Introduction
Around 3.5 billion people around the globe are infected by intestinal parasites, 24% affected by helminths, which places them as one of the most prevalent parasitosis in the world, especially in tropical and subtropical zones1. These infections are denominated as “unattended diseases” due to the impact that social determinants of health have on their occurrence. Furthermore, a prevalence of 20 - 30% intestinal parasites has been established in the Latin-American population, predomi nantly in rural regions with lower socioeconomic classes2-4. In Colombia, prevalence varies according to the geographic region, parasite type, and living conditions. The National In testinal Parasite Survey (NIPS) states that 29,6% children be tween the ages of 7 to years were infected by geohelminths, greater part by Trichuris trichiura (18,4%), Ascaris lumbricoi des (11,3%), and hookworms (6,4%)5.
Orofecal contamination is the most common transmission route. Helminths, which are present in fecal matter, invade the soil and through it, they disseminate. By contrast, pro tozoa transmit through contact with hands, water, and food that has been contaminated with fecal matter6,7. There are multiple risk factors associated with intestinal parasites, most part associated with socioeconomic status, lifestyle, and age8,9. Among these are poverty, contaminated water sources, in adequate residue disposal, lack of footwear, absent aqueduct and sewers systems, deficient food and hand hygiene, as well as inadequate household flooring9-11.
Intestinal parasites can arise due to ingestion of cysts and eggs, or due to larvae’s transdermal penetration. Each patho gen has its own development cycle within the human body, and while some limit their infection to the intestinal tract, some may invade other organs such as the brain and the lungs12-14. However, in general, these parasites’ replication takes place in the digestive system15-16.
Clinical manifestations of intestinal parasites are unspecific and vary from asymptomatic patients to severe intestinal ob struction or perforation, depending on multiple factors such as patient immunity, nutritional status, socioeconomic sta tus, parasite numbers and class, among others15. Abdominal discomfort, nausea, vomiting, and weight loss are amid the more typical symptoms1.
Additionally, geohelminths are capable of causing acute pul monary lesions known as Loeffler syndrome, which is charac terized by abundant expectoration, fever, and eosinophilia, secondary to the bronchial pathway being invaded by lar vae13. Moreover, hookworms can easily be recognized by cu taneous changes inflicted by larvae migration15. Parasite in fections, even in absence of symptoms, have been associated with independent conditions such as anemic syndromes (as with hookworms), physical and psychomotor development Ayapel is located at the eastern side of the department of Córdoba (Colombia), along the shores of a swamp with its same name, at an altitude of 32 meters and with an average yearly temperature of 34 °C and 87% relative humidity. Its re nown for being immersed in a biodiverse zone in regards to fauna and flora, which is now a days threatened by pollution, mercury contamination and deforestation18.
El Cedro, one of Ayapel’s localities, situated at the margin of Ayapel swamp, is around 5 km away from Ayapel’s urban area on water, and 21 km away by land. It has approximately 3,000 inhabitants of which 1,150 live in its urban center, while the rest live in more distant and rural areas. Due to its geo graphic location, social conditions, and lacking infrastructure, El Cedro faces a high risk of flooding which impacts local agriculture and economy and limits accessibility to Ayapel’s town center. On the other hand, El Cedro’s inhabitants have no access to local health facilities, an adequate aqueduct or sewage system, or an established garbage disposal service18.
Due to this precarious situation, identifying the intestinal parasite profile of children younger than 10 years’ old who inhabit this locality and describing their sociodemographic conditions is considered pertinent in order to intervene and follow up both these children and their respective families.
Materials and Methods
Type of study and sampling
A cohort follow-up study took place between 2017-2018. A convenience sampling in children from 1 to 10 years of age resides in El Cedro - Ayapel. Open invitation to participa te in this study was divulged through a community leader to all of the locality’s inhabitants. The cohort was interve ned with education about the control of intestinal parasitosis and received pharmacologic therapy with Albendazole and Secnidazole at the beginning of the follow-up, seven and 12 months after fecal testing (Figure 1).
Participants
Eligible candidates were children from 1 to 10 years of age who lived in El Cedro. The father, mother, or tutor of each minor who participated in the study was required to assist to the community center where the study’s objective was laid out and both assent and consent were obtained. Additio nally, a plastic container was handed to each guardian and image-based instructions were imparted for fecal matter sampling at home.
Data and fecal matter sampling
Sociodemographic data and risk factors were obtained through a primary source survey that was filled out by the child guardian assisted by the study’s investigators, and that was previously validated by the NIPS 2012-20145. Each fe cal matter sample was correlated by name with its respective survey. The samples were transported at 4 °C and sent for processing and analysis at the Instituto Colombiano de Me dicina Tropical (ICMT- Sabaneta, Antioquia).
Interventions: education and pharmacologic therapy
After carrying out the survey and obtaining the initial sam ples, each guardian was provided with Ivermectin (6 mg/mL at a dose of 1 drop per kilogram) for both the minor and his/ her co-inhabitants due to the numerous amount of children concomitantly affected by scabies in the first intervention; afterwards, thorough medical checkups were performed on infants older 1 to 10 years old. Seven months later, phar macologic treatment was provided with Albendazole and Secnidazole. Twelve months after the first samples were taken, the sampling process was replicated, and educatio nal instructions were imparted focused on risk prevention. Additionally, patients had a new medical checkup and anti-parasitic treatment with Albendazole and Secnidazole were once again administered (Figure 1).
Microbiological analysis of water samples
Water samples were collected from three frequent extraction zones of water that was latter used for human consumption: school, community center, and the main water tank. Samples were submitted for microbiological examination by the ICMT.
Statistical analysis of collected data
Data quality control was performed on every survey, and this information was later processed on Microsoft® Excel 2011, initially along with the coprological results to latter be ex ported to IBM® SPSS22 for both univariate and bivariate analysis. For the latter, Chi-square test was performed using a confidence level of 95,0% and a 5,0% margin of error with a p value ≤ 0,05 interpreted as being statistically significant.
Results
In the initial sampling, 47 minors participated, of which 61,7% were male, and the average age was 5,7 years. 51,1% of caretakers had achieved less than an elementary educa tion. 72,3% of families had a monthly salary below $87 do llars (04/04/2017), and 97,9% of homes were classified in the lowest socioeconomic status.
46,8% of survey respondents mentioned the lack of nearby garbage disposals. On a similar note, the most frequented human waste disposal method were toilets connected to septic tanks (78,7%). While 89,4% evidenced insects and ro dents in their home surroundings, around 48,9% mentioned sharing a living environment with pigs, 75,7% of which roa med freely. Additionally, those who were surveyed said that the predominant flooring in the household was soil based in 51,1% of their homes.
Regarding hand hygiene, 91,5% of caretakers allegedly was hed their hands before preparing every meal, and 93,6% af ter going to the bathroom. 93,6% always washes fruits and vegetables, and 24,3% add hypochlorite to water that is used for human consumption.
When asked about the minor’s eating habits, 57,4% of those who responded to the survey mentioned the consumption of pork meat, 12,7% of chicken and poultry, 53,2% of beef, and 10,6% of fish. 38,2% of respondents referred that the minor usually walks around barefoot, and 89,4% constantly play in the ground. 12,7% does not wash its hands after defecating and 6,4% does not wash its hands before each meal.
Concerning gastrointestinal symptoms in the past two weeks before the survey, 27,7% refer diarrhea, 8,5% vomiting, 48,9% abdominal pain, and 34,0% fever. Additionally, half of care takers indicate that on previous occasions, a physician diag nosed the minor with a parasite infection, and of these, only 25% had a coprological exam taken.
86,5% of respondents mentioned previous use of pharma cologic de-worming treatment, and 28,8% referred using it in the last 3 months, Albendazole being the most common pharmaceutical used for this purpose (55,8%) followed by Pyrantel (11,5%). 80,8% of those who responded to the sur vey were affiliated to the subsidized social security system.
The microbiological study of water sources identified the presence of E. coli in all three sites that were sampled; addi tionally, coliforms were isolated from the central water tank while levels greater than 100 colony forming units CFU/dL of mesoaerobic bacteria were found in the central tank and in the school (Table 1).
Table 1 Microbiological water analysis results.
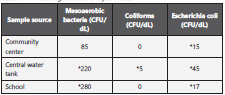
Reference values obtained from resolution 2115 of 2007 (21). (CFU/dL) = Colony forming units by deciliter *Levels above the permitted reference point for safe human consumption
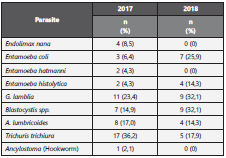
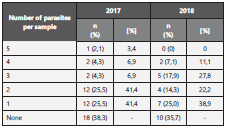
Pertaining to coprological results, in the first collection cycle, 47 samples were obtained, of which 61,7% had at least one type of parasite and 36,2% had two or more. Specifically, among the isolated parasites, Trichuris trichiura eggs were the most frequently present (36,2%) followed up by Giardia lamblia cysts, Ascaris lumbricoides eggs, and Blastocystis spp (Table 2 and 3).
For the second sampling, a year after the first one, 28 of the 47 initial samples were collected. In this occasion, 64,3% had at least one type of parasite and 39,3% had two or more. The most frequently reported parasites were Giardia cysts and Blastocystis spp (32,1%), followed by E. coli cysts in 25% (Ta ble 2 and 3).
14,3% and 10,7% of cases that were positive for G. lamblia and T. trichiura eggs, respectively, in the first collection cycle were also positive in the second sampling. 36,2% of the initial samples and 39,3% of the second samples had more than one type of parasite; additionally, one sample had up to 5 types of different parasites (Table 3).
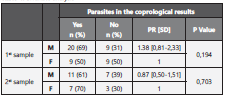
After comparing those that in the first sample referred having received pharmacologic treatment during the last 3 months, and those who didn’t, 75% and 60% respectively had at least one type of parasite. Regarding differences according to gender, in the first cycle 69% of males and 50% of females were infected by at least one type of parasite. On the second cycle, 51,1% of men and 70% of women were infected. There were no statistically significant differences for neither of the se results (Table 4).
Discussion
The pediatric population of El Cedro is in an adverse envi ronment due to negative social determinants of health in the context of a low income and poorly educated population ex posed to a plethora of risks that may deteriorate their health, even more than it may affect other children in the region as reported by the NIPS5,22. This precarious situation and the lack of state presence in the community can partly be explained due to its geographic location at the shores of the Ayapel swamp, which represents an obstacle for reaching Ayapel’s town center and accessing public infrastructure and services, including waste management. This problem is exacerbated by a political setting plagued by corruption and irresponsible use of public finances in the region19-21.
With this survey, most respondents indicate having a low so cioeconomic status with scarce financial resources that can be destined for maintaining the household. Additionally, the re is space lacking for the safe disposal of garbage and waste, and the presence of insects and rodents is a common find. These can act as infectious reservoirs, and their presence in side the living environment is considerably larger than it is in other populations in the region22-24. On a similar note, close contact with pigs is significantly greater, being almost 40% more than reported in the same biogeographic province. This has been previously recognized as a potential risk fac tor for infection by Blastocystis spp., which is considered as a zoonoses of porcine origin, and although its pathogenicity is not clearly established, its presence has been correlated with contaminated water sources25-27 .
Other helminthiasis risk factors have been identified in El Cedro’s inhabitants, such as close contact with soil and in adequate waste disposal. This represents a latent threat for the population due to the dumping of sewage water in the streets, which helps perpetuate the reproductive cycle of di fferent parasites; both for geohelminths with transdermal in vasion upon contact with tainted soil, as with protozoa, due to higher probability of orofecal transmission.
Hand hygiene habits in both the minors and caretakers are purportedly strict as is evidenced in the surveys; however, this may not coincide with what is reported in the coprolo gical analysis, due to the fact that in both sampling cycles, around 60% of children were infected by some type of pa rasite. Additionally, due to the low sensitivity that results from a single coprological exam, these results may be un derestimating actual infection rates28-30. This can be tackled in various manners; the population’s low educational level, lacking sanitary living conditions, and even local traditions may instill a misconception of what adequate hand hygiene habits may be, taking into account that even when more than 90,0% manifested washing their hands frequently, along with the fruits and vegetables they eat, the vast majority of minors had intestinal parasites.
Another finding that must be analyzed are the results from the water samples, which come to show that the main wa ter tank, which acts as the reservoir from which most of the population gets its drinking water, is contaminated by coli forms, which are indicators of microbiological contamination. Furthermore, there were positive Escherichia coli findings in all three sampling sites, which acts as a precise marker for fecal matter contamination19.
On the matter of symptoms presented in the course of the previous 2 weeks before the survey, the prevalence of abdo minal pain, diarrhea, and fevers is alarming, and surpasses the findings of the NIPS5. These symptoms may be caused by the intestinal parasites, and as a result, may lead to prolon ged absentness at school, and in the long term, may have a negative impact in the physical and mental development of the child12,31.
When reviewing the results of the coprological sampling, the prevalence of helminthiasis in the first cycle of sampling is above levels reported both nationally and regionally, which makes El Cedro, a locality with a high risk of intestinal para sites due to it being above 50% of global prevalence. This si tuation, as defined by the WHO, requires strict pharmacologic prophylaxis regimen32; however, results obtained in this stu dy, although limited, help establish an initial conclusion that determines that therapeutic and prophylactic pharmacologic intervention in these types of populations in which risk factors are not impacted head on, has little to no effect in reducing the prevalence of intestinal parasites in a long term33.
The most prevalent geohelminth T. trichiura is followed by A. lumbricoides. This is similar to the region’s prevalence as well as the statistics derived by Medina et al. in a group of children attending a public kitchen in Medellín, Antioquia5,34. This suggest common pathogens in Colombia’s pediatrics population can be used to furthermore optimize prevention and treatment strategies.
In this study, protozoa are isolated in around 60% of samples; Giardia lamblia and Blastocystis spp. are the most prevalent, especially in the second sampling cycle, surpassing levels re ported in the same province and reaching numbers like those reported by Londoño et al. in Calarcá, Quindío in a population of similar size, and in which association was established bet ween close contact with animals without previous deworming and the appearance of intestinal parasites35. This could suggest a potential public health strategy that could reduce morbidity associated with said infection in El Cedro’s population.
Giardia’s presence in samples indicates contaminated wa ter and food sources as the main transmission route20. The studied population presents a very similar prevalence of this specific protozoa as what was established by Giraldo-Gómez et al. who emphasizes Giardia’s role in the development of malnutrition, alongside learning and developmental delays36. As such, in long term, fighting for cleaner water sources and the infrastructure needed for aqueduct and sewers may not only help in reducing this population’s intestinal parasites but in the long run may have a positive impact in its children’s academic and occupational future.
Previous studies have established a significant association between the presence of helminths such as A. lumbricoides and T. trichiura and even among some protozoa; moreover, it has been reported that they may be a synergic associa tion between parasites, and some have described that having a large array of different parasite types may be linked with more numerous isolated parasites in each sample17. Further more, the NIPS established food insecurity, inadequate waste disposal, and nearby garbage dumps as factors associated with multiparasitic infections5. These factors may help iden tify modifiable health determinants and impact on multiple parasitic infections at the same time.
Although results from previous studies have been controver sial in proposing gender as a possible risk factor, this study suggests that both male and female patients have similar results regarding intestinal parasites, lacking a statistically significant difference between the two5,17. As such, gender-based approach to this issue may not be a priority.
Finally, the ample variety of parasites isolated in both occa sions highlights the importance of opting for a pharmaceu tical agent with a wide spectrum, recognizing each individual population’s parasitological profile and considering the pre valence of geohelminths and/or protozoa. Specifically, regar ding El Cedro, this is the first study that is focused on laying out the basis of knowledge about the epidemiological profile regarding intestinal parasites in its pediatric population, while also aiming to identify those risk factors that can be potentially intervened to obtain a more efficient and holistic treatment.
This study’s main limitation was its reduced sample size, which hindered the possibility of carrying out a more detai led statistical analysis. Additionally, being limited to a single coprological exam is not ideal due to its low sensibility, espe cially with S. stercolaris and Ancylostoma (hookworm), which justifies taking serial three sample approach and molecular techniques to identify parasites that may not be visible with light microscopy30.
Findings of this study evidence high prevalence of intestinal parasites with helminths and protozoa which exceeds levels reported on national level, focusing a population exposed to multiple risk factors which have a negative impact in limiting efficiency of educative and pharmacologic interventions. This backs the idea that to have more efficient communitarian in terventions, public health officials and physicians must not only have a therapeutic approach but must also integrate strategies focused on facing the different obstacles regar ding the community’s social determinants of health.