) and CDC category (http://dx.doi.org/10.22354/in.v26i1.1022) were explored. In table 2, several important outcomes are compared at the program entry and after the follow-up period had elapsed. It is noteworthy, that 18 pairs of data were processed for analysis of immuno-virological outcomes shown in table 2. Pairs of data with missing va lues at baseline or during follow-up, were excluded from the analysis. As a result, relative risk (RR) and absolute risk reduc tion (ARR) statistics were calculated based on proportions of patients that differ from those shown in baseline and follow-up columns of table 3. In such columns, the percentage of the totality of available patients at a time is shown.
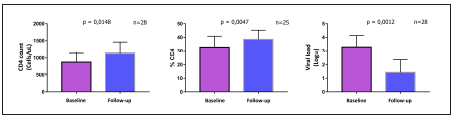
Figure 1 Immuno-virological control marker changes in pediatric patients of an HIV program in Bogota, Colombia. Changes in immuno-virological control markers are observed during follow-up period. Bars represent means and corresponding 95% confidence intervals. n: number of pairs of observations.
Table 2 HIV program follow-up effect on different nutritional outcomes of pediatric patients in Bogota, Colombia.
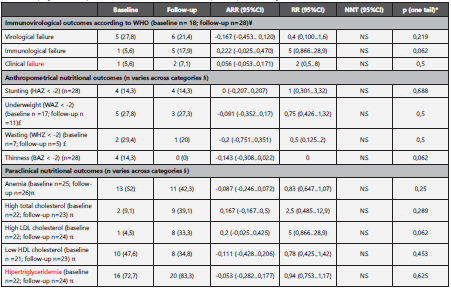
Effect size of HIV program on different clinical and paraclinical outcomes. ¥: Baseline n is lower because of exclusion of ART naive patients; however, for ARR and RR estimating purposes, the number of pairs of data was 18. £: Baseline n is lower because patients exited the age group they belonged to during follow-up. §: Pairs of data with missing values at baseline or during follow-up were excluded from analysis. π: Baseline and follow-up n differ because of missing data. *A mid-p value of McNemar test was used, because the number of discordant pairs was lower than 25. ARR: Absolute risk reduction; RR: Relative risk; NNT: Number needed to treat; n: number of observations.
Baseline anthropometrical evaluation revealed that 4 out of 28 patients were stunted (14,3%; 95%CI: 1,3 - 27,2), 2 of them severely stunted (HAZ < -3); 2 out of 7 patients were wasted (28,6%; 95%CI: 0 - 62), 1 of them severely wasted (WHZ < -3); and 5 out of 17 patients were underweight (27,8%; 95%CI: 7,1 - 48,5), 1 of them severely underweight (WAZ < -3). Ac cording to BAZ, 4 out of 28 patients suffered thinness (29,6%; 95%CI: 12,4 - 46,8), 1 of them was severely thin (BAZ < -3). Changes in anthropometrical variables after clinical follow-up interval are shown in figure 2.
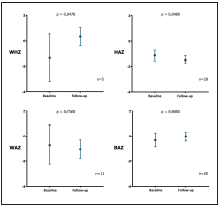
each measure. WHZ: Weight for height Z score; HAZ: Height for age Z score; WAZ: Weight for age Z score; BAZ: BMI for age Z score; n: number of pairs of data
Figure 2. Changes in Z scores of different anthropometrical indices during clinical follow-up of pediatric patients of an HIV program in Bogota, Colombia. .
Anemia was found in 2 out of 8 girls and 11 out of 17 (64,7%) boys, adopting hemoglobin cut-off points from a reference laboratory (<12 g/dL for girls and <14 g/dL for boys). Con sidering reference values for diagnosis of dyslipidemia in children13, baseline elevations in total cholesterol (TC), LDL cholesterol (LDLc) and triglycerides (TG) in 2 out of 22 (9,1%), 1 out of 22 (4,6%) and 16 out of 22 (72,7%) patients, respecti vely. A low HDL cholesterol (HDLc) value was found in 10 out of 21 (47,6%) patients. No patients had an estimated glome rular filtration rate (eGFR) below 60 mL/min, making use of the last update of Schwartz formula9.
After a paraclinical follow-up interval, an increment in mean value of TC, mainly due to LDLc was found (p=0,0063), with no significant changes in HDLc, TG or hemoglobin. Serum creatinine increased during paraclinical follow-up interval (p=0,0019), parallel to a decrease in mean eGFR from 116 mL/min to 105 mL/min (p=0,0109). Finally, mean changes of several clinical-anthropometrical and paraclinical nutritional diagnosis are shown in table 2.
Discussion
In this work, malnutrition was found to be a frequent comor bidity in pediatric patients living with HIV in Bogota, Colom bia. The WHO Nutritional Landscape Information System has defined prevalence cut-off points to stablish the impact that several nutritional diagnosis have on the public health of a na tion14. According to this information, the prevalence of wasting found in this work is categorized as critic (≥15%), proportion of patients with underweight is catalogued as high (20-29%), while the percentage of patients with stunting seems to be low (<20%). In contrast, after a comparison with previous data from other Colombian studies, it is likely that nutritional pano rama of those patients may have improved over time.
The percentage of children with underweight that was found more than a decade ago in different Colombian cities (70,4 - 72%)4,5 is high in comparison with the upper limit of the confidence interval found in the present study (27,8%; 95%CI: 7,1 - 48,5). Likewise, a previous proportion of children with stunting of 67% in 20054, is high in comparison with the cu rrently found one (14,3%; 95%CI: 1,3 - 27,2). Nonetheless, a prevalence of wasting found in that same work from Cali4 seems to be similar to the one reported in this research (28,6%; 95%CI: 0 - 62). Several reasons explain this impro vement in the nutritional status. According to the Nutritional Situation National Survey of Colombia (ENSIN, from its Spa nish initials), Between years 2005 to 2015 pediatric chronic malnutrition decreased 5,2% for children under 5 years and 6,5% for children between 5 and 12 years of age15. In the same way, between years 2017 and 2018 there was a para mount 24% increment in ART national coverage for children between 0 and 14 years16. In view of the above, it is plausi ble that malnutrition prevalence in children living with HIV in Bogotá may be decreasing, although further research is required to confirm such assertion.
In the present research there were no clinically significant lon gitudinal changes in anthropometrical markers, which is in contrast with previous findings from published cohort studies that show a sustained improvement of every Z score (WHZ, HAZ, WAZ and BAZ) throughout the first year from start of ART, with a subsequent stabilization of the markers during the following 5 years of follow-up17,18. In this way, the current work is consistent with previous findings because of, except for ART naive patients, 18 out of 21 patients (85,7%) were already re ceiving ART with a therapy duration greater or equal to one year, and therefore they were out of the nutritional benefit window of the first year from ART starting point.
There was no significant variation in the percentage of pa tients with therapeutic failure during follow-up and the proportion was comparable with the one found in two Thai prospective cohorts and a study from Netherlands, notwiths tanding the use of different definitions19,20. However, it is no teworthy that the percentage of patients with undetectable viral load was so low even after the paraclinical time interval had elapsed (35,7% 95%CI: 18-53,5). A meta-analysis of 12 publications, with a total of 1497 patients from developing countries, estimated in 70% (95%CI: 67 - 73) the proportion of patients with undetectable viral load at one year from ART start21. One possible explanation of this contrast is the de tection limit of viral load assay, which is < 20 copies/mL for the present study. If the cut-off point from the above cited studies (< 50 copies/mL) is used instead, the proportion of patients with viral suppression increases to 53,6%. The rea son for the finding of a low proportion of patients with unde tectable viral load could be explored in future local studies.
The fact that the finding of a percentage CD4 increment (ΔCD4) of 5,9% (95%CI: 1,6 - 10) was inferior to the 14% (95%CI: 12 - 16) reported in the Ciaranello, et al meta-analy sis, is explained because of the exclusion of studies with ART experienced patients in the cited meta-analysis, which stand for the majority of the studied population in the present work. Previous research has confirmed a marked increase in CD4 percentage throughout the first year of ART, a change that is much lower and seems to stabilize in subsequent years of therapy continuation19,20; as observed in this work, whose ART experienced population had a median therapy duration of 5,5 years (ICR: 1,6 - 9,1) at HIV program entry.
It is well known that protease inhibitors (PI) increase very low density lipoprotein cholesterol (VLDLc) production in the li ver and decrease peripheral retrieval of triglycerides22. Des pite that in the present work, more than 80% of patients received LPV/r (http://dx.doi.org/10.22354/in.v26i1.1022), It is remarkable that the percentage of patients with hypertriglyceridemia and total hypercholesterolemia is similar to the one found in an observational study, whose children population recei ved treatments without PI in 100% of cases23. It is likely that, because of the use of different cut-offs for diagnosis of dys lipidemia13, It may not be possible to draw valid conclusions from this comparison. In contrast, a previously found preva lence of low HDLc of 3,7% in patients treated with nevirapine (NVP) based regimens24 is much lower than the one found in this study, which was estimated around 50%. Anti-atheroge nic properties attributed to NVP-based regimens lead to an associated increase of HDLc, in comparison with other ART regimens25. After paraclinical follow-up interval had elapsed only LDLc increased significantly, which is explained because of the frequent use of PI; being this pharmacological group an independent predictor of hypercholesterolemia26.
Prevalence of anemia found in the current study seems to be high, but this is related with the chosen analytical cut-off point. If a lower diagnostic cut-off of 10 g/dL is selected and cautious interpretation of confidence intervals is given, then the proportion of anemic children results comparable (8%; 95%CI: 0 - 18,6) with a 22,3%% (95%CI: 18,5 - 26%) found in a meta-analysis of 3524 Ethiopian children with HIV27. In contrast, a high prevalence of anemia between 62,2 and 70% has been reported in African HIV-positive and ART-naive children28,29. The above supports the concept that ART con tributes to improve anemia, as was evidenced in EuroSIDA cohort, that detected a 19,3% decrease in the proportion of anemic patients at one year from ART starting30.
Among limitations of this work, a low number of patients is highlighted; which notwithstanding, does not hinder the hypothesis-generating capacity of a study designed with an exploratory nature. Numerous nutritional, anthropometrical and paraclinical variables were not found in accessed clinical records, which limits the descriptive potential of the whole nutritional panorama. As a retrospective study, the possibi lity of a confusion bias is not excluded, and a multivariable analysis to deal with that systematic error was not conside red appropriate due to a low number of patients. A selection bias is possible, given that all patients belong to a subsided insurance regimen and come from a unique HIV program in the city, which must be kept in mind for generalization of the findings. Finally, an information bias in clinical records is also possible, although paraclinical information was corroborated directly in the clinical laboratory database and a double audit to typed information was done. In the same way, it is stressed that there were no missing data in the principal variables; by which, it is not probable that the conclusions of the current work may be affected because of missing values.
In conclusion, undernutrition is frequent among children li ving with HIV in Bogotá and it is plausible that its prevalence had decreased through time, hand-in-hand with a change in other development indices of the country. Inclusion of pa tients in an HIV program was associated with an improve ment in medical control of the disease, with stable therapeu tic failure rates that are comparable to what has been found in other parts of the globe. However, frequent problems such as anemia and dyslipidemia remain being a therapeutic cha llenge for this group of patients in Colombia.














