Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de Salud Pública
Print version ISSN 0124-0064
Rev. salud pública vol.14 no.1 Bogotá Jan./Feb. 2012
The cost-effectiveness of enzyme replacement therapy (ERT) for the infantile form of Pompe disease: comparing a high-income country's approach (England) to that of a middle-income one (Colombia)
Costo-Efectividad de la terapia del reemplazo enzimático (TRE) para la forma infantil de la enfermedad Pompe: Perspectiva económica comparativa de un país ingresos altos (Inglaterra) vs uno de ingresos medios (Colombia)
Héctor E. Castro- Jaramillo
London School of Hygiene and Tropical Medicine/Faculty of Public Health and Policy, London, United Kingdom. Epidemiologia Clínica y Bioestadística Pontificia Universidad Javeriana/ Departamento de Bogotá, Colombia. hector.castro@lshtm.ac.uk
Received 25th November 2010/Sent for Modification 22th October 2011/Accepted 12th January 2012
ABSTRACT
Objectives Determining the cost-effectiveness of enzyme replacement therapy (ERT) for the classical infantile form of Pompe disease (complete acid a-glucosidase deficiency-related) in two different settings: England and Colombia. Pompe disease is very rare (1:40,000 births incidence).
Methods A literature review was made and historic databases searched for National Health Service (NHS) reimbursed costs in England and by health insurers in Colombia; expert opinion was elicited. Two Markov models were constructed for comparing both countries; alive with symptoms and dead were the transition states used. Patients aged < 6 months receiving ERT were assumed to have 75 % survival rate and better health-related quality of life (HR-QoL) compared to those without treatment (0.700 HR- QoL using the EQ-5D scale).
Results The incremental cost-effectiveness ratio (ICER) per quality-adjusted life year (QALY) gained was £234,307.7 for England and £109,991 for Colombia. Uncertainty about Anal HR-QoL with ERT, disease progression and cost from palliative care had the biggest impact on the ICER in both models. If ERT costs were reduced to 10,000 times per dose and HR-QoL was 0.750-0.820 ICER, then £165,000 could be attainable for England and £65,000 for Colombia. Transaction costs per case in Colombia were high.
Conclusions ERT was more effective than no ERT in treating infantile Pompe disease, but high levels of uncertainty still remain about survival and progression rates and QoL in the long-run. ICERs were high compared to CE thresholds. Manufacturers' ERT costs and monopoly had a major impact on Anal CEA results.
Key Words: Glycogen storage disease type II, Pompe disease, cost and cost analysis, cost- benefit analysis, quality of life (source: MeSH, NLM).
RESUMEN
Objetivo Determinar la costo-efectividad de la TRE como indicación para la forma clásica de la enfermedad de Pompe (relacionada con deficiencia completa de a-glucosidasa ácida) desde dos perspectivas, Inglaterra y Colombia. La enfermedad de Pompe es muy rara (incidencia 1:40000 nacimientos).
Métodos Revisiónbibliográfica y bases de datos para calcular costosasociados al tratamiento en NHS en Inglaterra, aseguradores de salud en Colombia y opinión de expertos. Dos procesos de Markov fueron construidos para comparar entre países; los estados de transición fueron: vivo sintomático y fallecido. En pacientes de < 6 meses de edad con TRE, se asume un incremento de 75 % de sobrevida y mejor calidad de vida comparada con los que no reciben TRE (HR-QoL 0.700 usando EQ-5D).
Resultados Inglaterra alcanzo ICER por QALY ganado £234307, 7 y Colombia £109991. Incertidumbre sobre HR-QoL con TRE, progresión de enfermedad y costo de cuidado paliativo tuvieron el mayor impacto en losICERs;sí el costo de TREfuera 10.000 menor y la HR-QoLalcanzara 0.750-0.820 ICERs de £165000y £65000 podrían obtenerse para Inglaterra y Colombia respectivamente. Los costos transaccionales en Colombia son representativos.
Conclusiones La TRE es más efectiva que no dar tratamiento, pero incertidumbre sobre tasas desobrevida, progresión y HR-QoLpermanecen en el largo plazo. Los ICERsson altos comparados a los umbrales establecidos de CE. Los costos de TRE y el podermonopolístico del fabricante tienen un impacto importante en los resultados Anales de CEA. Investigación adicional debe realizarsea futuro.
Palabras Clave: Enfermedad de Almacenamiento de Glucógeno Tipo II, glucogenosis generalizada, Glucogenosis 2, Enfermedad por Deficiencia de Maltasa Ácida, Enfermedad de Pompe, Enfermedad por Deficiencia de Lisosoma alfa-1,4-Glucosidasa, deficiencia de maltasa ácida, análisis costo- beneficio, costo efectividad, calidad de vida (fuente: DeCS, BIREME).
Pompe disease is a very rare autosomal recessive disorder which was first described in 1932 by JC. Pompe (a Dutch pathologist); it is also known as acid maltase deficiency or glycogen storage disease type II (1). The disease is characterised by acid a-glucosidase deficiency leading to progressive glycogen accumulation in cardiac, skeletal and respiratory muscle tissue which could result in cardio-respiratory deficiency and death.
There are two clinical forms of the disease, the classical infantile form which is usually lethal and related to complete a- glucosidase deficiency and the milder and later onset form that may present at any age (sub-categorised as non-classical infantile, childhood, juvenile and adult) usually related to partial enzyme deficiency (1-4).
The incidence of all combined forms of Pompe disease is 1:40,000 births worldwide (5,6). It is not precisely known how many people suffer from it, but it has been assumed that no more than 5,000-10,000 people worldwide are affected. It occurs in people of all races, but occurs more frequently in African Americans (1/14,000 births cf 1/60,000 adults and 1/100,000 Caucasian children) (7). Consequently, Pompe disorder has been classified as an orphan disease, such denomination being used for rare conditions (less than 200,000 people in North-America and no more than 5 in 10,000 in Europe).
Feeding problems, failure to gain weight, muscular weakness, motor development impairment, cardiac problems, respiratory difficulty and airway inffections are frequently reported as being the main symptoms during the first months of life (mean 1.9- 2.1 months) and most infants die within the first year (92-95 %) usually after a short period of inpatient care (median 2.8- 4.0 months) from cardiorespiratory insufficiency (7,8).
Late-onset Pompe disease predominantly presents as a slowly progressive proximal myopathy affecting young and middle aged individuals; the symptoms are mostly related to mobility problems and limb girdle weakness, such disability varying amongst patients. Diagnosis is not straightforward and may take more than 5 years in up to 28 % of patients (8).
Treatment was merely palliative and supportive before enzyme replacement therapy (ERT) was introduced; however, a recombinant a-glucosidase (myozyme) was approved in 2006 as the first and only drug to be used for enzyme replacement. Evidence showed that a-glucosidase significantly prolonged total and ventilator-free survival in patients suffering from the classical infantile form. Patients with severe deficiency and early onset of enzyme deficiency (before 6 months of age) seem to have shown the most marked benefit (9).
A 20mg/kg ERT scheme every two weeks is expected to increase survival rates per year from 73 % (45-92, 95 % CI) to 89 % (68-100, 95 % CI) according to some clinical studies (10). Cox proportional-hazards regression models have shown that a-glucosidase has reduced the risk of death by 99 % (0.01 hazard ratio; 0.00-0.10 95 %CI; p<0.0001) in patients aged < 6 months and 71 % (hazard ratio: 0.29; 0.11-0.81 95 % CI; p<0.018) for patients aged > 6 months to < 36 months. a-glucosidase-treated patients had improved their cardio-myopathy, growth, weight gain, motor development and functional status. According to the manufacturer at least 76 % survival rate is expected for patients aged < than 6 months who are being treated. No long-term assessment of health-related quality of life (HR-QoL) has been made for live patient populations receiving ERT.
Although all patients treated with myozyme could develop adverse events from treatment, the commonest mild to moderate adverse reactions requiring intervention have been infusion-related (up to 51 % of cases during the 2 hours following infusion); clinical trials and post-marketing safety experience with myozyme have shown that about 1 % of patients developed anaphylactic shock and/or cardiac arrest during myozyme infusion requiring life-support measures and another 14 % have developed other major allergic reactions.
METHODS
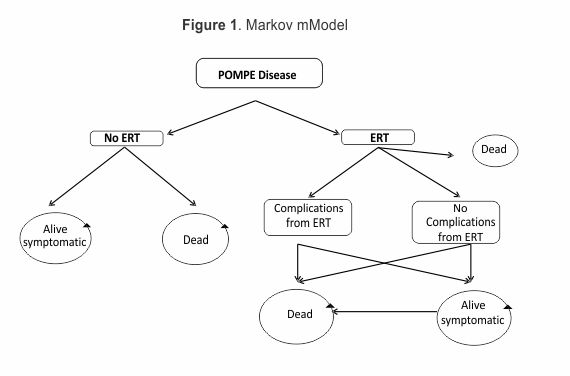
Two Markov models from were constructed from a health system's perspective based on patient transition state (alive-symptomatic and dead); since the disorder is always progressive and symptomatic after being diagnosed, no alive-asymptomatic transition state was considered. ERT was assumed to partially restore patients' quality of life (QoL). Motor development impairment and respiratory insufficiency progression were expected to occur in the model in the long-run.
Most parameters for populating the models were derived from published literature. Each cycle was considered to be yearly based and the model was run for 20 cycles from experts' opinion regarding expected life expectancy (9). Discount rates were 5 % for both costs and effects in each cycle. Effectiveness was calculated in quality-adjusted life years (QALY) gained and cost in 2010 GBP (£). Markov models were run using TreeAge pro 2009.
Eight studies were reviewed concerning the natural course of disease; one of the main comparative reviews of historic cohorts came from a 2003 Dutch study (8) which gave a 92 % mortality rate (109/119) during the first year of life after birth for children suffering Pompe disease and who received no ERT. Some assumptions were made when constructing the decision model. Since death usually occurs after a 78 day period of inpatient palliative and supportive care, such average length of stay was introduced and multiplied by average cost per day in calculating the average cost of supportive and palliative care, assuming that all patients would be placed in an intensive care unit (ICU) before death. Two other studies determined ERT effectiveness rate, assuming a 75 % survival rate per year for this model (9, 10).
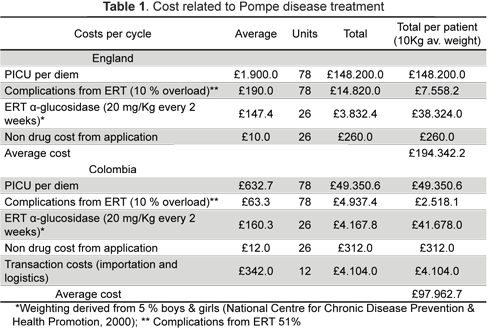
Costs were calculated for inpatient palliative and supportive care in a paediatric intensive care unit (PICU). ERT was given every two weeks involving 20 mg/Kg myozyme doses (26 per cycle); ERT infusion non-drug costs and the cost of treating complications arising from ERT application were also included. A 51 % probability for complications arising from ERT application was included (11) and 10 % extra was loaded for inpatient care assuming that any complication would require extra care should complications arise. Assuming a marked deviation from normal weight gain curves for boys and girls with Pompe disease, 5 % weight was used as a reference for calculating total ERT dose per case (12).
All costs related to diagnosis were not included, assuming that children would enter the model after being diagnosed and average outpatient specialist's consultations were assumed to be similar for both groups as disease progression would mostly likely lead to them being treated as inpatients, hence costs related to out-patient care other than ERT infusion were neglected. Inpatient care costs, ERT cost and costs from treating complications from ERT application in England were obtained from National Health Service (NHS) and healthcare resource group (HRG) sources, parliament reports and the British National Formulary for Children (BNF-C) for different years. Non-drug ERT application costs were obtained from a 2003 Dutch study (13). All costs were calculated at 2010 prices, using inflation rates reported by the UK Office for National Statistics (14, 15). Average costs incurred by third-party payers from the Colombian Social Health Insurance Scheme (SHI) were calculated; COOMEVA EPS, one of the biggest insurers, shared historical data regarding average inpatient care, ERT, non-drug and ERT treatment complication costs. They were adjusted to 2010 values according to the Colombian Statistics Department (DANE) inflation Figures and exchanged from Colombian pesos to British Pounds to ease comparison (16,17). An extra £ 4,104 cost per cycle was added to the Colombian model arising from third-party payers' importation costs and logistics (£ 342 per month). Table 1 gives a summary of costs.
Three scenarios were assumed for classifying symptomatic patients still alive and suffering from Pompe's disease. EuroQol Group's EQ-5D would give the worst-off state scenario as being health state 22 322 (0.189 utility value), 22 221 middle state (0.587 utility value) and 21 211 best-off state (0.814) (18). Since there was a 50 % chance of moving from best-off or middle state to worst-off state (with or without ERT), two main health states were combined (0.189*0.5) + (0.587*0.5)=0.388 and (0.587*0.5)+(0.814*0.5)=0.700 (18). Assuming that ERT would favour higher HR-QoL, the higher combined value was assigned to being alive with ERT and the lower value to being alive without ERT.
RESULTS
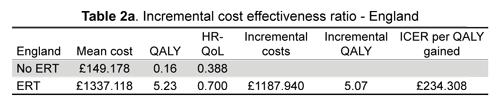
No ERT gave £149.178 per 0.16 QALYs gained and ERT £1,337.118 per 5.23 QALYs gained after 20 years in the English model; the incremental cost-effectiveness ratio (ICER) per QALY incremental cost-effectiveness ratio was £234,307.7.
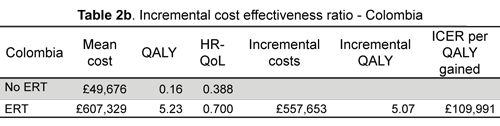
No ERT resulted in £49.676 per 0.16 QALYs gained and ERT £607.329 per 5.23 QALYs gained after 20 years in the Colombian perspective model; the ICER per QALY was £109,991.
Both one-way and two-way sensitivity analysis were used for reflecting alternative structural assumptions.
Sensitivity analysis 1. ERT would have a differential impact on health state. ERT may not resolve all disease symptoms and patients may remain, at least, in a mild disease state. All patients were assumed to have 0.700 QoL with ERT treatment in the present model. Some literature has shown that cardiac hypertrophy may become resolved after ERT but other complications like inability to achieve normal motor development, feeding problems and mild respiratory symptoms may persist or even progress, thereby having an expected impact on HR-QoL value. HR-QoL could be much lower than expected if glycogen deposits continue, even with ERT.
Sensitivity analysis 1 included 0.100 to 0.900 utility values. ERT ICER substantially decreased the more optimistic the assumption was about HR-QoL. The ICER was nearly to £180,000 in the English model at 0.900 and sloped to £2,015,000 when the value was 0.100. The Colombian ICER was 0.900 close to £85,000 and sloped up to nearly £946,000 when the value was 0.
Analysis 2. Changes in drug cost. ERT is a relatively costly treatment. Base-case analysis estimated that a patient would cost £3,833 annually per Kg for England and £4,166.5 for Colombia. Sensitivity analysis 2 revealed the extent to which ERT unit cost drives the cost-effectiveness model. The cost per Kg varied between £0 and the final estimates included for each country. The results showed a mild impact on ICER ranging from £200,000 at £0 cost per Kg up to £234,000 at actual cost in England and from £73,000 at cost per Kg of £0 up to £110,000 at actual cost in Colombia.
Analysis 3. Additional transaction costs being faced by the Colombian health system and derived from additional importation and transportation costs. Ranging from £0 up to £41,040 (10 times the actual importation cost of £4,104), transaction costs were hypothetically simulated and increased this result so that ICER fell from its actual £110,000 to £104,000 at no importation cost and ICER would increase up to £164,000 if importation cost rose 10 times. Analysis 4. Cost of treatment compared to Effectiveness. Two-way sensitivity analysis showed that if ERT costs were 10,000 times lower (nearly £0.38 for England and £0.41 for Colombia), ICER would become decreased to £165,000 for England and £65,000 for Colombia, but only if the utility derived from treatment (HR-QoL) reached at least 0.820 for England and 0.750 for Colombia. Monte Carlo simulation was used for probabilistic sensitivity analysis (PSA) to emulate a cohort of 1,000 patients with Pompe disease being treated with ERT and no ERT.
DISCUSSION
Clinical evidence supports the clinical effectiveness of ERT with a-glucosidase (myozyme) in the short-run for patients suffering the early onset (classical) form of Pompe disease. Survival rates during the first year of life would be expected to improve from 8 % without treatment to 40 % and even closer to 100 % with ERT; decreased cardiac hypertrophy, major milestones regarding motor development and later progression of respiratory insufficiency could increase life expectancy at birth for this population by up to at least 20 years according to experts' opinion (1-3,8,9).
In spite of such optimistic findings, there is still very limited evidence and lack of long-term data to prove that HR-QoL would be close to life lived in full health (a utility value of 1), or if patients receiving ERT could live a productive and less-dependent life. Disease progression rate with ERT has not been sufficiently studied and it may be the case that the more severe the disease, the greater the chances of demanding costly inpatient and intensive palliative care with its subsequent negative impact on the model's results. If Pompe disease could be stabilised by ERT or even regressed, then a new transitional state of being alive and asymptomatic whilst receiving ERT could be introduced into future models.
Two models were run to compare a high income country (England) having a taxation-based health system, a single payer (the NHS), a strong culture and health technology assessment (HTA) with a middle income country (Colombia) having a social health insurance scheme, many purchasers and a very recent history of HTA. An additional difference and paradoxical finding was that average ERT cost per case in Colombia might be 9 % more costly than in England, probably from health insurers' segmented purchasing power compared to a stronger monopsonistic purchaser in the UK (associated with additional £4,104 transaction importation and nationalisation costs per case).
ICERs per QALY were still very high in both cases, £234,000 in England and £90,000 in Colombia compared to "established" cost-effectiveness thresholds per QALY or YL gained as decision rules in some other countries [for instance £30,000 in the UK, USD$50,000 in the USA, NZ$20,000 in New Zealand, US$69,000 in Sweden, AU$ 42,000 in Australia and so on](19,20), especially as low income (LIC) and middle income (MIC) thresholds have not been set or discussed by their societies (19). However, it is clear that healthcare decisions are not made on the appeal of arbitrary numbers; cost-effectiveness thresholds are still objective national benchmarks related to each society's affordability criteria.
The results from this model agreed with those of Scottish NHS 2007 (Scottish Medicines Consortium) where cost per QALY gained for children <6 months was £244,450; this 2010 version was £234,308 per QALY gained (England). SMC advice in 2007 was not to recommend using a-glucosidase (myozyme) in Scotland for treating Pompe disease (9). Nevertheless, this model's level of correspondence had several limitations, basically because of limited data sources. Real worldwide disease prevalence is not clear, utility values for different states of the disease (mild, moderate or severe) with or without ERT have not yet been elicited. EQ-5D despite having cultural and geographical differences was applied in both models to assign values; using expert prefferences, a child-friendly version ofEQ-5D might be more suitable for elucidating patients' preferences. Uncertainty about future clinical attainment or deterioration still remains. It was also assumed that as chronic disease impact on weight gain will be persistent, then a 5 % percentile line was used throughout; however, it is unclear if weight gain curves would become closer to normal distribution following chronic ERT treatment. Probability of death following anaphylactic shock from ERT application was calculated at 50% (1 % overall risk). It was also assumed that since Pompe disorder is progressive and once diagnosed it is always symptomatic, no alive-asymptomatic transition state was considered. Costs from diagnostic phase and out-patient care were seen as irrelevant due to coinciding with previous economic studies concerning lysosomal storage disorders (LSD) (21). Additional limitations were related to inflation rates and trends for both countries, and the possible impact on currency exchange rates that the ongoing economic crisis may have had on international and pharmaceutical markets.
This analysis ran two comparative models to assess ERT cost-effectiveness with a-glucosidase for the classical infantile from of Pompe disease. The results showed that ERT was clinically more effective than no ERT in treating the disorder but high levels of uncertainty still remain about life expectancy, survival rates, progression and quality of life regarding long-term treatment.
ICER gave £234,000 for England and £110,000 for Colombia, such figures still being very high compared to tacit or explicit international decision-making thresholds. Costs related to inpatient and palliative care had the greatest expected impact on overall costs due to the aggressive natural course of disease. Pompe disease treatment is categorised as orphan drug treatment, therapeutical options are still limited and costs related to research and developing new medicines high, hence manufacturers' prices and monopolistic power still have a large impact on final CEA results; paradoxically, MIC might be facing higher fees than HIC when reimbursing a-glucosidase. Issues related to fragmented purchasing power and context regulation should be born in mind when assessing the evidence in detail.
Comparing different countries' perspectives is not about what is right and wrong but about informing what can be learned from one setting to another. Since HTA does not travel well across borders, local data becomes essential in decision-making and it seems there is ample space for further research in this field in developing countries. CE thresholds become benchmarks whenever budgetary constraints hamper/support decision-making, but they need to be used alongside other social and technical tools.
Since there are no clinical comparators for ERT other than supportive and palliative care for Pompe disease, further economic evaluation should include considering the real costs of inpatient and out-patient care for both patients with and without ERT. Ethical considerations will only allow comparisons with historic non-ERT cohorts and patients receiving ERT; a big enough international sample of patients treated with a-glucosidase might be hard to obtain and expensive, hence economic modelling appears to be a useful research tool for assisting decision-makers.
Patients around the world are receiving treatment regardless of sustainability and lack of data regarding long-term QoL with ERT, so additional research, especially from broader perspectives than the purchaser's, is advisable to ensure awareness about broader societal aspects of treatment to report on potential social welfare losses or gains when ERT is used in the long-run.
Acknowledgments: The author would like to thank Faisal Latif and Ayotunde Uko for helping in gathering data, Cora Peterson and Dr Alec Miners for their initial comments and advice on the analysis' general approach and Dr Virgilio Barco and his team from COOMEVA EPS in Colombia for kindly sharing information for the Colombian Markov model.
REFERENCES
1. Hirschhorn R, Reuser AJJ. Glycogen storage disease type II: acid-glucosidase (acid maltase) deficiency. In: Scriver CR, Beaudet A, Sly WS, Valle D, eds. The Metabolic and Molecular Bases of Inherited Disease. Vol. III. New York, NY: McGraw-Hill; 2001. pp. 3389-3420. [ Links ]
2. Engel AG, Gomez MR, Seybold ME, Lambert EH. The spectrum and diagnosis of acid maltase deficiency. Neurology. 1973;23:95-106. [ Links ]
3. Wokke JH, Ausems MG, van den Boogaard MJ, Ippel EF, van Diggelene O, Kroos MA, et al. Genotypephenotype correlation in adult-onset acid maltase deficiency. Neurol. 1995;38:450-454. [ Links ]
4. Slonim AE, Bulone L, Ritz S, Goldberg T, Chen A, Martiniuk F. Identification of two subtypes of infantile acid maltase deficiency. J Pediatr. 2000;137:283-285. [ Links ]
5. Ausems MG, Verbiest J, Hermans MP, Kroos MA, Beemer FA,Wokke JH, et al.Frequency of glycogenstorage disease type II in The Netherlands: implications for diagnosisand genetic counselling. Eur J Hum Genet. 1999;7:713-716. [ Links ]
6. Martiniuk F, Chen A, Mack A, Arvanitopoulos E, Chen Y, Rom WN, et al. Carrier frequency for glycogen storage disease type II in New York and estimates of affected individuals born with the disease [letter]. Am J Med Genetics. 1998:79:69-7. [ Links ]
7. Ziótkowska-Graca, B, Kania,A, Zwoliñska,G, Nizankowska-Mogilnicka, E. Via Medica Polish Pneumonology and Allergology 2008, vol. 76, no 5, pages 396-399 Pol. Pneumonol. Allergol.2008; 76: 396-399. [ Links ]
8. Van den Hout HM, Hop W, Van Diggelen OP, Smeitink JA, Smit GP, Poll-The BT, Bakker HD, Loonen MC, de Klerk JB, Reuser AJ, Van der Ploeg AT. The Natural Course of Infantile Pompe Disease:20 Original Cases Compared With 133 Cases From the Literature: 10.1542/peds.112.2.332 Pediatrics. 2003;112;332-340. [ Links ]
9. NHS Scotland Scottish Medicines Consortium [Internet]. Alglucosidase alpha 50mg powder for concentrate for solution for infusion (Myozyme). Reference No. (352/07). Myozyme 2007. Available at http://www.scottishmedicines.org.uk Searched on April 15th 2010 [ Links ]
10. Kishnani PS, Corzo D, Nicolino M, Byrne B, Mandel H, Hwu WL, et al. Recombinant human acid a-glucosidase: Major clinical benefits in infantile-onset Pompe disease. Neurology 2007; 68: 1-11. [ Links ]
11. Genzyme Corporation. All rights reserved. MYOZYME and Genzyme are registered trademarks of Genzyme Corporation. (Revised 01/09) 2009. MZ-US-P003-01-09. [ Links ]
12. National Center for Health Statistics in collaboration with the National Center for Chronic Disease Prevention and Health Promotion [Internet]. Growth charts. (2000). Available at http://www.cdc.gov/growthcharts18. Searched on April 20 2010. [ Links ]
13. Van Zanten A, Engelfriet PM, Van Dillen K, Van Veen M, Nuijten MJC, Polderman KH. Research Importance of non-drug costs of intravenous antibiotic therapy. Critical Care. December 2003; 7:184-190. [ Links ]
14. The UK Parliament [Internet]. House of Commons. Intensive care Unit Costs. Published via web on June 15th 2005.. Available at: www.publications.parliament.uk/pa/cm200506/cmhansrd/vo050615/text/50615w38.htm Searched on April 20th 3010 [ Links ]
15. Office for National Statistics UK [Internet]. Consumer price index.. Available at www.statistics.gov.uk/instantfigures.asp for inflation rates Searched on April 20th 2010 [ Links ]
16. Departamento Administrativo de Estadística Colombia-DANE [Internet]. Indice histórico de precios al consumidor. Available at http://www.dane.gov.co/index.php?option=com_content&view=article&id=103&Itemid=76 Searched on April 15th 2010. [ Links ]
17. Bank of Canada [Internet]. Currency converter. Year 2003 on wards. Available at: www.bankofcanada.ca/cgi-bin/famecgi_fdps currency converter 2003 Searched on April 20th 2010. [ Links ]
18. Dolan P, Gudex P, Kind P, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ.1998 March 7; 316(7133): 736-741. [ Links ]
19. Eichler HG, Kong SX, Gerth WC, Mavros P, Jbnsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: How are cost-effectiveness thresholds expected to emerge? Value in Health. 2004. Vol 7. 5: 518-528. [ Links ]
20. Devlin N, Parkin D. Does NICE have a cost-effectiveness threshold and what other factors influence its decisions? A binary choice analysis. Health Econ. 2004. 13: 437-452 [ Links ]
21. Connock M, Burls A, Frew E, Fry-Smith A, Juarez-Garcia A, McCabe C, et al. The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher's disease: a systematic review. Health Technol Assess. 2006 Jul;10 (24):136- 40. [ Links ]