Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista de Salud Pública
versión impresa ISSN 0124-0064
Rev. salud pública vol.16 no.2 Bogotá mar./abr. 2014
https://doi.org/10.15446/rsap.v16n2.31690
http://dx.doi.org/10.15446/rsap.v16n2.31690
Artículos/Investigacíon
ErbB2+ metastatic breast cancer treatment after progression on trastuzumab: a cost-effectiveness analysis for a developing country
Tratamientos para cáncer de seno metastásico ErbB2+ en progresión Post-Trastuzumab: Análisis de costo-efectividad para un país en vía de desarrollo
Liliana Chicaíza-Becerra1, Mario García-Molina1, Oscar Gamboa2 y Carlos Castañeda-Orjuela1
1 Universidad Nacional de Colombia, Bogotá, Colombia. lachicaizab@unal.edu.co; mgarciamo@unal.edu.co; cacastanedao@unal.edu.co
2 Instituto Nacional de Cancerología, Bogotá, Colombia. oa_gamboa@yahoo.es
Received 7th August 2012/Sent for Modification 15th October 2013/Accepted 18th November 2013
ABSTRACT
Objective Breast cancer (BC) and metastatic breast cancer (MBC) are significant causes of deaths amongst women worldwide, including developing countries. The cost of treatment in the latter is even more of an issue than in higher income countries. ErbB2 overexpression is a marker of poor prognosis and the goal for targeted therapy. This study was aimed at evaluating the cost-effectiveness in Colombia of ErbB2+ MBC treatment after progression on trastuzumab.
Methods A decision analytic model was constructed for evaluating such treatment in a hypothetical cohort of ErbB2+MBC patients who progressed after a first scheme involving trastuzumab. The alternatives compared were lapatinib+capecitabine (L+C), and trastuzumab+a chemotherapy agent (capecitabine, vinorelbine or a taxane). Markov models were used for calculating progression-free time and the associated costs. Effectiveness estimators for such therapy were identified from primary studies; all direct medical costs based on national fees-guidelines were included. Sensitivity was analyzed and acceptability curves estimated. A 3 % discount rate and third-payer perspective were used within a 5-year horizon.
Results L+C dominated its comparators. Its cost-effectiveness ratio was COP $49,725,045 per progression-free year. The factors most influencing the results were the alternatives' hazard ratios and the cost of trastuzumab.
Conclusion Lapatinib was cost-effective compared to its alternatives for treating MBC after progression on trastuzumab using a Colombian decision analytic model.
Key Words: Cost-benefit analysis, breast neoplasm, receptor, epidermal growth factor, Colombia (source: MeSH, NLM).
RESUMEN
Objetivo El cáncer de seno (CS) y cáncer de seno metastásico (CSM) son importantes causas de muerte entre las mujeres a nivel mundial y en países en vía de desarrollo. En estos últimos los costos de los tratamientos son aún más preocupantes que en países de alto ingreso. La sobreexpresión de ErbB2 es marcador de pobre pronóstico y objetivo de terapias dirigidas. Se evaluó la costo-efectividad de los tratamientos de CSM ErbB2+ en progresión post-trastuzumab en Colombia.
Métodos Se desarrolló un modelo analístico de decisiones para evaluar los tratamientos en una cohorte hipotética de CSM ErbB2+ que progresaron después de un primer esquema con trastuzumab. Las alternativas comparadas fueron: lapatinib+capecitabina (L+C), y trastuzumab más un agente quimioterápico (capecitabina, vinorelbinao un taxano). Se usaron modelos de Markov para calcular el tiempo libre de progresión y los costos asociados. Estimaciones de efectividad fueron identificadas de estudios primarios. Se incluyeron todos los costos médicos directos basados en los manuales tarifarios nacionales. Se realizaron análisis de sensibilidad y curvas de aceptabilidad. Se descontaron costos y resultados a una tasa anual de 3 %, la perspectiva de análisis fue del tercer pagador y el horizonte de 5 años.
Resultados L+C domina a sus comparadores con un razón de costo-efectividad de COP $49 725 045 por año libre de progresión. Los factores que más influencian los resultados son los hazard ratios de las alternativas y el costo de trastuzumab.
Conclusión Lapatinib es costo-efectivo comparado con sus alternativas para el tratamiento del CSM después de la progresión con trastuzumab en el escenario colombiano.
Palabras Clave: Análisis de costo-beneficio, cáncer de seno, receptores del factor de crecimiento epidérmico, Colombia (fuente: DeCS, BIREME).
Around 45 % of the more than 1 million breast cancer (BC) diagnoses and 55 % of BC-related deaths every year occur in developing countries (1). The 5-year recurrence-free mean survival rate worldwide is 60 % (2). Almost 50 % of BC patients develop metastatic disease (3). Retrospective analysis shows a decrease in metastatic breast cancer (MBC) incidence (4) and longer survival due to the introduction of new agents and targeted therapy (5); however, such tendency may be hindered in developing countries due to their lower income and other urgent health-related problems, such as infectious diseases.
MBC treatment is not seen as being curative but mainly palliative, concentrating on an improvement in the progression-free survival and patients' quality of life (6).
Due to the development of inactivating-only targeted drugs, epidermal growth factor receptors (EGFR) and hormone receptors represent significant biological markers in BC and MBC treatment. EGFRs (ErbB1 and ErbB2) have been found to be overexpressed in around 25 % of primary BC (7-10); it is associated with poor prognosis (lower recurrence-free (RF) and overall survival (OS) rates) (7-12).
Trastuzumab alone or combined with first-line chemotherapy in phase III clinical trials has given better overall response (OR), time to progression (TP) and OS rates than chemotherapy alone in ErbB2+MBC patients (13-16). Evidence of trastuzumab use in Erb B2+MBC which has progressed after trastuzumab-based therapy mainly consisted of retrospective analysis and limited-sized phase II studies (17-30). One phase III study (31-32) found that trastuzumab + capecitabine had better OR, clinical benefit (CB) and TP rates than capecitabine alone.
Lapatinib is an oral, small molecule which selectively and reversibly inhibits the tyrosine-kinase signalling pathways for ErbB2+ErbB1 and EGFR which are useful in MBC cases where resistance to trastuzumab has developed (18, 29). L+C has been shown to be superior to capecitabine alone in patients who have previously been treated with trastuzumab (33-34). This study was aimed at assessing the cost-effectiveness of L+C compared to trastuzumab plus chemotherapy for ErbB2+MBC in Colombia.
MATERIALS AND METHODS
The alternatives assessed here were (L+C) compared to trastuzumab + capecitabine (T+C), trastuzumab + paclitaxel (T+P), trastuzumab + docetaxel (T+D), and trastuzumab + vinorelbine (T+V). Trastuzumab-based strategies represent current practice in Colombia, although the different alternatives coexist.
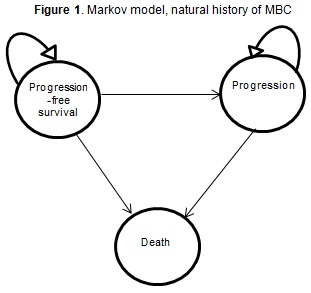
A three-state Markov model for the natural history of MBC was constructed (Figure 1). The model simulated a cohort of MBC women whose cancer had already progressed after a trastuzumab first scheme. Initial state was called progression free survival (PFS). Patients were treated with L+C or trastuzumab plus chemotherapy, according to the alternative, until they progressed again (and moved on to the progression (P) state). Only palliative care was administered from then on.
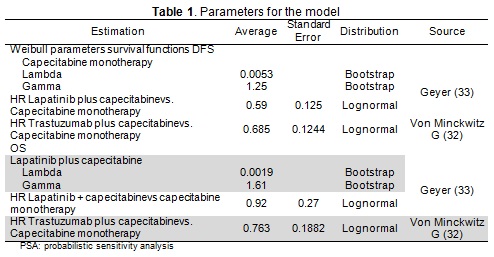
Indirect comparisons were made in the absence of head-to-head studies. The sources of evidence for estimating probability were the ErbB2+ subgroups of clinical trials comparing T+C to capecitabine (32) and L+C to capecitabine (33). Disease-free survival (DFS) and OS rates were estimated as Weibull functions for each chemotherapy alone from the survival curves reported in the literature and multiplied by the hazard ratios (HR) from trastuzumab and lapatinib studies.
The models supposed all types of chemotherapy to be equally effective, only differing regarding their cost and adverse events. Table 1 gives the parameters used in the model and their sources.
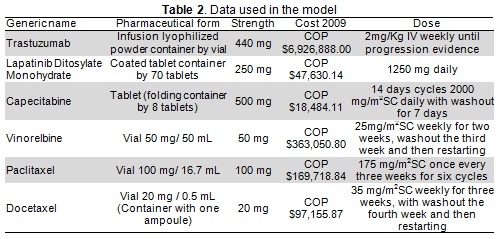
ErbB2+metastatic breast cancer care protocols and adverse events in a Colombian setting were validated by peer consensus. Direct care costs were identified for each event based on the Colombian Social Protection Ministry's SISPRO database drug cost information system and national fees guidelines (SOAT 2009) (Table 2).
Progression-free time was the outcome and discount rate 3%. The perspective adopted was that of the third payer, including all direct medical costs. The time horizon was 5 years. Incremental cost-effectiveness ratios were calculated. Deterministic and probabilistic sensitivity analysis included acceptability curves for each alternative.
RESULTS
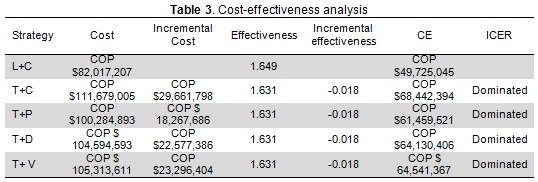
L+C was the most effective and least expensive alternative (i.e. dominant) (Table 3). L+C cost-effectiveness ratio was COP $49,725,045 per progression-free year (average Colombian exchange rate in 2009 was COP $2,156 per dollar).
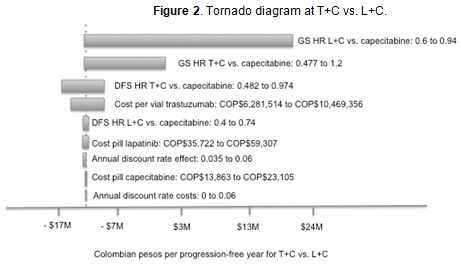
Four variables accounted for 99.4 % of variability in the results: T+C cf capecitabine OS and DFS HR, L+C cf capecitabine OS and the cost per vial of trastuzumab (Figure 2). Tornado analysis results for the other alternatives were very similar (data not shown).
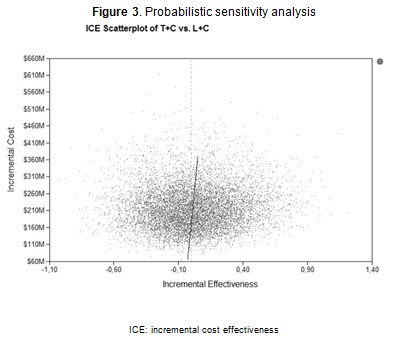
A probabilistic sensitivity analysis with 10,000 simulations (Figure 3) showed that L+C was dominant for 50.13 % of the simulations and was still cost-effective in the remaining 49.87 % cases, as the ICER for the alternatives exceeded the three times per-capita GDP threshold (three times COP $11,216,656). Similar results were produced when trastuzumab was accompanied by taxane or vinorelbine (data not shown).
DISCUSSION
This cost-effectiveness analysis showed that the lapatinib-based strategy was dominant in MBC treatment after ErbB2+ progression on trastuzumab compared to trastuzumab with capecitabine, taxane or vinorelbine.
A benefit not included in this analysis was that the lapatinib strategy only required oral administration, implying a positive impact on patients' quality of life. A cost-utility analysis was not made as there are no evaluations of states of health have been made in Colombia; instead, the result was expressed in DFP years because OS was not significantly different in the alternatives.
Our analysis did not include other strategies as comparators because they are not accepted in Colombia as oncological treatment. The results were subject to commercial and regulatory practice determining the prices and packing-sizes used in Colombia.
Although there was no cost-effectiveness threshold for Colombia, we used WHO recommendations in terms of annual per capita GDP; however, as lapatinib dominated the alternatives currently being used in Colombian medical practice, the result was robust regarding the threshold.
This study's limitations included treatment-effectiveness data being obtained from international studies which obviously used different populations to the Colombian population. If treatment effectiveness and complication frequency were significantly different from that considered in this study, the results would also have been different. Given a lack of head-to-head studies comparing the alternatives being studied, the comparisons in this analysis were indirect and based on HRs from existing randomized clinical trials; the results would thus vary for direct comparison. Due to data being unavailable for determining chemotherapy effectiveness, a strong assumption was required in taking the same effectiveness, implying that therapy would differ only regarding cost and adverse events.
Prior economic analysis has dealt with trastuzumab in ErbB2+MBC progression (35-46) but only two have compared it to L+C (47, 48). One found that L+C ICER exceeded the threshold per QALY gained for the USA; the other found that L+C was dominant for the UK, as it provided greater QALY at lower cost compared to T+C or T+V or trastuzumab only. Another analysis comparing L+C cf T+C for Mexico (49) was based on results from an interrupted clinical trial (50). So far no full-length article has been published for a developing country. This is the first complete cost-effectiveness analysis comparing two human epidermal growth receptor inhibitors in advanced MBC for a developing country.
Current trastuzumab-based practice relies on using the same treatment, even after patients have developed resistance to it. Developing countries must have access to cost-effective alternatives thereby enabling ErbB2+metastatic breast cancer to be faced while keeping the fiscal burden at bay.
Acknowledgments: The authors are grateful to José Urrego and Mabel Moreno for their help in collecting cost data.
Conflict of interest: All authors state that this research was funded by Glaxo Smith Kline; however, the independence of the results was ensured and no person from the firm took part in any study phase.
Authors' contributions: LC, MG and OG were responsible for the concept and design, OG and CC for data collection and assembly, LC, MG and OG for data analysis and interpretation, LC, MG, OG and CC for writing the manuscript and LC, MG, OG and CC for final approval.
REFERENCES
1. Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al, eds. Cancer incidence in five continents. Vol. IX. Lyon, France: International Agency for Research on Cancer; 2007. (IARC scientific publications no. 160). [ Links ]
2. Gasparini G, Longo R, Torino F, Morabito A. Therapy of breast cancer with molecular targeting agents. Ann Oncol. 2005 May;16Suppl 4:28-36. [ Links ]
3. Lippman ME.Breast Cancer.In: Wiener C, Fauci A. Harrison's Principles of Internal Medicine.New York: McGraw Hill;2008. [ Links ]
4. Martin M, Mahillo E, Llombart-Cussac A, Lluch A, Munarriz B, Pastor M, et al. The "El Alamo" project (1990-1997): two consecutive hospital-based studies of breast cancer outcomes in Spain. Clin Transl Oncol. 2006 Jul;8(7):508-18. [ Links ]
5. Chia SK, Speers CH, D'Yachkova Y, Kang A, Malfair-Taylor S, Barnett J, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007 Sep 1;110(5):973-9. [ Links ]
6. Smith I. Goals of treatment for patients with metastatic breast cancer. Semin Oncol. 2006 Feb;33(1 Suppl 2):S2-5. [ Links ]
7. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177-82. [ Links ]
8. Nicholson S, Wright C, Sainsbury JR, Halcrow P, Kelly P, Angus B, et al. Epidermal growth factor receptor (EGFr) as a marker for poor prognosis in node-negative breast cancer patients: neu and tamoxifen failure. J Steroid Biochem Mol Biol. 1990 Dec 20;37(6):811-4. [ Links ]
9. Gullick WJ, Love SB, Wright C, Barnes DM, Gusterson B, Harris AL, et al. c-erbB-2 protein overexpression in breast cancer is a risk factor in patients with involved and uninvolved lymph nodes. Br J Cancer. 1991 Mar;63(3):434-8. [ Links ]
10. Tsutsui S, Ohno S, Murakami S, Hachitanda Y, Oda S. Prognostic value of epidermal growth factor receptor (EGFR) and its relationship to the estrogen receptor status in 1029 patients with breast cancer. Breast Cancer Res Treat. 2002 Jan;71(1):67-75. [ Links ]
11. Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707-12. [ Links ]
12. Borg A, Tandon AK, Sigurdsson H, Clark GM, Ferno M, Fuqua SA, et al. HER-2/neu amplification predicts poor survival in node-positive breast cancer. Cancer Res. 1990 Jul 15;50(14):4332-7. [ Links ]
13. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER-2 for metastatic breast cancer that overexpresses HER-2. N Engl J Med. 2001 Mar 15;344(11):783-92. [ Links ]
14. Pegram M, Forbes J, Pienkowski T, Valero W, Eiermann G, Von Minckwitz M, et al. BCIRG 007: First overall survival analysis of randomized phase III trial of trastuzumab plus docetaxel with or without carboplatin as first line therapy in HER-2 amplified metastatic breast cancer (MBC). J Clin Oncol. 2007; 25:18S. (Abstract LBA 1008). [ Links ]
15. Robert N, Leyland-Jones B, Asmar L, Belt R, Ilegbodu D, Loesch D, et al. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2 overexpressing metastatic breast cancer. J Clin Oncol. 2006; 24:2786-2792. [ Links ]
16. Mackey JR, Daufman B, Clemens M, Bapsy P, Vaid A, Wardley A, et al. Trastuzumab prolongs progression-free survival in hormone.dependent and HER-2-positive metastatic breast cancer. Breast Cancer Res Treat. 2006;100(suppl 1):3a. [ Links ]
17. Gelmon KA, Mackey J, Verma S, Gertler SZ, Bangemann N, Klimo P, et al. Use of trastuzumab beyond disease progression: observations from a retrospective review of case histories. Clin Breast Cancer. 2004. Apr;5(1):52-8; discussion 9-62. [ Links ]
18. Tripathy D, Slamon DJ, Cobleigh M, Arnold A, Saleh M, Mortimer JE, et al. Safety of treatment of metastatic breast cancer with trastuzumab beyond disease progression. J Clin Oncol. 2004 Mar 15;22(6):1063-70. [ Links ]
19. Bartsch R, Wenzel C, Altorjai G, Pluschnig U, Rudas M, Mader RM, et al. Capecitabine and trastuzumab in heavily pretreated metastatic breast cancer. J Clin Oncol. 2007 Sep 1;25(25):3853-8. [ Links ]
20. Bullock K, Blackwell K. Clinical efficacy of taxane-trastuzumab combination regimens for HER-2-positive metastatic breast cancer. Oncologist. 2008 May;13(5):515-25. [ Links ]
21. Stemmler HJ, Kahlert S, Siekiera W, Untch M, Heinrich B, Heinemann V. Prolonged survival of patients receiving trastuzumab beyond disease progression for HER-2 overexpressing metastattic breast cancer (MBC). Onkologie. 2005; 28: 582-586. [ Links ]
22. Tokajuk P, Czartoryska-Arlukowicz B, Wojtukiewicz MZ. Activity of Trastuzumab-based therapy beyond disease progression in heavily pretreated metastatic breast cancer patients-single institution experience. Proc Am Soc Clin Oncol. 2006; 24: 614s. [ Links ]
23. Extra JM, Antoine EC, Vincent-Salomon A, Bergougnoux L, Campana F, Namer M. Favourable effect of continued trastuzumab treatment in metastatic breast cancer: results from the French Hermine cohort study. Breast Cancer Res Treat. 2006; 100(suppl 1): S102. [ Links ]
24. Bartsch R, Wenzel C, Altorjai G, Pluschnig GJ, Locker M, Rudas RM, et al. Trastuzumab plus capecitabine in heavily pretreated patients with advanced breast cancer. Proc Am Soc Clin Oncol. 2007; 25: 45s. [ Links ]
25. Fugimoto-Ouchi K, Muiyazaki T, Sekiguchi F, Mori K. Preclinical study of continuous administration of trastuzumab as combination therapy after disease progression with trastuzumab monotherapy. Proc Am Assoc Cancer Res. 2005; 45: 1195. [ Links ]
26. Morabito A, Longo R, Gattuso D, Carillio G, Massaccesi C, Mariani L, et al. Trastuzumab in combination with gemcitabine and vinorelbine as second-line therapy for HER-2/neu overexpressing metastatic breast cancer. Oncol Rep. 2006; 16: 393-398. [ Links ]
27. Fountzilas G, Razis E, Tsavdaridis D, Karina M, Labropoulos S, Christodoulou C, et al. Continuation of trastuzumab beyond disease progression is feasible and safe in patients with metastatic breast cancer: a retrospective analysis of 80 cases by the Hellenic Cooperative Oncology Group. Clean breast cancer. 2003; 4: 120-125. [ Links ]
28. Montemurro F, Faggiuolo R, Redana S. Continuation of trastuzumab beyond disease progression. J Clin Oncol. 2005; 23: 2866-2868. [ Links ]
29. Montemurro F, Donadio M, Clavarezza M, Redana S, Jacomuzzi ME, Valabrega G, et al. Outcome of patients with HER-2- positive advanced breast cancer progressing during trastuzumab based therapy. Oncologist. 2006; 11: 318-324. [ Links ]
30. Antoine EC, Extra JM, Vincent-Salomon A, Bergougnoux L, Campana F, Namer M. Multiple lines of trastuzumab provide a survival benefit for women with metastatic breast cancer: results from the Hermine cohort study. Eur J Cancer Suppl. 2007; 5: 213. [ Links ]
31. Von Minckwitz G, Zielinski C, Maarteense E, Vogel P, Schmidt M, Eitmann H, et al. Capecitabinev scapecitabine + trastuzumab in patients with HER-2-positive metastatic breast cancer progressing during trastuzumab treatment: The TBP phase III study (GBG26/BIG 3-05) (abstract 1025). J Clin Oncol. 2008; 26 (suppl 15s): 47s. [ Links ]
32. Von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German breast group 26/breast international group 03-05 study. J Clin Oncol. 2009 Apr 20;27(12):1999-2006. [ Links ]
33. Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER-2-positive advanced breast cancer. N Engl J Med. 2006 Dec 28;355(26):2733-43. [ Links ]
34. Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008 Dec;112(3):533-43. [ Links ]
35. Phillips KA, Marshall DA, Haas JS, Elkin EB, Liang SY, Hassett MJ, et al. Clinical practice patterns and cost effectiveness of human epidermal growth receptor 2 testing strategies in breast cancer patients. Cancer. 2009 Nov 15;115(22):5166-74. [ Links ]
36. Poncet B, Bachelot T, Colin C, Ganne C, Jaisson-Hot I, Orfeuvre H, et al. Use of the monoclonal antibody anti-HER-2 trastuzumab in the treatment of metastatic breast cancer: a cost-effectiveness analysis. Am J Clin Oncol. 2008 Aug;31(4):363-8. [ Links ]
37. Lidgren M, Wilking N, Jonsson B, Rehnberg C. Cost-effectiveness of HER-2 testing and trastuzumab therapy for metastatic breast cancer. Acta Oncol. 2008;47(6):1018-28. [ Links ]
38. Neyt M, Huybrechts M, Hulstaert F, Vrijens F, Ramaekers D. Trastuzumab in early stage breast cancer: a cost-effectiveness analysis for Belgium. Health Policy. 2008 Aug;87(2):146-59. [ Links ]
39. Lidgren M, Jonsson B, Rehnberg C, Willking N, Bergh J. Cost-effectiveness of HER-2 testing and 1-year adjuvant trastuzumab therapy for early breast cancer. Ann Oncol. 2008 Mar;19(3):487-95. [ Links ]
40. Fagnani F, Colin X, Arveux P, Coudert B, Misset JL. Cost/effectiveness analysis of adjuvant therapy with trastuzumab in patients with HER-2 positive early breast cancer. Bull Cancer. 2007 Jul 1;94(7):711-20. [ Links ]
41. Shiroiwa T, Fukuda T, Shimozuma K, Ohashi Y, Tsutani K. The model-based cost-effectiveness analysis of 1-year adjuvant trastuzumab treatment: based on 2-year follow-up HERA trial data. Breast Cancer Res Treat. 2008 Jun;109(3):559-66. [ Links ]
42. Garrison LP, Lubeck D, Lalla D, Paton V, Dueck A, Perez EA. Cost-effectiveness analysis of trastuzumab in the adjuvant setting for treatment of HER-2-positive breast cancer. Cancer. 2007 Aug 1;110(3):489-98. [ Links ]
43. Millar JA, Millward MJ. Cost effectiveness of trastuzumab in the adjuvant treatment of early breast cancer: a lifetime model. Pharmacoeconomics. 2007;25(5):429-42. [ Links ]
44. Norum J, Olsen JA, Wist EA, Lonning PE. Trastuzumab in adjuvant breast cancer therapy. A model based cost-effectiveness analysis. Acta Oncol. 2007;46(2):153-64. [ Links ]
45. Kurian AW, Thompson RN, Gaw AF, Arai S, Ortiz R, Garber AM. A cost-effectiveness analysis of adjuvant trastuzumab regimens in early HER-2/neu-positive breast cancer. J Clin Oncol. 2007 Feb 20;25(6):634-41. [ Links ]
46. Liberato NL, Marchetti M, Barosi G. Cost effectiveness of adjuvant trastuzumab in human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2007 Feb 20;25(6):625-33. [ Links ]
47. Le QA, Hay JW. Cost-effectiveness analysis of lapatinib in HER-2-positive advanced breast cancer. Cancer. 2009 Feb 1;115(3):489-98. [ Links ]
48. Delea T, Tappenden P, Sofrygin O, Karnon J, Amonkar M, Browning D, et al. Cost-effectiveness (CE) of lapatinib plus capecitabine (L+C) in women with HER2+ metastatic breast cancer (MBC) who received prior therapy with trastuzumab (TZ) based on updated survival data from EGF100151. J Clin Oncol 26: 2008 (May 20 suppl; abstr 6559). [ Links ]
49. Anaya P, Aldaco F. Cost effectiveness analysis of lapatinib/capecitabine (LC) versus trastuzumab/capecitabine (TC) in patients with metastatic breast cancer ERBB2 after progression to the first scheme of trastuzumab. Value in Health. 14 (3): A165, 2011. [ Links ]
50. Cameron D, Casey M, Oliva C, Newstat B, Imwalle B, Geyer CE. Lapatinib Plus Capecitabine in Women with HER-2-Positive Advanced Breast Cancer: Final Survival Analysis of a Phase III Randomized Trial. The Oncologist. September 2010. vol. 15 no. 9 924-934. [ Links ]