Cancer refers to a set of chronic, degenerative diseases with disorderly cell growth that can spread to other parts of the body 1. This is an important public health problem worldwide, with an estimated annual incidence of 14 million cases, accounting for approximately 8.2 million deaths per year 2.
Mouth cancer is among the ten most frequent types of cancer in the Brazilian population and is the sixth leading cause of cancer in the world 2. Squamous cell carcinoma (SCC) is a malignant tumor that originates in the stratified squamous epithelium; it is the most common tumor of the head and neck region, accounting for approximately 90% of malignant tumors in the oral cavity 3,4. SCC is more common in men between the fifth and seventh decades of life and the tongue is the most commonly affected anatomical site 5-7.
SCC has a multifactor etiology that has not yet been fully clarified 8. Smoking and alcoholism are considered the main etiopathogenic factors and the combination of these two habits increases the risk of developing cancer 9-11. However, oral SCC can also develop in individuals without either of these habits 12,13.
Even though examining the oral cavity is easy, most cases of mouth cancer are detected in late stages. 8 This situation affects the type of treatment used, as therapeutic procedures vary depending on the stage and histological factors 14. Thus, the early detection of a malignant tumor increases the odds of patient survival 15.
The aim of the present study was to identify the profile of patients diagnosed with oral SCC and treated at an oncology reference center, and the factors associated with the clinical staging of the disease in a six-year period in the city of Campina Grande (northeastern Brazil).
MATERIALS AND METHODS
A descriptive, analytical, cross-sectional study was conducted with a census sample nested in a global, accumulated survival study of patients with oral SCC in a six-year period. A total of 293 medical charts of patients treated at an oncology reference center (Paraíba Assistance Foundation Hospital) in the city of Campina Grande (Paraíba, Brazil) between 2000 and 2006 were analyzed.
An individual clinical chart designed specifically for this study was used to collect data. The data on the patient charts were duly recorded, including socio-demographic profile, tumor characteristics, clinical staging and proposed treatment. The inclusion criteria were a diagnosis of oral SCC at the aforementioned reference center between 2000 and 2006, registration at the hospital and patient records with the results of the histopathological exam.
Oral SCC with confirmation by histopathological exam was the dependent variable. The independent variables were location of the tumor, socio-demographic factors, lifestyle habits, clinical staging (TNM) and grouping based on the recommendations of the American Joint Committee on Cancer 16. Initial staging or early diagnosis was recorded for cases in which the tumor was in Stage I or II at the time of diagnosis. Advanced staging or late diagnoses were recorded for cases in which the tumor was in Stage III or iv at the time of diagnosis 16.
This study was approved by the Human Research Ethics Committee of the State University of Paraíba (Brazil) under process number 0223.0.133.000-11 in compliance with Resolution 196/96 of the Brazilian National Board of Health. Data were collected with the authorization of the department chief and the hospital administration.
A simple descriptive analysis was performed to characterize the sample. Bivariate Poisson regression with robust variance was used to determine associations between the independent variables (sex, age, race and lifestyle habits) and clinical staging of the tumor (p<0.05). Multivariate Poisson regression models were constructed with variables achieving a p-value of <0.20 in the bivariate analysis, as well as variables considered epidemiological determinants. The Statistical Package for Social Sciences (SPSS for Windows, version 18.0, SPSS Inc, Chicago, IL, USA) was used for the organization and analysis of the data.
RESULTS
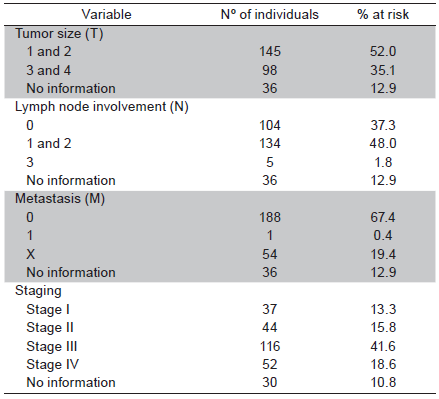
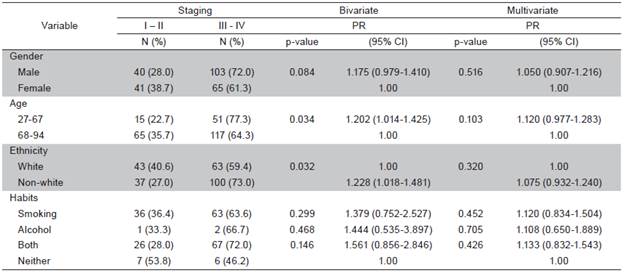
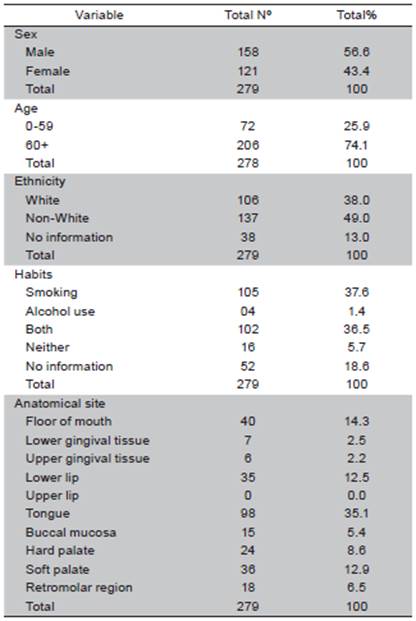
Fourteen patient charts (4.8%) were excluded from the sample due to the lack of histopathological results that confirmed the diagnosis of oral SCC. Out of 279 cases analyzed, 158 (56.6%) were men and 121 (43.4%) were women (ratio: 2 to 1.5, respectively). The mean age was 65 years in men and 71 years in women. In 206 cases (74.1%), age was over 60 years. The overall mean age was 67 years (range: 27 to 94 years). Non-white individuals predominated in the sample (49.0%). At the time of diagnosis, a large part of the sample smoked (37.6%) and 36.5% used both tobacco and alcohol. The tongue was the most frequently affected anatomical site (98 cases; 35.1%) (Table 1). A total of 52% cases were classified at T1 and T2; 48% of cases presented metastasis in adjacent lymph nodes (N1 and N2) and only one case of distant metastasis was found. Most cases were in advanced stages: 41.6% in Stage III and 18.6% in Stage IV (Table 2). In the bivariate analysis, the following variables were associated with clinical staging: sex, age, race and lifestyle habits. However, none of these variables remained in the final multiple Poisson regression model (Table 3).
Table 1 Distribution of oral SCC ethnicity, anatomical site and cases according to sex, age, smoking and alcohol use
DISCUSSION
Squamous cell carcinoma is the most frequent malignant tumor found in the oral cavity and affects more men than women 15,17,18. In the present study, the male to female ratio was 2:1.5. The larger proportion of men is explained by the more frequent use of alcohol and smoking in this population 8, although, the literature commonly describes an even larger sex difference 19-21. In the absence of tobacco abuse, the female sex has a greater prevalence rate of cancer of the head and neck 13, but women generally also have a greater survival rate because such tumors are often diagnosed earlier 22.
The mean age in the overall sample was 67 (65 in men and 75 in women) and the most affected age group was 60 to 79 years. A small number of cases occurred in younger individuals, which is coincides with data described in the literature 23,24. The lower rate of occurrence among younger individuals is explained by shorter exposure time to risk factors, such as alcohol and tobacco, in comparison to older patients, as the increase of age leads to a greater accumulation of the harmful effects of carcinogenic agents 7,8.
In this study, non-white individuals were more affected by SCC (55%) than whites (45%), which differs from what is commonly described in the literature. 25,26 However, information on ethnicity was missing in a significant number of cases, which hinders the interpretation of this finding. Nonetheless, race does not appear to be a determinant factor for mouth cancer 27.
On the other hand, 105 individuals (37.6%) were smokers, 36.5% used both tobacco and alcohol, and 5.7% of cases had neither of the two habits. The combination of tobacco and alcohol is common 28 and a risk factor for mouth cancer. Indeed, patients who are not smokers have tumors with a lower frequency of genetic alterations 9. Therefore, the control of these habits is the best strategy regarding the long-term perspective of this disease 3.
The tongue was the most affected anatomical site (35.13%), followed by the palate (21.50%), floor of the mouth (14.34%) and lip (12.54%). These findings are in agreement with data described in previous studies 7,20,29-30.
A total of 65.5% of the patients received a late diagnose (Stages III and IV), while 32.5% were diagnosed early (Stages I and II). This situation is common with regard to oral SCC. 5,8,30 The larger number of patients in advanced stages is caused by the fact that such tumors are asymptomatic in early stages, as well as several difficulties of a social nature and a lack of awareness 31.
Clinical staging at the time of treatment was not significantly associated with sex, age, ethnicity, smoking or alcohol use. These findings may be related to the fact that clinical staging does not depend on these variables 30. An advanced tumor stage at the time of diagnosis may be explained by the delayed seeking of treatment due to the absence of pain or minimal pain in the early phase of tumor growth, unawareness of the disease, fear of the diagnosis, socioeconomic difficulties and the lack of knowledge among some healthcare professionals regarding clinical aspects related to oral SCC, leading to a delayed or mistaken diagnosis 32,33.
Although not all patients charts had been filled out correctly, which may have led to information bias, these findings demonstrate that the late diagnosis of oral SCC is common. Thus, there is a need to implement public policies directed at periodic prevention campaigns, greater awareness on the part of the population regarding mouth cancer and the importance of early diagnosis. Moreover, investments should be made to train healthcare professionals to correctly identify or treat oral SCC or refer the patient to an appropriate center.
The results of the present study demonstrate a higher incidence of SCC in men, older patients, non-white individuals and smokers. Clinical staging was not associated with any of the variables analyzed•