Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista de Salud Pública
versión impresa ISSN 0124-0064
Rev. salud pública v.13 n.5 Bogotá sep./oct. 2011
Nasal carriage of Panton Valentine leukocidin-positive methicillin-resistant Staphylococcus aureus in healthy preschool children
Colonización nasal de Staphylococcus aureus Meticilino Resistente portador de la Leucocidina Panton-Valentine en preescolares
Juan Rebollo-Pérez1, Cindy Ordoñez-Tapia2, Carmen Herazo-Herazo2 and Niradiz Reyes-Ramos1
1Facultad de Medicina, Campus de Zaragocilla, Universidad de Cartagena, Cartagena, Colombia jeanrebollo@gmail.com,nreyesr@unicartagena.edu.co
2Universidad de Sucre, Sincelejo, Colombia. pao_2387@yahoo.es,c_herazo22@hotmail.com
Received 14th January 2011/Sent for Modification 6th August 2011/Accepted 23th September 2011
ABSTRACT
Objective Determining the prevalence of nasal carriage of S. aureus, both sensitive to methicillin and resistant to it, in preschool children and evaluating the presence of Panton-Valentine leukocidin genes in the isolates.
Methods This was a cross-sectional study in which cultures from anterior nares were obtained from healthy preschool children. Isolates were identified as S. aureus based on morphological and biochemical tests. Antibiotic susceptibility profiles were determined by the disk diffusion method. All the isolates were further analyzed by multiplex PCR to determine the presence of mecA and PVL genes; methicillin-resistant isolates were also SCCmec typed by multiplex PCR.
Results Overall S. aureus nasal colonization prevalence was 38.5 % and 4.8 % for methicillin-resistant strains. All the methicillin-resistant isolates carried the genes for PVL; two isolates possessed the SCCmec type IV, two were SCCmec type I and one was SCCmec type II. Conclusion This study revealed high PVL-positive, methicillin-resistant S. aureus colonization prevalence in healthy preschool children from Cartagena, which may play a key role in the epidemiology of community-associated infection by methicillin-resistant S. aureus in healthy children from this particular geographical area.
Key words: Nasal carriage, Staphylococcus aureus, methicillin resistance, healthy children (source: MeSH, NLM).
RESUMEN
Objetivo Determinar la prevalencia de colonización nasal de S. aureus, tanto sensible como resistente a meticilina, en niños preescolares y evaluar la presencia de los genes de la leucocidina Panton-Valentine en estos aislamientos.
Métodos Estudio de corte transversal en el que se realizaron cultivos de flora nasal de niños preescolares. Los aislamientos fueron identificados como S. aureus con base en su morfología y pruebas bioquímicas. La susceptibilidad a antibióticos se determinó por el método de difusión en disco. Todos los aislamientos fueron analizados por PCR múltiple para determinar la presencia de los genes mecA y PVL, y para la tipificación del casete cromosómico SCCmec de los aislamientos resistentes a meticilina.
Resultados La colonización nasal por S. aureus fue 38,5 %, y la de cepas meticilino-resistentes fue 4,8 %. Todos los aislamientos SARM portaban los genes para PVL, dos portaban el elemento SCCmec tipo IV, dos fueron tipo I y uno fue tipo II.
Conclusión Encontramos una alta prevalencia de colonización por cepas meticilino-resistentes, PVL-positivos en la población estudiada, lo que podría jugar un papel clave en la epidemiología de las infecciones por S.aureus meticilino-resistente en esta área geográfica.
Palabras Clave: Colonización nasal, Staphylococcus aureus, resistencia a meticilina, niños sanos (fuente: DeCS, BIREME).
Staphylococcus aureus is a bacterium that behaves both as human commensal and as a pathogen frequently causing clinically important infections ranging from skin abscesses to life-threatening infections such as bacteremia and pneumonia (1,2). The anterior nares represent the most common site for staphylococcal colonization (3,4), longitudinal studies having shown that about 30 % of individuals are S. aureus nasal carriers (5). Nasal carriage of this bacterium has been identified as a risk factor for developing community-acquired and nosocomial infections, more than 80 % of isolates originating from the nose; therefore, it has been widely accepted that the bacterium plays a key role in the infection's pathogenesis and epidemiology (6,7).
Methicillin-resistant Staphylococcus aureus (MRSA) has emerged as an important pathogen since 1969; it was first considered to be confined to hospitals and medical centres and nowadays as being presented in community settings. Community-acquired MRSA (CA-MRSA) strains have been identified as being highly clonal and virulent, responsible for around 30 % of S. aureus infections (8) and are probably the most important challenge to routine clinical practice regading managing infectious diseases. Although the nasal carriage of MRSA has been reported to have a low frequency in healthy pediatric populations (0.2 % - 2.2 %), several recent reports have documented increasing MRSA nasal colonization prevalence in healthy children (9-11). A cross-sectional study was carried out in preschool institutions to ascertain the prevalence of nasal carriage of S. aureus and MRSA in preschool children and to evaluate the possible risk factors involved in such carriage. A high PVL-positive MRSA colonization prevalence was found in healthy preschool children in the study population.
MATERIALS AND METHODS
Study design and population
This cross-sectional study was conducted from June to November, 2009. Anterior nares from 104 children aged 2 to 6 years were sampled. Written questionnaires regarding their demographics and medical history (antibiotic use and the presence of respiratory infection, skin infection or allergies) were completed by the children's parents; these parents gave their written consent. This study was approved by the University of Cartagena's Ethics Committee.
Specimen collection and microbiological methods
Nasal samples were obtained with a sterile cotton swab and placed in modified Stuart transport medium (OXOID, England); they were then transported to our microbiology lab and processed within 4 hours. S. aureus isolates were identified by previously described methods (12). Antibiotic susceptibility was assessed by the disc diffusion method following Clinical Laboratory Standards Institute (CLSI) guidelines (13). The antibiotics tested were: cefoxytin, erythromycin, clindamycin, gentamycine, vancomycin and rifampin; the double disk diffusion test (D test) was performed in the same plate by placing the clindamycin and erythromycin disks 15 mm apart.
DNA extraction
Genomic DNA from all isolates was extracted using the following protocol. Around 3-5 colonies were suspended and washed with Tris 0.5 M and then homogenized in TE buffer (10 mM Tris, 1 mM EDTA), heated at 100 ºC for one hour and immediately frozen at -35 ºC for 20 minutes, thawed at 65 °C and finally centrifuged at 13,000 rpm for 15 minutes. The supernatant containing the bacterial DNA was deposited in a fresh tube and stored at -20 ºC for further analysis.
Detecting mecA, nuc and LuK-PV genes by multiplex PCR
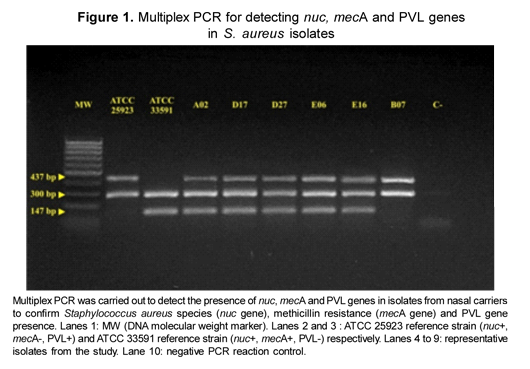
Multiplex PCR of all the S. aureus isolates was carried out using three sets of oligonucleotides: MecA1F - MecA2R (14) which amplifies a 147 bp fragment from the mecA gene, Nuc1F - Nuc2R (15) which amplifies an S. aureus-specific fragment of about 300 bp from the nuc gene and LukPV1F - LukPV2R (16) which amplifies a 437 bp fragment from the lukS/lukF-PV genes.
DNA was amplified in a 25µL reaction volume containing 12.5 µL PCR mix (PCR Master Mix, Promega), 0.2 µM of each primer and 5 µL of template DNA. PCR reactions were carried out in a Perkin-Elmer thermocycler in the following conditions: an initial denaturing cycle at 94°C for 5 min, followed by 30 cycles of 94°C for 1 min, 50 °C for 1 min and 72 °C for 2 min, with a final 10 min extension step at 72 °C.
The S. aureus reference strains ATCC 33591 (mecA positive; nuc positive, PVL negative) and ATCC 25923 (mecA negative; nuc positive, PVL positive) were used as control strains. DNA template was replaced by ultrapure water in negative PCR reaction controls. All PCR products were analyzed on 2 % agarose gel and visualized with ethidium bromide staining using a UV light transilluminator.
SCCmec typing by multiplex PCR
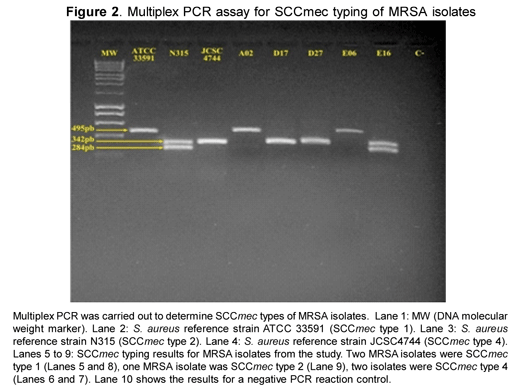
All MRSA isolates were SCCmec typed using a multiplex PCR strategy, according to previously described protocols (17) which included 3 sets of primers amplifying different sized fragments for each SCCmec type.
Statistical analysis
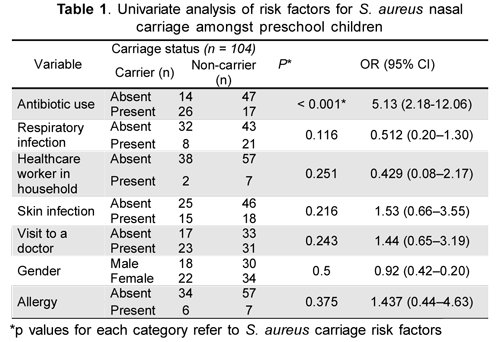
Microsoft Office Excel was used for recording data and exported to SPSS statistical software (version 18.0) which was used for final data analysis. Univariate analysis was applied to determine colonization association with potential risk factors using Fisher's exact test. A < 0.05 p value was defined as being statistically significant.
RESULTS
A total of 104 preschool children from three different preschool institutions, ages ranging from 2 to 6 years, participated in the study. Participating children's average age was 4.01 years (SD: 1.15) and 48 of them (46.2 %) were male.
S. aureus nasal colonization prevalence was 38.5 % and 5 children (4.8 %) were colonized with MRSA strains. MRSA isolates' frequency amongst all S. aureus isolates was 12.5 %. PVL genes were present in all the MRSA strains, while just one MSSA strain had the PVL genes (Figure 1), for an overall 5.8 % PVL-positive S. aureus prevalence. SCCmec typing showed that two isolates carried SCCmec type 4, two carried SCCmec type 1 and one carried SCCmec type 2 (Figure 2).
No association was found between S. aureus nasal carriage and gender, ethnicity, visit to a doctor, household contact with hospital staff, having a skin or respiratory infection and household number. However, there was an association between S. aureus nasal carriage and antibiotic use during the past 3 months (5.13odds ratio, 2.18- 12.06 confidence interval, p = 0.00011) (Table 1).
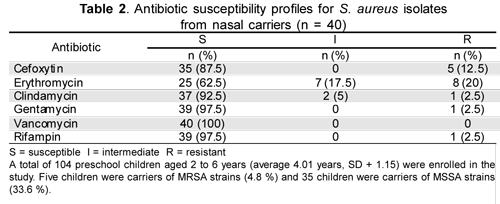
Antibiotic susceptibility tests indicated that 20 % of the isolates were erythromycin-resistant, one isolate (2.5 %) was clindamycin resistant and the D test showed no inducible clindamycin resistance. All the isolates were sensitive to vancomycin and five isolates were resistant to 2 or more antibiotics. Table 2 shows the isolates' antibiotic sensitivity pattern.
A total of 104 preschool children aged 2 to 6 years (average 4.01 years, SD + 1.15) were enrolled in the study. Five children were carriers of MRSA strains (4.8 %) and 35 children were carriers of MSSA strains (33.6 %).
DISCUSSION
S. aureus is an important pathogen associated with nosocomial and community-acquired infection. Several reports have documented an increase in infections caused by methicillin-resistant S. aureus, mostly affecting children in several geographic regions. Nasal colonization by S. aureus has been considered an important risk factor for infections that could threaten a carrier's life.
High MSSA and PVL-positive MRSA nasal carriage rates were found (38.5 % and 4.8 %, respectively) which may represent a risk for colonized children and the rest of the community, taking into account that these types of strain are implicated in invasive S. aureus infections (18,19). Because S. aureus is spread through contact, school institutions, where children are in close contact with each other, may play a role in these strains' transmission and spread in the community and preschool children may be an important reservoir and source for the transmission of strains causing skin and soft tissue infections.
The MRSA strains found in this study carried the three SCCmec types, revealing the diversity of MRSA strains dispersed in our community. Although CA-MRSA strains have been reported as mainly carrying SCCmec type 4, two isolates were also found carrying the type 1 SCCmec and one carrying the type 2 SCCmec which have been previously associated with nosocomial settings (20); this may represent evidence of S. aureus strains' changing epidemiology and distribution.
Previous studies have suggested that environmental factors like prior antibiotic use, contact with a healthcare facility, poor socio-economic conditions and overcrowding are involved in the increase of CA-MRSA nasal carriage (21,22). However, an association was only found between prior antibiotic use and carriage state; there was no association between contact with a healthcare facility and MRSA carriage in this study, according to statistical analysis.
There was a high erythromycin resistance rate in this study and five isolates were resistant to more than 2 antibiotics; three of them were MRSA strains. This is an important finding because multi-resistant strains which are now dispersed in the community represent a challenge to routine clinical practice in the management of infectious diseases. Our results support the view that MRSA strains may spread from a hospital setting to the community, because three of the 5 MRSA isolates found in this study had features pertinent to nosocomial strains, such as SCCmec types 1 and 2 along with multi-drug resistance. Therefore, studies addressed at tracking S. aureus strains' epidemiology and distribution are needed and may serve as the basis for designing strategies for controlling their spread and dissemination from (or to) a hospital or community setting.
Acknowledgments: The Universidad de Cartagena financed Niradiz Reyes PhD, the main investigator. The authors wish to thank the three preschool institutions which participated in the study (H.I.C Skinner II, H.I.C El Labrador and H.I.C España).
Conflicts of interest: The authors declare that they do not have any conflict of interest related to this study.
REFERENCES
1. Miller LG, Perdreau-Remington F, Rieg G, Mehdi S, Perlroth J, Bayer AS, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005; 352(14): 1445-53. [ Links ]
2. Mongkolrattanothai K, Boyle S, Kahana MD, Daum RS. Severe Staphylococcus aureus infections caused by clonally related community-acquired methicillin-susceptible and methicillin-resistant isolates. Clin Infect Dis. 2003; 37(8): 1050-8. [ Links ]
3. Casewell MW. The nose: an underestimated source of Staphylococcus aureus causing wound infection. J Hosp Infect. 1998; 40 Suppl B: S3-11. [ Links ]
4. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997; 10(3): 505-20. [ Links ]
5. Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005; 5(12): 751-62. [ Links ]
6. von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med. 2001; 344(1): 11-6. [ Links ]
7. Lo WT, Lin WJ, Tseng MH, Wang SR, Chu ML, Wang CC. Methicillin-resistant Staphylococcus aureus in children, Taiwan. Emerg Infect Dis. 2006; 12(8): 1267-70. [ Links ]
8. Kaplan SL, Hulten KG, Gonzalez BE, Hammerman WA, Lamberth L, Versalovic J, et al. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Infect Dis. 2005; 40(12): 1785-91. [ Links ]
9. Creech CB, Kernodle DS, Alsentzer A, Wilson C, Edwards KM. Increasing rates of nasal carriage of methicillin-resistant Staphylococcus aureus in healthy children. Pediatr Infect Dis J. 2005; 24(7): 617-21. [ Links ]
10. Gorwitz RJ, Kruszon-Moran D, McAllister SK, McQuillan G, McDougal LK, Fosheim GE, et al. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001-2004. J Infect Dis. 2008; 197(9): 1226-34. [ Links ]
11. Lo WT, Lin WJ, Tseng MH, Wang SR, Chu ML, Wang CC. Risk factors and molecular analysis of panton-valentine leukocidin-positive methicillin-resistant Staphylococcus aureus colonization in healthy children. Pediatr Infect Dis J. 2008; 27(8): 713-8. [ Links ]
12. Bettin A, Suarez P, Bedoya A, Reyes N. Staphylococcus aureus in residents from a nursing-home in Cartagena. Rev Salud Publica (Bogotá). 2008; 10(4): 650-7. [ Links ]
13. CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard-Tenth Edition CLSI document M02-A10. Wayne, PA: Clinical and Laboratory Standards Institute; 2009. [ Links ]
14. Zhang K, McClure JA, Elsayed S, Louie T, Conly JM. Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2005; 43(10): 5026-33. [ Links ]
15. Brakstad OG, Aasbakk K, Maeland JA. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J Clin Microbiol. 1992; 30(7): 1654-60. [ Links ]
16. Lina G, Piemont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999; 29(5): 1128-32. [ Links ]
17. Oliveira DC, de Lencastre H. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2002; 46(7): 2155-61. [ Links ]
18. Labandeira-Rey M, Couzon F, Boisset S, Brown EL, Bes M, Benito Y, et al. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007; 315(5815): 1130-3. [ Links ]
19. Adler A, Temper V, Block CS. Panton-Valentine Leukocidin-producing Staphylococcus aureus. Emerg Infect Dis. 2006; 12: 1789 - 1790. [ Links ]
20. Wei Qi ME, O'Brien F, Imhof A, Ruef C, McCallum N, Berger-Bachi B. Molecular Epidemiology of Methicillin-Resistant Staphylococcus aureus in Zurich, Switzerland (2003): Prevalence of Type IV SCCmec and a New SCCmec Element Associated with Isolates from Intravenous Drug Users. Journal Of Clinical Microbiology. 2005; 43(10): 5164-5170. [ Links ]
21. Saxena S, Singh K, Talwar V. Methicillin-resistant Staphylococcus aureus prevalence in community in the east Delhi area. J Infect Dis. 2003; 56(2): 54-6. [ Links ]
22. Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis. 2003; 36(2): 131-9. [ Links ]