Dengue fever (DF), with its severe dengue forms 1, is the most important mosquito-borne human viral disease affecting children throughout the world, including Colombia 2. The disease is caused by four different dengue virus (DENV) serotypes that are transmitted by Aedes spp. mosquitoes. An estimated 390 million annual DENV infections occur in the world's tropical and subtropical regions 3. Consequently, DENV infections pose a high economic burden in endemic areas 4,5. In DENV-endemic countries, mandatory notification of DENV infections, by means of a surveillance system, is requested to control the disease and to reduce the associated morbidity and mortality 6.
Public health surveillance systems have been defined as the ongoing systematic collection, analysis and interpretation of health data, essential to the planning, implementation and evaluation of public health practice, closely integrated with the timely dissemination of these data to those who need to know 7. The Colombian National System for Public Health Surveillance (Sistema de Vigilancia en Salud Pública -SIVIGILA, in Spanish) was established in 2006 and is regulated to provide systematic and timely information on the event dynamics of different diseases or conditions that affect public health by means of the SIVIGILA computer program 8. For DENV surveillance, SIVIGILA members must report case incidences and classify them as DENV infections without warning signs, DENV infections with warning signs, or severe DENV infections, according to the 2009 WHO guidelines 1, in order to observe quality, accuracy and timeliness standards to guide prevention and control strategies 9.
The information flow in the system begins at the primary public health facilities (clinics/hospitals), known as Primary Units of Data Generation (PUDG). The PUDG then send the information either to the Notifying Municipal Units (NMU) or the state Notifying Departmental Units (NDU). The data are finally transferred to the Colombian National Institute of Health (NIH), which regulates and coordinates the system 9-11. The system requires the PUDG physicians to identify and notify the NMU/NDU about each DENV case via a 24-hour telephone reporting system and a paper form that is transferred by the SIVIGILA electronic system to them 9-12. The NMU/NDU manage, analyze, investigate and provide weekly notifications of all cases reported by time and place to the NIH via the SIVIGILA system. A team of experts at the NMU/NDU then coNDuct DENV infected case detection and mosquito vector-species larvae surveillance and control in hot spots for reported DENV cases.
The NIH supervises and monitors data collection, provides technical support, uses the information received to assess/evaluate the epidemiology and produces weekly epidemiological reports, and provides and improves annually updated protocols and goals for surveillance, control and prevention programs. The NIH also reports to the Colombian Ministry of Health and to the Pan American Health Organization 8.
Because early detection, notification of cases by time and place, and accurate diagnosis, monitoring and treatment of cases are essential to prevent outbreaks, this work assesses the suitability of these parameters in the PUDG according to the public health surveillance system guidelines of the Centers for Disease Control (USA) 13, in a large DENV hyper-endemic city (Barranquilla) in the Colombian Caribbean coast 14,15. To our knowledge, this is the first report that critically evaluates a DENV surveillance system before the emergence of other clinically similar arboviruses such as chikungunya and Zika virus, for which there are no routine differential diagnostic assays in the Americas, including Colombia 16-19.
MATERIALS AND METHODS
Study Site
Barranquilla is located on the northern Colombian Caribbean coast. It is one of the country's principal sea-port cities and had a population of 1 386 865 during the study period. From 2009 to 2013, the numbers of DENV infections per 10 000 inhabitants reported were 7.3, 12.38, 4.66, 6.25 and 27.91, respectively 20.
Evaluation of the PUDG for physicians' performance to diagnose, manage and report DENV infections
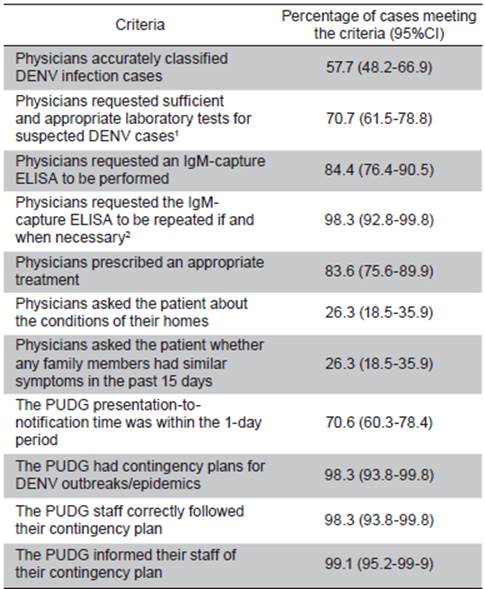
A questionnaire was used to investigate eleven criteria to evaluate the performance of the physicians at eleven PUDG (Table 1). An expected value of 50% was used to calculate a suitable sample size for the analysis of the PUDG case files. In this study, a sample size of 96 was required to calculate the percentage with a ±10% confidence interval and 95% confidence level. The results from the evaluation of these files were double-entered and then exported to Stata 13.1 for analysis.
Performance of the PUDG in reporting to the Barranquilla NMU from 2009 to 2013
The DENV infection case forms reported electronically by each of the eleven PUDG were examined to assess the timeliness, positive predictive values (PPV) and completeness of the data.
The timeliness of the DENV infection case notifications was assessed by calculating the presentation-to-notification time. The PPV were calculated based on the number of confirmed/suspected DENV cases reported. The completeness of the data was assessed in reports with required missing data points and by comparing the DENV infection cases reported by the NMU and the NIH.
RESULTS
Evaluation of physician and clinics/hospital performance to diagnose, manage and report cases at the PUDG level
The physicians' clinical activities were evaluated using those reported in 117 files from 10% (11/114) of the PUDG in Barranquilla (Table 1). In these analyses, physicians accurately classified only 57.7% of the patients according to the NIH protocols, and of those 83.6% received appropriate treatment, 70.7% received correct and sufficient laboratory tests, 84.4% had an IgM-capture ELISA performed on their serum samples, and for 98.3% of the patients who required it, the physicians requested the repetition of an IgM ELISA after day 5 of the onset of symptoms. Ideal timely notification (i.e., within one day) was achieved in 70.6% of the clinics/hospitals via telephone and SIVIGILA. A contingency plan for the DENV infection cases appeared to be well-established at the PUDG (98.3%): it was properly informed to the staff (99.1%), and a large part of the staff (98.3%) followed it. However, the PUDG physicians questioned only 26.3% of the patients about their home conditions or the recent presence of similar disease symptoms in family members or friends.
Analysis of surveillance data reported by the PUDG in Barranquilla to the NDU about cases from 2009 to 2013
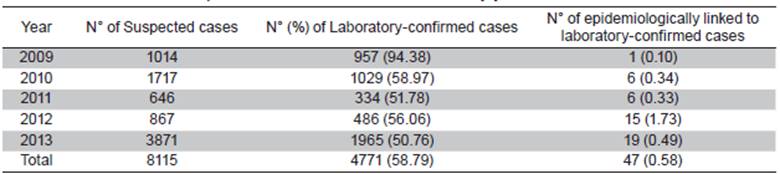
The numbers of suspected DENV infection cases reported by SIVIGILA during 2009-2013 in Barranquilla was 8 115 (Table 2). Of these cases, 54.4% (4 411/8 115) occurred in males, the mean patient age was 17.7 years (SD = 15.7) of whom 70.2% (5 685/8 115) were less than 19 years old; based on the patient phenotype, most of the suspected cases (86.9%: 7 071/8 115) occurred in mestizos. The highest incidence of suspected DENV infections were reported in 2009 (n=1 014), 2010 (n=1 717) and 2013 (n=3 871). The IgM-capture ELISA (IgM test) was requested in 73.4% (5 953/8 115) of the forms and 58.8% of those tests were positive.
Table 2 Suspected and confirmed DENV cases by year from 2009 to 2013
Cases confirmed by laboratory-based positive IgM-capture ELISA vs. cases confirmed by having contact with a DENV-infected relative or living in an area which had a very recent DENV outbreak/epidemic
Amongst the suspected DENV-infected patients, 66.1% (5 362/8 115) showed no warning signs, 27.9% (2 265/8 115) showed warning signs, 0.4% (29/8 115) had severe dengue, 0.03% (2/8 115) had severe dengue with shock, and 0.04% (3/8 115) died. Overall, 32.7% (2 650/ 8 115) of these suspected DENV-infected cases were hospitalized, of which 40.5% (1 074/2 650) had DENV infections without warning signs, 58.7% (1 556/2 650) had warning signs, 0.8% (20/2 650) had severe dengue, 28.9% (766/2 650) went to a first level health facility, while 31.6% (837/2 650), 23.8% (631/2 650) and 15.8% (419/2 650) went to second, third, and fourth level healthcare facilities, respectively, the first level being the most general level of health care, and the fourth level, the most complex.
Timeliness
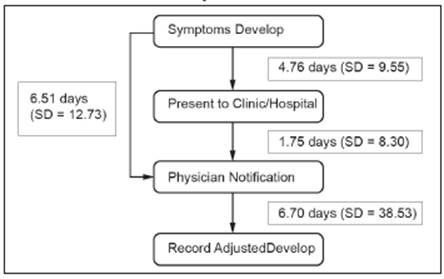
The time taken from the visit of the patient to the notification of DENV infection cases was used as an indicator of the system's efficiency. The first time period evaluated was the time from symptom onset to visit at a PUDG. On average, a patient visited the PUDG at 4.8 days (SD = 9.55) of fever (Figure 1). However, a minority of patients (18.8%) visited the PUDG on days 0-2, while the majority (57.5%= 4 665/8 115) did so on days 3-5, followed by 19.6% on days 6-8 of fever.
Although it is mandatory to report each case within 1 day after the visit of the patient, the average visit-to-notification time was 1.75 days (SD = 8.3; range: 0-367 days). However, most cases (70.6%; 5 729/8 115) were reported within one day, followed by 15.5% (1 257/8 115) and 8.7% (702/8 115) of cases reported within 3 days or 7 days after presentation, respectively.
The percentage of cases reported by the PUDG with a visit-to-notification time of 1 day was 71.3%, 74.0%, 68.3%, 72.5% and 70.3% from 2009 to 2013, respectively, and therefore no apparent trend was observed. While the highest number of cases (n=3 871) resulted from an outbreak (n=2 432 cases) during the fourth quarter of 2013, it did not dramatically affect the average PUDG visit-to-notification time. No significant difference was noted regarding the timely reporting by private and public PUDG, since 69.6% and 71.2% of cases of each type were reported within 1 day, respectively (data not shown).
Surprisingly, 12.9% of the DENV infection case reports were subsequently completed or modified, resulting in an average case report completeness of 16.7 days (SD = 38.5; range: 0-409 days).
Completeness (Data quality)
One or more of the following variables was absent from more than 25% of the case forms: a) PUDG type, b) whether the patient knows that s/he had dengue before, c) whether relatives or friends were infected in the previous 15 days, or d) treatment facility level. In addition, some minor discrepancies between the numbers of cases reported by the Barranquilla NDU and the NIH were found.
Positive Predicative Value
An average of 58.79% (4 771/8 115) of the cases were confirmed by a positive IgM-capture ELISA during the five-year (2009-2013) period, while only 0.58% (47/8 115) were confirmed through an epidemiological nexus/ link (Table 2).
Despite a DENV epidemic occurring in the final quarter of 2013 that involved 2 432 patients, which increased the cases to the highest annual incidence, that year had the lowest PPV (50.2%) of the five-year study period.
DISCUSSION
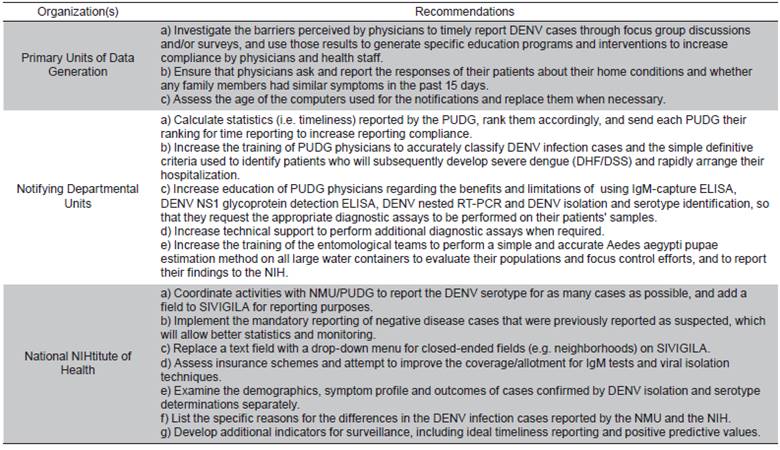
To our knowledge, this is the first study to evaluate a national DENV surveillance system and suggest recommendations. To control DENV disease in the population, physicians should accurately and promptly diagnose and treat patients, while mosquito vector populations must be effectively reduced. These study results showed that additional measures are required to: a) determine the DENV serotypes in the patients and whether they were related to primary or secondary DENV infections, b) correctly request additional laboratory tests, and c) coNDuct more exhaustive interviews to DENV-infected patients about their potential epidemiological links. Further comments and recommendations concerning the components of the Barranquilla DENV surveillance system are.
Simplicity
Simplicity relates to both the structure and the ease of operation of the surveillance system 13. In this regard, the PUDG staff reported cases via telephone as well as using a form that was later submitted to SIVIGILA. The additional work that implied collecting case data on a paper form and transferring it to the SIVIGILA electronic system may account for the lack of compliance. During this study, a NDU staff member spent up to three days downloading all of the PUDG forms to be sent to the NIH, which was, of course, a particular concern. Consequently, PUDG physicians were advised to directly enter the data for each DENV-infected patient in an electronic format at the beginning of the notification process (Cuadro 1).
Flexibility
The DENV system can process a large number of cases, which was considered a very valuable attribute 11, as demonstrated by its ability to deal with the dramatically increase of DENV infection cases in 2013. However, the lowest PPV for this five year study period probably resulted from the increased numbers of false positive cases that presented during that DENV outbreak.
This SIVIGILA system is also used to report other notifiable diseases and is flexible and rapid enough to incorporate the recently introduced chikungunya virus reports by sending a downloadable update to all of the PUDG and NMU to update SIVIGILA.
Timeliness
It has been suggested that the timely visit-to-notification of cases process should be evaluated regularly 21. A 1-day visit-to-notification of DENV infection cases allows for a faster identification of impending DENV outbreaks, so that appropriate control/preventative programs can be quickly implemented. Because the PUDG physicians reported an average of only 70.6% of cases to the NMU within 1 day of visit to the health care facility, further improvement is urgently required.
Furthermore, reporting only 5.25% of these DENV infection cases one week after consultation was deemed unacceptable. In consequence, NMU should, on the one hand, perform regular DENV infection case visit-to-notification assessments for each of the PUDG, rank them according to their performances, and disseminate the results to encourage improved performances, and on the other, use focus groups or surveys to investigate the barriers perceived by PUDG physicians to timely report DENV infection cases and employ those results to establish educational programs to increase 1-day compliance (Cuadro 1).
Completeness/positive predictive value (Data Quality) The Colombian NIH has clearly stated that it is essential to provide certain case variables to SIVIGILA 12. Therefore, it is also essential for the PUDG physicians to provide accurate and complete data for each DENV infection case. With that in mind, this study found four concerns.
Firstly, the correct spelling and complete entry of the neighborhood names of the place of residence of each DENV case varied dramatically and required excessive data cleaning to compare DENV infection case frequencies by neighborhood. A consistent neighborhood classification coding system done using a drop-down menu in the electronic SIVIGILA notification form is extremely necessary to avoid confusion (Cuadro 1).
Secondly, the low PPV obtained in this study indicated that improvement in DENV-infected case confirmation and/or reporting is pressingly required. It was previously shown that up to 35% of the patients who experienced secondary DENV infections in Barranquilla did not produce detectable DENV-specific IgM antibodies during the entire (acute and convalescent) disease period, and most cases identified during DENV outbreaks were caused by secondary DENV infections 14. Accordingly, many of the IgM ELISAS used for patient confirmation might have produced false negative results, and some false positive DENV infections might have also been obtained in some patients who, instead, developed DENV infections weeks to several months earlier 14. To circumvent these issues, serum samples can be subjected to RT-PCR to either confirm the presence of DENV RNA, to a secreted DENV nonstructural-1 (NS1) glycoprotein detection assay 22 or to DENV isolation using mosquito cell-lines and specific MAbs 14,15.
Alternatively, paired serum samples, collected from suspected DENV-infected patients 2-14 days apart, could be used to confirm a rise in the DENV-specific IgM or IgG titers (14, 15). While these additional methods would dramatically increase the PPV of the cases in the PUDG, there may be limitations due to time, required expertise, and human and financial resources. Hence, a central resource to perform these assays is essential to increase the PPV. In this regard, the NMU should provide additional technical support to the PUDG by performing these assays in the central NMU facility, but this would require additional technical support and NIH funding (Cuadro 1). Moreover, the NMU and the NIH should investigate the subsidization or complete funding of these DENV laboratory tests through patient insurance.
The DENV serotype in each case can be determined using a DENV nested RT-PCR or isolation and identification with DENV serotype-specific monoclonal antibodies 14,15. Because some strains of each DENV serotype have different pathogenic potentials 15, it is also advisable to identify which strains produce severe symptoms and focus control efforts to avert potential severe dengue/ DHF/DSS outbreaks (Cuadro 1).
Finally, it is highly advisable that PUDG physicians report the number of suspected cases that had negative confirmation test results, which would greatly aid the statistical analyses performed by the NIH.
Stability
This DENV infection case reporting system relies on phone calls and SIVIGILA forms and was found to be reliable because the PUDG and NMU staff noted that the SIVIGILA software did not present frequent failures or errors. However, some of the computers used to report disease cases via SIVIGILA were more than five years old and had potential issues with their processing abilities. Consequently, the computers used at all levels of the SIVIGILA disease reporting system should be inspected and replaced when required (Cuadro 1). In addition, the NIH should determine why there are discrepancies between the cases reported by the NDU and the NIH (Cuadro 1).
Control Response
It is urgent that the NIH and NMU provide simple and definitive clinical criteria for use in DENV infected patients during the early acute phase (<72 hours of fever), so the PUDG physicians can promptly identify the few patients who will later develop severe dengue/DHF/ DSS. These patients can be timely hospitalized and treated to lessen or prevent the onset of severe forms of the disease 15 (Cuadro 1).
Even though this surveillance system provides the location of a case by time and space, it is known that dengue transmission does not occur necessarily at the place of residence. Furthermore, the problems of reducing the impact of this urban vector-borne disease in Barranquilla and elsewhere rely on the known deficiencies in the design and implementation of vector control programs.
Aedes aegypti populations could be better determined in the pupal stage, that is, the last aquatic stage, rather than larvae surveys in local and international evaluations 23-24. Because large domestic water-storage containers were found to be their main breeding sites (up to 98%), a simple, rapid, robust and accurate single water-surface sweep estimation method coupled with calibration factors was developed 27,24. This method was suitable to rapidly and accurately estimate the pupae numbers without any sediment disturbance in all types of water (20 liter to multi-1 000 liter) containers at different water levels 24. Considering its importance for identifying productive breeding sites to coNDuct interventions to lessen or prevent DENV, as well as chikungunya and Zika virus outbreaks, that method should be implemented by the NMU and performed by local health technicians/field inspectors. In addition, the results should be reported to the NIH, so that hot-spot neighborhoods can be identified for targeted interventions (Cuadro 1).
The recommendations for the PUDG, the NMU and the NIH suggested to be included in the Colombian DENV case surveillance, based on the observations from this study, are fully presented in Cuadro 1 ♠