Introduction
The hepatitis E virus (HEV) is currently a public health problem, both in developed and developing countries1. Said virus is considered an emerging zoonotic pathogen and one of the principal causes of acute viral hepatitis throughout the world; with a high risk of developing chronic infection in immunosup pressed patients. However, the global burden of the HEV infec tion has not been evaluated exhaustively2. Since ancient times, registries are available of outbreaks of viral hepatitis globally. In Latin America, it is considered that, due to this health event, high mortality occurred in the pre-Hispanic population, in the territory now known as Central America3.
Although this virus generates a high disease burden globally, in Colombia it is still not deemed a public health problem4; being the most-common cause of acute viral hepatitis in the world, without direct-acting antiviral treatment available. Ac cording to a recent WHO report, 20-million people are infec ted with HEV annually, resulting in 44,000 deaths1.
In 1955 in Delhi, India, an HEV epidemic was reported with approximately 29,000 cases5; thereafter, outbreaks of hydric origin continued to be reported and cases in great num bers were reported as non-A and non-B, which is why this disease was denominated as enteric non-A non-B hepatitis (ENANBH). Later, by late 1978, another epidemic outbreak was again reported also of hydric origin in the Kashmir va lley with 52,000 cases and 1,700 deaths; the symptomatology was similar to that of hepatitis A and negative for hepatitis A and hepatitis B, confirming them as ENANBH6. In 1981, a Soviet military camp in Afghanistan had a hepatitis outbreak; for its study, a volunteer ingested a concentrate of feces sam ples from infected soldiers, causing him acute hepatitis. The serum from the volunteer was negative for the hepatitis A vi rus (HAV) and hepatitis B virus (HBV), which suggested a new pathogen responsible for this infection7. This article aims to offer a review of the topic on the hepatitis E virus, from the last six years, to describe current general aspects of the he patitis E virus, its genome, currently identified transmission routes, and thus contribute to its visibility, for its prevention and control.
Hepatitis E virus
The HEV is formed by an icosahedral particle without en velope of about 32 nm; withstands the acidic and alkaline conditions of the intestinal tract, facilitating the fecal-oral transmission path.
The HEV is a positive-strand, class IV RNA virus belonging to the genus Hepevirus, only member of the family Hepeviridae8.
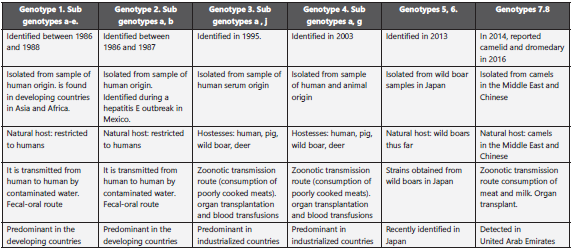
This family is made up of the genera: Orthohepevirus that in fects terrestrial vertebrates and Piscihepevirus, which infects fish. The Orthohepevirus is comprised of four species, A - D, with different host types. The genus Orthohepevirus A has the HEV variants that infect humans. China, between 1986 and 1988, had a great epidemic affecting 120,000 people and the genotype identified was described as genotype 1, which can be found in Asia, Africa, and South America. In Mexico, during an outbreak between 1986 and 1987, genotype 2 was detected, although it can also be found in Nigeria and Chad. These two genotypes are related and restricted to humans; their transmission is related with deficient sanitation and contaminated water, only in humans9,10.
Genotypes 3 and 4 were identified in 1995 and 2003, respec tively. The HEV genotype 3 is disseminated globally and is prevalent in industrialized countries. The HEV genotype 4 is limited to east Asia, including Japan, China, and South Korea; its reservoir includes pigs, boars, deer, and rabbits, it circula tes among humans, pigs, rabbits, deer, and mongooses; VHE4, identified in humans and pigs8,9. In many parts of China, the HEV genotype 4 (HEV-4) has emerged as the most-common genotype that causes acute hepatitis. Recently, it was shown to cause persistent infections in transplant receptors11,12,13.
The HEV5 and HEV6 represent strains identified in boars from Japan, where unique nucleotide sequences were found pre viously designated as HEV of genotype 5 and genotype 6; however, it is important to bear in mind that not all boar HEV isolates belong to genotypes 5 and 6; most of the HEV isolates from boar are classified as genotypes 3 and 4 with zoonotic capacity14 (Table 1).
In 2014, HEV was reported in camelids and dromedaries from Dubai and then, in 2016, in Bactrian camels from Xinjiang (China); in the genomes of samples obtained from these camels, nucleotide differences were found > 20% compa red with the complete HEV genomic sequences available. For this reason, two new genotypes were proposed: HEV-7 for dromedary camel (DcHEV) and HEV-8 for Bactrian camel (BcHEV). A case of chronic HEV-7 infection in a liver trans plant receptor, frequent consumer of camel products, indica tes the zoonotic potential of these new genotype15.
Genomic organization
The HEV has a diameter from 27 to 34 nm and a genome consisting of three open reading frames (ORF). The 5′ non-translated region (NTR) (27 nucleotides) is covered with a 7-methylguanine and is followed by ORF1, which encodes the non-structural proteins (NS) necessary for replication. The ORF2 encodes the central protein of the viral capsid, whi le ORF3 partially overlaps ORF1 and encodes a protein like viroporin. This virus’ genome hosts a 3′ NTR (65 nucleotides) that ends with una poly (A) tail (Figure 1.) (16,17.
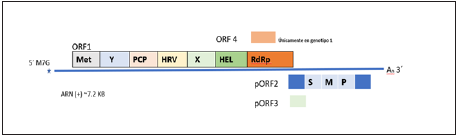
Hepatitis E virus (HEV) is a positive polarity single stranded RNA of approximately 7.2kb; with consisting of three open reading frames: ORF1, ORF2 ORF3; once it has entered the cell and the viral capsid is eliminated, it is translated by the host’s ribosomes in order to produce the poly protein ORF1, which forms the non-structural region of the virus and allows its replication. Only in HEV genotype 1 is it associated with ORF1 ORF4, which is believed to enhance the activity of RdRp, which is a non-structural protein that is part of ORF1, when translated into a protein. This is initially transcribed to a negative polarity strand as a template for the production of new virions and also transcribes into a shorter subgenomic ARN that contains ORF2 and ORF3. ORF2 is translated into capsid protein (pORF2) and ORF3 protein (pORF3), a viroporin, which is required for virion exit (17) .
Figure 1. Genomic structure of the hepatitis E virus. (Modified from Nimgaonkar et al., 20186
These reading frames have different sizes and functions; ORF1 is the largest viral gene, encodes for a non-structural polyprotein of 1,690 amino acids that permit viral replication of the genome. It encodes the non-structural proteins of the virus, including the RNA-dependent RNA polymerase (RdRp), RNA helicase, and methyltransferase, it contains other doma ins with little characterization, such as the “X” domain and the “Y” domain, the hypervariable region (HVR) and a cysteine protease like papain (PCP) (6.
The ORF2 encodes the preORF2 structural subunit and co rresponds to the viral capsid protein, being the virion’s main structural component; this protein is highly immunogenic. The production of the currently available vaccine has focused on this protein and uses the ORF2 gene from a genotype 1 strain18. The ORF2 has 1,983 nucleotides, starts at nucleotide 37 downstream of the ORF1 stop Codon and overlaps on the nucleotides of ORF319. Recently, it was demonstrated that the HEV produces three different forms of ORF2: the first form is described as ORF2i, component of infectious particles; the second is secreted ORF2g (ORF2 glycosylated); and the third is ORF2c (ORF2 split off) that is not associated with infectious particles but found as antigen in serum of infected patients20.
The ORF3 is the smallest of the entire genome, with functions yet unknown; it is translated from a sub-genomic RNA that makes up a protein of approximately 115 amino acids. It has been found in viral particles present in serum from patients and in cell cultures; it is necessary for the release of the viral particles21,17.
The ORF4 was recently identified as a new reading frame that is fully embedded within ORF1, exclusively in genotype-1 strains. The transitory expression of ORF4 produces a protein of molecular weight of 20 kDa; this ORF4 protein interacts with the RdRp, helicase and X viral proteins, stimulating viral RdRp activity and reinforcing viral replication22,19.
The HEV was denominated “quasi-enveloped” in 2016, found in enveloped and not enveloped forms. It is eliminated in fe ces as a non-enveloped virus, but HEV taken to cell culture has a lipid cover. This form of appearing “quasi-enveloped” with lipid cover, in the blood circulation, confers it protec tion against neutralizing antibodies against the ORF2 pro tein (capsid protein) and the ORF3 protein, which allows it to evade the humoral immune response6. Although both vi ral forms are infectious, the non-enveloped virus is 10 times more infectious than the quasi-enveloped form16.
HEV replication
Systems to cultivate HEV in vitro have been developed only recently, and the viral replication mechanism continues being hypothetical. Analysis of the genome has been performed and, through analogies with other known viruses, the HEV replication cycle has been proposed23.
On the HEV’s mechanism of entry to the cell, little is known and the receptor is still unknown, but it has been shown that host factors are involved in the entry into the cell of naked HEVs, such as heparan sulfate proteoglycan, which partici pate in the cellular binding of many enveloped and not en veloped viruses and, in this case, to human hepatoma cells; GRP78, also known as binding immunoglobulin protein (BiP) molecular chaperone in the endoplasmic reticulum also in volved in the entry of the virus into the cell. Asialoglycopro tein (ASGP) are galactose receptors found principally on the surface of hepatocytes and ATP5B (5β subunit of ATP syntha se) that, although it is a mitochondrial protein, a fraction is expressed on the cell surface and is implicated in viral infec tions 24. Binding to the cell surface and entry to the host cell are the initial and basic moments in the cycle of viral infec tion. The expression of specific membrane components that allow viral attachment to susceptible host cells is determined by viral tropism in the target cell25.
The hepatitis E virus can present itself as a non-enveloped virus, where the capsid cover interacts with the surrounding environment, or as a quasi-enveloped virus (eHEV), where the capsid is coated with an exosome membrane; both forms are infectious, however, it is considered that the non-enve loped virus is a much more infectious form than the quasi-enveloped presentation26.
The HEV and eHEV use different mechanisms to enter the cell. The entry of eHEV is believed to depend on the degra dation of its membrane in the lysosome27.
For eHEV, the virus enters the cell through clathrin-depen dent and dynamin-dependent receptor-mediated endocyto sis, a GTPase that mediates membrane fission during endo cytosis and enables degradation of lysosomes coat lipids6,28. After endocytosis, the viral genome is released in the cyto plasm and the positive sense HEV RNA is translated by host factors into the ORF1 polyprotein from ORF1, which contains the RNA-dependent RNA polymerase (RdRp); the RdRp then transcribes full-length negative sense complementary viral RNA, which serves as a template for full transcription of po sitive sense RNA and a 2.2-kb subgenomic RNA29. When this sub genomic RNA is translated, the ORF2 and ORF3 proteins are produced. Thereafter, upon continuing, the cycle produ ces the viral assembly and the release of the new virions. Du ring this process, the HEV virion can be released as a “quasi-enveloped” virion (eHEV) in exosome membranes toward the blood stream or pass with its lipid cover through the bile duct, where it degrades and is released in its “naked” form in the stool. When the released eHEV enters the blood stream, its quasi-enveloped form protects it neutralizing antibodies against pORF2 and pORF3 (Figure 2).
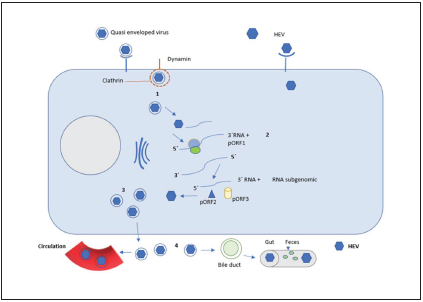
The non-enveloped virus has a more efficient replication and a mechanism of entry to the cell different from that of the enveloped virus6; for the quasi-enveloped virus, the replication cycle is described in the following manner: 1: the cycle starts with entry of the viral particle onto the cell through endocytosis by using a clathrin- and dynamin-dependent receptor; upon entering the cell, the lipid cover is degraded by lysosomes and leaves mRNA exposed, the polyprotein ORF1 (pORF1) is translated into a single RNA of positive polarity and then transcribed into an RNA of negative polarity with its full length; 2: negative-polarity RNA allows positive-polarity RNA to be transcribed to generate virions and a subgenomic, shorter RNA containing the capsid protein ORF2 (pORF2) and the protein ORF3 (pORF3) a viroporin essential for viral release. 3. Subsequently, there is the assembly of the capsid and covering of the virion with the lipid membrane (possibly derived from the trans-Golgi network bound for the cell surface). 4: viruses are released and take two routes, one goes into the bloodstream as eHEV, where the envelope protects it from being neutralized by antibodies to pORF2 and pORF3, it is also considered that this form is less efficient to infect the cell; the other route is to enter the bile duct, where the lipid envelope is degraded and released in the feces.
Figure 2 Schematic representation of the HEV replication
Extrahepatic replication
The target cell of HEV par excellence is the hepatocyte; however, extrahepatic replication of the virus has been de monstrated. Among the extrahepatic manifestations asso ciated with the infection due to HEV, there are neurological manifestations, like the Guillain-Barré syndrome, neuralgic amyotrophy, encephalitis, myelitis, myositis, vestibular neu ritis, peripheral neuropathy, Bell’s palsy and multiple mono neuritis, aplastic anemia, acute thyroiditis glomerulonephritis at the renal level, manifesting as proliferative membranous glomerulonephritis and cryoglobulinemia; anemia and acute pancreatitis; however, the pathogenesis of these events is unknown and further studies are necessary to permit defi ning these associations30.
Clinical aspects of the hepatitis E infection
Outbreaks of hepatitis E are a serious public health problem in developing countries. The disease causes acute infections, principally in young adults. The mortality rate is approxima tely 2%; however, it can exceed 20% in pregnant women in some regions of India31.
The HEV infection is usually asymptomatic, jaundice occurs in 5% to 30% of infected patients 26. The prodromal phase with its nonspecific symptomatology, which includes fever, nau sea, vomits, and anorexia, can last up to one week. Symptoms tend to resolve spontaneously after a few days to a week. However, in the presentation of outbreaks, the mortality rate varies from 0.5% to 4.0%32.
Despite starting as an asymptomatic event most of the times, there are reports of progression of the infection to the chronic form with hepatic damage and cirrhosis, especially in immuno suppressed patients, solid organ transplant receptors, carriers of the human immunodeficiency virus (HIV) and people with hematologic malignancies33. During gestation, studies have demonstrated that especially during the second and third tri mesters, there is greater affectation; the third trimester is the stage during which 30% of maternal mortality occurs. The HEV infection during pregnancy is also associated with high inciden ce of premature births and vertical transmission, as well as other complications, like disseminated intravascular coagulation34.
Epidemiological behavior
Infection due to the hepatitis E virus (HEV) is a cause of viral hepatitis of global distribution; it is hyperendemic in tropi cal regions, causing outbreaks in these countries, especially after great floods or in refugee camps. It is estimated that the number of symptomatic infections due to HEV in tropical countries is above 3 million per year35. The global burden of hepatitis E reports approximately 20-million cases annually of which 3.3-million cases are symptomatic and 60,000 deaths are attributed to HEV genotypes 1 and 26.
The first well-documented hepatitis E epidemic took place in India between 1955 and 1956, affecting 29,000 people. It was determined that the cause was due to the contamination of water sources. The epidemiological patterns vary according to the region where hepatitis E is endemic if compared with those that are not; in endemic areas, epidemics are more fre quent. This behavior has been observed in China, India, Asia, the Middle East, and Africa36.
It has been found that genotypes 1 and 2 are the principal causes of epidemic acute hepatitis and endemic in develo ping countries. Under poor hygienic conditions, these geno types are transmitted among humans via fecal-oral means and via contaminated water. However, in developed coun tries, genotypes 3 and 4 are of zoonotic origin, with trans mission through foods and through contact with the infected animal. It is possible for transmission through blood transfu sions and organ transplants37. It is a particularly severe disea se when it occurs during pregnancy38 (Table 2).
Table 2 Epidemiological behavior of the hepatitis E virus
| Country | Date | Genotype | Source of infection | Transmission path | Population at risk | Behavior |
|---|---|---|---|---|---|---|
| India | 1955 - 1956 The first well-documented hepatitis E epidemic. | 1 Asian strains | Contamination of water sources. poor hygienic conditions | Transmitted between humans fecal-oral path | Humans. Responsible for severe hepatitis in pregnant patients and infants 39 | Are the principal causes of epidemic acute hepatitis and endemic in developing countries. Epidemic China, India, Asia, Oriente Medio y África. Are found exclusively in humans. Considered endemic in some regions of Asia and Africa; was also detected in Cuba and Venezuela. Uruguay autochthonous HEV. |
| Mexico | 1980s, Outbreak in the where HEV-Gt2 was Identified 40 | 2 A single Mexican strain | Contamination of water sources. poor hygienic conditions | Humans Responsible for severe hepatitis in pregnant patients and infants | Are found exclusively in humans. Mexico and Africa | |
| USA | 1997. From the first known case of HEV in an individual who had not recently travelled outside the continental US 41 | 3 (HEV US-1) | Zoonotic origin, have been found in humans and animals such as pigs, boars, and deer | Transmission through foods and through contact with the infected animal. It is possible for transmission through blood transfusions and organ transplants | older people, immunocompromised individuals, patients with chronic liver diseases, workers contact with HEV-infected animals | Is the most prevalent genotype in Europe. Sporadic cases in industrialized countries. |
| China | 1999 Human HEV strain obtained from Chinese HEV patients 42. | 4 | Zoonotic origin, have been found in humans and animals such as pigs, boars, and deer | older people, immunocompromised individuals, patients with chronic liver diseases, workers contact with HEV-infected animals | Sporadic cases in China, Japan, and Taiwan | |
| Japan | 2014 Japan43 | 5-6 | Wild boars | Possibility of zoonotic infection | humans | the pathogenicity, epidemiology, remain unclear |
| Dubai | 2014, reported dromedary 44 | 7 | Dromedary camel | consumption of camel products | humans | unknown |
| Xinjiang (China) | 2016 reported Bactrian camels45 | 8 | Bactrian camel | consumption of camel products | humans | epidemiology, zoonotic potential, and pathogenicity of the virus were unknown |
Immune response
The immune response during acute hepatitis has been stu died in both infected volunteers and non-human primates; findings suggest an incubation period of approximately 4 to 6 weeks since the infection until the onset of symptoms. Vi remia during infection due to HEV persist for approximately one month after the symptoms appear in healthy individuals; viral RNA in feces has been detected in studies with patients since the first week after the start of the disease up to 28 days later and in serum from the same patients, since the first until the sixth week. Immunoglobulin IgM anti-HEV was detected on the first week after the symptoms appeared and diminis hed during the following six weeks46.
Seroconversion against the hepatitis E virus was demonstra ted by a volunteer who ingested a feces mixture contamina ted with ENANBH; said volunteer developed antibodies du ring the early phase of the disease and using his serum it was possible to visualize spherical virus particles of approximately 27 to 30 nm in fecal samples. With these same particles, the same particles were visualized in samples of cases documen ted in Asia, Africa, and North America, leaving evidence that the HEV in the different regions of the world were related47.
The mechanism of HEV immunopathogenesis remains diffi cult to identify. The innate immune response forms the first line of defense against viral infections, including HEV. The retinoic acid inducible gene I (RIG-I) detects pathogen-as sociated motif patterns (PAMP) in viral RNA to induce inna te antiviral immune responses. It has been shown that the RIG-I path plays an important role during the HEV infection. However, the RNA-HEV motifs recognized by RIG-I are still unknown48.
Type I interferon (IFN) is also in the first line of defense and once the cell is infected, the virus genome is recognized by pathogen recognition receptors and induces activation of in tracellular signaling cascades. It has been found that some of the genes encoded for HEV have the capacity to interrupt the signaling cascades for antiviral immune responses and do not permit production of cytokines / chemokines. Currently, the HEV evasion mechanisms are being studied49.
Pathogenesis of hepatitis E
The hepatitis E virus is an important human pathogen that causes acute and chronic infection. Currently, the replication and pathogenesis mechanisms are not well known50.
It is still not clear how through fecal-oral transmission, the virus particles reach the liver. Recent research has indicated that, in primary cultures of intestinal cells, RNA-HEV and ORF2 antigen were detected in the intestinal crypts of a pa tient with chronic infection. This information suggests that, upon replicating in the intestinal tract, it circulates in quasi-enveloped form; reaches the liver through blood circulation and there it replicates in hepatocytes, the virus is released and through circulation it returns as a quasi-enveloped virus or without the lipid cover that is eliminated by the bile salts and, thus, is liberated in the feces. Bearing in mind that the HEV does not produce cytopathic effect, liver damage may be due to cytotoxic T lymphocytes and natural killer cells51.
In immunocompetent individuals, most infections due to HEV take place asymptomatically; it is unknown how many of tho se exposed have seroconversion to anti-HEV and how many of those infected do not have clinical signs of HEV infection. Chronic infection due to hepatitis E may, in the long term, produce acute liver failure in some patients or chronic liver failure in patients with underlying liver disease. Prior studies have demonstrated that infections due to HEV increase the risk of death up to 70% in cases of a previous liver disease. The typical symptomatology of acute liver failure over chro nic hepatitis, lead to acute deterioration of liver function with clinical complications, like ascites, hepatic encephalopathy and/or hepatic coagulopathy52.
In experimental works, the RNA-HEV has been detected in feces one week before the onset of symptoms and up to two weeks after; in serum of almost all patients, it was possible to detect it two weeks after the start of the disease and continue with posi tivity from 4 to 16 weeks. In experimental processes, at hepatic level, in experiments, HEV antigens - indicative of viral replica tion - can be visualized after seven days of infection. The RNA-HEV levels in serum and feces are quite high from the beginning of the infection and fall sharply at the end of it; simultaneously when a high antibody response is given to the vigorous anti extremely high rate of mortality due to fulminant liver failure in pregnant women (20% - 30%) are unknown17.
The HEV infection in immunosuppressed individuals may take place in chronic manner and lead to potentially deadly cirrhosis. In solid organ transplant receptors, the infection may also occur in chronic manner, especially for liver trans plant receptors. A differential diagnosis to consider in these patients with elevated transaminases and which present re jection, must include hepatitis E51.
The parenteral route in HEV infection, through blood transfu sion, constitutes an important form of HEV transmission. The strains related with hepatitis E in these cases are those from ge notypes 3 and 4; this post-transfusion risk must be considered17.
Vaccine
Hepatitis E is increasingly recognized globally as an infection that contributes as global disease burden, but it is underes timated. The subpopulations associated with severe diseases and death include pregnant women, patients with basic liver diseases, and the elderly52.
Prevention of HEV infection through vaccination is based on the capsid protein, given that it is highly immunogenic and elicits effective neutralizing antibodies53.
Hecolin® is currently the only vaccine authorized for the preven tion of hepatitis E; it was authorized in China and launched in 2012. This vaccine is developed by Xiamen Innovax Biotech Co., Ltd.; however, many obstacles exist for its application54,55.
The World Health Organization (WHO) has drafted a first do cument indicating its position on the vaccine against hepati tis E, focused principally on the availability of evidence about the Hecolin® vaccine, the only vaccine against hepatitis E that is currently authorized. The vaccine, which is currently only licensed for use in China in people from 16 to 65 years of age, with high risk of HEV infection, according to occupa tion or lifestyle, protects against symptomatic infection due to HEV, with an extremely high rate of efficacy against the HEV genotype 4. The WHO considers that data on the pro tection against genotype 1 are limited and against genotypes 2 and 3 are not available. However, scant information on the vaccine’s behavior globally leads the WHO to avoid recom mending its introduction for routine use in national vacci nation programs, although leaving it to the consideration of local authorities regarding the possibility of deciding to use the vaccine based on the local epidemiology56.
Conclusions
In summary, Infection by the hepatitis E virus is a serious pu blic health problem in many developing countries, especially in those where there are displaced groups, because the rou tes of transmission between humans are the contamination of water sources and water sources. poor hygienic conditions. It is also an important health problem for pregnant women due to specific strains and for those immunosuppressed or organ transplant recipients. Little is known about its viral cycle and its cell receptors; Greater understanding is needed on the ac tivity and role of ORF1, ORF2, ORF3, and ORF4 polyproteins relative to genotype 1, which will allow more information for vaccine development. Finally, it is to be recognized that more information is needed on the clinical behavior of the disease, and epidemiological data that help to understand the trans missibility of the virus and the relationship with its hosts.