Dengue is an epidemic disease transmitted to humans by the bite of infected mosquitoes (Aedes aegypti and Aedes albopictus) and it is caused by Dengue Virus (DENV) 1. Approximately 40 % of the population of the world is at risk of pathogen transmission 2. In Mexico, all DENV serotypes (DENVI - 4) have been isolated. Additionally, disease transmission in most of Mexican states has been reported 3.
Mexican health services are obligated to report suspected cases of dengue using a web-based platform from the National Epidemiological Surveillance System (SINAVE) 4,5. The 1997 World Health Organization (WHO) dengue case definition was used during 2014 and suspected cases were classified as dengue fever (DF), dengue hemorrhagic fever (DHF) or dengue shock syndrome (DSS) 6.
Timely diagnosis and supportive initial treatment reduce dengue mortality and may potentially prevent additional cases among contacts 7. Since the spectrum of clinical manifestations of dengue is wide, early diagnosis may be difficult to detect 8,9. This study aimed to evaluate the association of clinical markers with acute laboratory-positive dengue infection using data from large case-control study conducted in an endemic area.
MATERIALS AND METHODS
Study population
A hospital-based case-control study was conducted in the state of Colima, Mexico, by using data from the SI-NAVE. Incident cases (n=2 732) -included individuals aged from 3 years old and on- were reported, from January to December in 2014, as clinically suspected cases of dengue (fever>2 of the following: headache, myalgia, arthralgia, retro-orbital pain or skin rash) that were subsequently and serologically confirmed as dengue infection cases. A multistage probabilistic sampling, according to age distribution of total confirmed cases from the database, was used in the selection of cases. Dengue serologic tests included ELISA (Enzyme-Linked Immunosorbent Assay), NSI (nonstructural protein 1), and igG/igM (immunoglobulin G/M); and were performed by staff from the State Laboratory of Public Health in accordance with normative standards 4.
Controls (n=2775) -included subjects notified as suspected dengue cases with a subsequent negative serology test- were matched to the cases (frecuency-matching) according to sex, five years old age groups, membership to a health care institution, and health jurisdiction of residence. Controls were randomly selected from individuals fulfilling the eligibility criteria.
Data regarding the clinical manifestations of study subjects were collected and included the initial disease classification (suspected DF, DHF or DSS); fever (yes/no); headache (yes/no); myalgia (yes/no); arthralgia (yes/no); retro-ocular pain (yes/no); skin rash (yes/no); persisting vomiting (yes/no); abdominal pain or tenderness (yes/ no); clinical fluid accumulation (ascites, edema or pleural effusion; yes/no); increased capillary fragility (petechiae, ecchymosis, hematoma or positive tourniquet test; yes/ no) and mucosal bleeding (gingival bleeding, epistaxis, hematemesis or melena; yes/no). Results of serology tests were also extracted from the database.
This study was approved by the National Commission for Clinical Research.
Statistical analysis
Summary statistics were used to compare cases and controls. To determine statistical association between the clinical manifestations and dengue, odds ratios (OR) and 95 % confidence intervals (CI) were estimated by means of unconditional logistic regression models. All analyses were conducted using Stata SE 11.0 (StataCorp, College Station, TX) and significance level was set at 5 %.
RESULTS
Table 1 shows the study population characteristics for selected variables. The mean age of cases and controls was 27, 8±18,0 and 26,9±17,8 years respectively, and this difference did not reach statistical significance. When compared with controls, cases were more likely to be classified at first healthcare contact as DHF/DSS patients requiring hospital admission. Cases also had a significant higher prevalence of retroocular pain (85,3 % vs. 83,0 %), skin rash (17,1 % vs. 9,1 %), persisting vomiting (15,3 % vs. 5,9 %), abdominal pain (14,3 % vs. 6,5 %), clinical fluid accumulation (2,4 % vs. 0,3 %), increased capillary fragility (9,1 % vs. 1,5 %) and mucosal bleeding (5,6 % vs. 1,3 %).
Table 1 Characteristics of participants by case control status
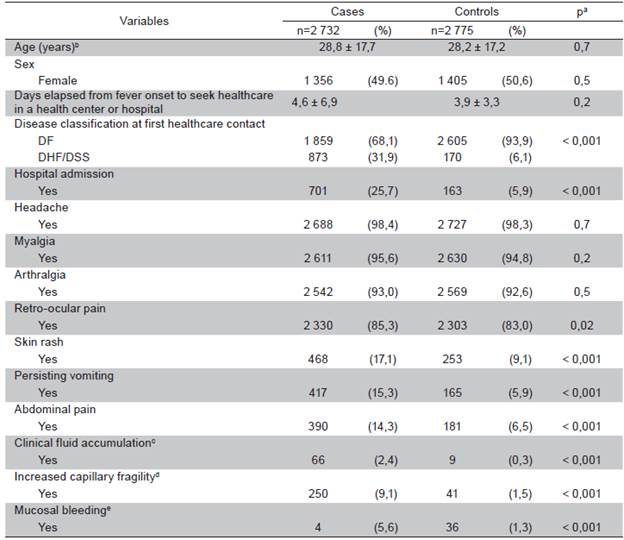
Relative frequency is shown (%) unless otherwise specified. Abbreviations: DF, dengue fever; DHF, dengue hemorrhagic fever; DSS, dengue shock syndrome; a From t-test or chi-square test as corresponding; b Arithmetic mean (standard deviation); c Ascites, edema or pleural effusion; d Petechiae, ecchymosis, hematoma or positive tourniquet test; e Gingival bleeding, epistaxis, hematuria, abnormal vaginal bleeding, hematemesis or melena.
In multiple analyses (Table 2), clinical markers associated with laboratory-positive dengue infection were skin rash (OR=I,7; 95 % CI 1,5-2,1), persisting vomiting (OR=I,8; 95 % CI 1,5-2,3) and increased capillary fragility (OR=I,8; 95 % CI 1,2-2,6).
Table 2 Bivariate and multiple analysis between clinical markers and laboratory-positive dengue infection

a Odds ratios adjusted (by design) by sex, 5-year age groups, membership to a health care institution and health jurisdiction of residence. b Odds ratios adjusted by sex, 5-year age groups, membership to a healthcare institution and health jurisdiction of residence, disease classification at first contact with healthcare and by the variables presented in the table. c Ascites, edema or pleural effusion. d Petechiae, ecchymosis, hematoma or positive tourniquet test. e Gingival bleeding, epistaxis, hematuria, abnormal vaginal bleeding, hematemesis or melena. a,b Unconditional logistic models were used.
DISCUSSION
We found that three clinical markers were associated with laboratory-confirmed dengue virus infection: skin rash, persisting vomiting and increased capillary fragility. Acute dengue illness is characterized by nonspecific signs and symptoms that are difficult to distinguish from other febrile illnesses 10. Moreover, in laboratory limited health care settings, a diagnostic algorithm based on clinical markers could improve early medical management and disease outcomes.
Previously published studies have described variations in clinical dengue features between them 11,12. Differences may be secondary to host response to infection 13.
Headache and retro-ocular pain are grouped in the WHO 2009 dengue case definition 14. The association of headache and retro-ocular pain with confirmed disease was not significant when they were analyzed combined (OR=I,6; 95 % CI 0,8-3,2; data not presented), which is consistent with a previously published study 15. Dengue-related ocular manifestations have been described in 10-40 % of confirmed cases 16,17.
Hospital admission rate was 25,7 % and 5,9 % in cases and controls respectively. This finding is lower to rates reported in other American or Asian populations (45 % - 80 %) 18,19. DENV-2 was the most frequent se-rotype isolated (87,8 %) and it has been associated with increased risk of developing DHF or DSS 20. In the study sample, 68,1 % and 31,9 % of cases were classified as DF and DHF as corresponding; no DSS cases were registered.
The autochthonous transmission of chikungunya virus and zika virus was first observed in Mexico on 2014 and 2015 respectively 21,22. There are clinical characteristics that may be helpful to distinguish between acute cases of dengue, chikungunya or zika infection in limited health-care settings 23,24.
There are some limitations in this study. First, cases and controls were selected from health services users and might not reflect the whole dengue-infected group. However, our results are useful in health care settings from dengue endemic areas. Second, data regarding dengue all warning signs -included in the 2009 WHO case definition- were not collected systematically by the analyzed surveillance system. In Mexico, the 1997 WHO case definition is used for epidemiological purposes. Third, this study was conducted in a population with high incidence of dengue infection, which means our findings may not be reproducible in a non-endemic area.
The results of this study suggest that clinical data may be used to identify acute dengue infection. To our knowledge, this is the first evaluation of interactions between age and clinical markers; further research is needed to understand better our findings ♦














