For many years, in epidemiology and public health, asymptomatic carriers have been defined as those people who behave like a vector: they transmit the disease; however, they may or may not develop signs and symptoms of the event that they potentially transmit. Therefore, they can do it during the incubation period, during convalescence or post-convalescence 1. As Gordis refers, a carrier is a person who has the infectious agent, but is not sick, nor have evidence of signs and symptoms of clinical manifestations. They can infect other people. A healthy carrier can have a limited duration or it can be chronic, in that sense it can last days, weeks, months or in rare cases, years 2.
There are not many diseases or cases in which asymptomatic carriers have been described, such as Hepatitis A, Typhoid Fever, among others. The case of Mary the Typhoid is very famous, a woman of British origin who is credited with at least three deaths and fifty people who fell ill with Typhoid Fever in New York City at the beginning of the last century 3,4. The asymptomatic carrier is challenging for public health and health services because, as with Mary, it can trigger an outbreak at a certain time and place.
Since December 31, 2019, the world has undergone changes in several areas. The first days they failed to identify the origin of the outbreak. On January 7,2020, the causative agent, a virus of the Coronaviridae family, was identified. The genetic sequence of the virus was shared on January 12,2020, called SARS-CoV-2 5-8. From the first month of starting COVID-19, it began to spread to other countries on the Asian continent and then to other continents.
The COVID-19 situation is very complex, since they have been reported from acute, pre-symptomatic and asymptomatic carriers to people with mild, moderate, severe and fatal symptoms 9,10 The former behave as vectors, which is a challenge for the detection of cases, isolation, follow-up, contact management, and in general all the epidemiological control that must be done with the health services of a territory 11.
COVID-19 has been the largest pandemic in the past 100 years. Despite the efforts made by the world scientific community, there is still no definitive cure. Due to the easy spread of the virus, multiple global questions remain to be answered, and within them is the role of people who transmit the virus without manifesting symptoms, and who, as previously mentioned, can be classified as asymptomatic and presymptomatic. The first are those who, even when carrying the virus, never develop the disease, that is, they remain completely asymptomatic during the course of the infection, and never manifest symptoms in any apparatus and system; presymptomatic patients transmit the pathogen agent a few days before symptoms begin 10,12.
This quick review of the literature had two objectives. First, to describe the epidemiological and sociodemographic characteristics of the asymptomatic carriers that have been reported, and to review the strategies used for the potential diagnosis and control of transmissibility at the family and community level.
METHODS
A systematic review approach was used. This, under the premise of including rationalized efforts to identify, organize/map and synthesize the findings 13,14. As a first measure, a process was carried out of translation into the documentary language and identification of a standard question format that responded to the information needs that guided this review. Consequently, the following questions were constructed: What are the epidemiological and demographic characteristics of people diagnosed with COVID-19 who remain asymptomatic during all the disease? What are the best strategies for diagnosis, prevention and control of the transmissibility of COVID-19 by asymptomatic people?
Eligibility criteria
Eligibility criteria inclusion were considered all studies published from January 1 to June 26,2020, conducted in humans, who remained asymptomatic throughout the clinical course of COVID-19. We searched for registries without methodological or language restrictions, the results of which focused on the clinical and sociodemographic characteristics of this population and/or the diagnostic or prevention and control strategies used to identify and reduce transmissibility. Records whose documentary type corresponded to letters to the editor, abstracts, editorials or errata faith were excluded.
Information sources
We considered some descriptors can be seen in table 1. These descriptors configured search equations adapted to next bibliographic databases (BDB): PubMed, Ovid, Scielo , Ebsco, Scopus, LILACS, Epistemonikos and Embase. Additionally, technical documents and reverse search in selected articles were considered (Table 1).
Table 1 Example of search equation, according to bibliographic databases
| Bibliographic database | Search equation |
|---|---|
| PubMed | ((((((asymptomatic[Title/Abstract]) OR “asymptomatic transmission”[Title/Abstract]) OR “Carrier State”[Title/Abstract]) OR “healthy carriers”[Title/Abstract])) AND ((“COVID-19”[Title/ Abstract]) OR “SARS-CoV2”[Title/Abstract])) AND diagnosis [Title/Abstract] |
Source: Own construction of the team of researchers.
Study selection
Four reviewers with postgraduate training in epidemiology and public health participated in this review. Through the application for the screening stage 15, peer re-viewers (jm, ca, fp) independently assessed the titles and summaries of the searches, depending on whether the included findings were relevant to the two objectives put forward. In the records without agreement, a third (sm) reviewer settled the conflicts. Upon passing the screening phase, in the eligibility stage a full-text records eligibility matrix was designed in excel to identify the articles to be evaluated in full text, selecting the records that had all the inclusion criteria considered in the review and the components of the guiding questions. This matrix was in-dependently completed for four groups of articles, one for each reviewer, and subsequently validated by consensus by all reviewers. Finally, when assessing the risk of bias of the selected studies, the articles that were subject to data extraction were included.
Data item and collection process
In consensus, the reviewers designed and piloted an information extraction matrix (excel) consisting of four fields.
Risk of bias in individual studies
Five criteria to establish the risk of bias were identified and weighed: absence of bias in the selection of participants, quality of the data record, absence of conflicts of interest or clear reporting of funding sources, absence of publication bias, and compliance with criteria of content and/or structure of the manuscript. The totality of articles selected for data extraction was divided into two groups, each of which was assigned to two pairs of reviewers, who independently rated each record and agreed the articles that ultimately formed part of the synthesis of findings of this review. The consistency of this stage was evaluated by the reviewers who have clinical and epidemiological experience.
RESULTS
Study selection
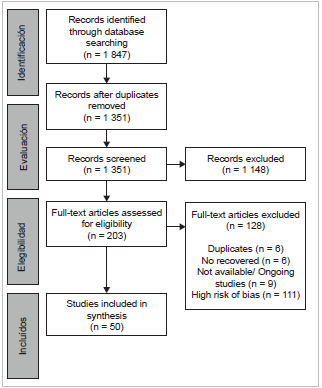
The literature search for this literature review included the different electronic BDBS reported in the methodology session. The search ran from January first to June 26-2020. It was carried out in various stages as shown in figure 1. In the identification stage, the keywords shown in table 1 were used, and a total of 1 874 articles were found.
Of these, 523 were eliminated in duplicates, leaving 1 351, which passed to the screening stage. They underwent a review of titles and summary by pairs and resolution of conflicts by a third reviewer, finding 20 duplicates and discarding 1.128 more that did not meet the inclusion criteria. At the end of this phase, 203 articles remained.
In the next stage, a full-text peer review was performed, 128 did not meet inclusion criteria, five were duplicates, two were not retrieved by language, and nine were excluded because they corresponded to research proto-cols. At the end of this phase, 59 articles remained, which went to the inclusion stage for quantitative synthesis and extraction of specific information. Researchers peer-re-viewed the full texts of records deemed eligible for inclusion; any discrepancies were resolved through discussion and consensus. In this phase, 1 duplicated and 8 with low methodological quality and high risk of bias were excluded, therefore leaving 50 articles (Figure 1).
Study characteristics
The vast majority of studies 37 were case studies and case series, one was a cross-sectional study, seven cohort studies (mostly retrospective) and five were systematic reviews (two with meta-analyses). They were published in indexed journals with high academic impact, with participants and researchers from countries such as China (n=25), the USA (n=6), Japan (n=3), Italy (n=3), and South Korea (n=3).
Synthesis of results
As described in the methodology, it was reviewed and verified that in the reviewed articles, asymptomatic people did not become symptomatic during the follow-up period. Of the 50 articles included, all showed at the end persons who fulfilled this characteristic; on average, the articles showed 73 participants, ranging from one to 878 participants. However, two-thirds of the studies had up to 50 participants. Regarding the sex of the participants, 39 of the articles reported both sexes, four reported only women, seven reported only men, and one did not report this variable. Regarding the population, about 23/50 of the articles reported adult population, thirteen reported a mixed population (adult and pediatric), eleven pediatric populations, three in pregnant women.
Six studies reported a history of diabetes, and seven reported hypertension history. Regarding the epidemiological designs that supported these studies, 28/50 studies selected were case series, five were systematic reviews, seven were cohort designs, and the rest were cross-sectional studies. The information gathering techniques were mostly through electronic clinical records, four reported face-to-face interviews. Most of the studies carried out descriptive analysis, that is, they presented reports with absolute and relative frequencies; few studies presented comparative analysis and/or bivariate tests or with statistical significance processes. In terms of prevention measures to avoid contagions, as individual measures, most of the studies do not mention the use of masks, three of the studies do mention it. Only three studies made mention of promoting healthy lifestyles. 13 studies shown basic supportive treatment and antivirals were administered to asymptomatic patients, the rest were not given treatment, they were only under observation. In 20 studies, they mention the identification of close contacts and testing (RT-PCR), and the follow-up of the contacts by telephone calls. In 22 studies, they mentioned the compulsory social isolation of asymptomatic (Table 2).
Table 2 Summary of studies included in the systematic review
| Authors | Countries | Population and participants | Sex | Age (range or average) | No. Part* | Study design | Co-morbidities | Main control and prevention measures |
|---|---|---|---|---|---|---|---|---|
| Lan, L.; et.al.16 | China | Children | Females/ Males(F/M) | 7 - 13 years old (y-o) | 4 | Case series (CS) | Non reported (NR) | Hospital isolation. Follow-up to close contacts and taking second test after 15 days. |
| Bai, K.; et.al.17 | China | Children | F/M | 11 y-o | 8 | CS | NR | Pharmacological treatment with interferon/ribavirin |
| Lee, Y.H.; et.al.18 | South Korea-SK | Both | F/M | 40,6 y-o | 371 | CS | Diabetes Mellitus (DM), High Blood Pressure (HBP) and Obesity | Preventive Isolation-PI, second test taken after 15 days. To declare a patient's recovery, two consecutive negative tests in 24 hours. |
| Lombardi, A; et.al.19 | Italy | Adults | F/M | 44,5 y-o | 17 | CS | NR | PI, identification of close contacts and testing** |
| Kronbichler, A; et.al.20 | Austria | Both | F/M | 31 y-o | 506 | SR-MA | NR | PI and monitoring of patients. |
| Khoury, R; et.al.21 | USA | Pregnancies | F | 18 - 47 y-o | 102 | Prospective cohort study- PCS | NR | Use of mask, PI and identification of close contacts and testing** |
| Patel, et.al.22 | USA | Adults | F | 82 y-o | 13 | Retrospective cohort study- RCS | NR | Use of mask, PI, second test and identification of close contacts and test** |
| Liu, Z.; et.al.23 | China | Adults | F/M | 40 y-o | 131 | PCS | NR | Identification of close contacts and testing** after 14 days. |
| Tabata, S.; et.al.24 | Japan | Adults | F/M | 45 - 75 y-o | 33 | Retrospective and descriptive study-RDS | DM and HBP | NR |
| Du, H.; et.al.25 | China | Children | F/M | 6 y-o | 24 | RCS | NR | Identification of close contacts and testing** after 14 days. Isolation in hospital |
| Zhang, L; et.al.26 | China | Children | F/M | 1 day to 17,5 y-o | 117 | SR-MA | NR | Carrying out of the test **after 14 days. Pharmacological treatment with interferon. |
| Alsofayan; Y.M.; et.al. 27 | Saudi Arabi | Both | F/M | 36 y-o | 142 | RDS | DM and HBP | PI. |
| Parri, N.; et.al.28 | Italy | Children | F/M | 6 y-o | 17 | CS | NR | NR |
| Wang, Y.; et.al-29 | China | Both | F/M | 39,3 y-o | 63 | RDS | DM and HBP | Pharmacological treatment with some oral antiviral with inhalation of interferon. To declare the recover y of a patient, two consecutive negative tests in 24 hours and a chest tomography with normal findings. |
| Zhen-Dong,Y.; et,al. 30 | China | Children | F/M | 7 y-o | 77 | Sistematic review-SR | NR | NR |
| Song, W.31 | China | Children | F/M | 11,5 months to 14 years | 8 | CS | NR | Second test**, symptomatic treatment, antipyretics, antivirals, traditional Chinese medicine. Follow-up to hospitalized patients. |
| Zhu, J.; et.al.32 | China | Both | F/M | 21 - 56 y-o | 878 | SR-MA | NR | Identification of close contacts, monitoring and PI. |
| Wang; L.; et.al.33 | China | Adults | M | 54 and 55 y-o | 2 | CS | NR | Second test**, quadruple treatment, antivirals. Follow-up to hospitalized patients. |
| Rivett; et.al. 34 | United Kingdom-UK | Adults | F/M | 34 y-o | 17 | CS | NR | Second Test**, telephone monitoring, identification of close contacts and testing** |
| Ujjan, I.; et.al.35 | Pakistan | Adults | F/M | 57,8 y-o | 343 | CS | NR | Second test**, follow-up of hospitalized patients and PI. |
| Chau, N.V.V.; et.al. 36 | Vietnam | Adults | F/M | 30 y-o | 13 | CS | NR | Second test**, education, in healthy lifestyles, telephone monitoring and PI. |
| London, V. et.al.37 | USA | Pregnancies | F | 30,5 y-o | 22 | RCS | NR | Second test** and education in healthy lifestyles. |
| Uechi, T.38 | Japan | Adults | F | 64 y-o | 1 | CS | HBP | Test** control and PI. |
| Wu, J.39 | China | Adults | F/M | 47,5 y-o | 9 | CS | NR | Test**s and identification of close contacts and test** |
| Jones, N.K.; et.al.40 | UK | Adults | F/M | 36,5 y-o | 8 | RDS | NR | PI. |
| Fusco, F.M.; et.al.41 | Italy | Adults | F/M | 43 y-o | 3 | Cross sectional study-CSS | NR | Use of mask and second test** 6-7 days after the first. |
| Cheung, Z.B.42 | USA | Adults | F/M | 67 - 90 y-o | 8 | RCS | DM and HBP | Pharmacological treatment with azithromycin, hydroxy-chloroquine, supplemental oxygen. |
| Wan, R.; et.al.43 | China | Adults | F/M | 36 - 45 y-o | 2 | CS | NR | Use of masks between 14 and 25 days. Pharmacological treatment. |
| Arons, M.M.; et.al. 44 | USA | Adults | NR | 78,6 y-o | 3 | CSS | NR | NR |
| Jiang, X.L.45 | China | Both | F | 0 - 53 y-o | 2 | CS | NR | PI. Phylogenetic analysis of surface samples such as rooms, kitchens and bath-rooms. |
| Castaganoli, R.; et.al. 46 | China | Children | M | 10 - 19 y-o | 3 | SR | NR | NR |
| Meng, H. et.al.47 | China | Adults | F/M | 40 y-o | 22 | RDS | DM and HBP | NR |
| Tan, Y.P.; et.al.48 | China | Children | F/M | 6 months | 2 | RDS | NR | Hospital isolation. |
| Albano, D.; et.al.49 | Italy | Adults | F/M | 64,5 y-o | 5 | PCS | Cancer | Three patients with PI for two weeks with daily temperature measurement. Two patients received pharmacological treatment. |
| Ji, t.; et.al. 50 | China | Both | F/M | 49 y-o | 50 | CS | NR | Follow-up, second test taken after two weeks and identification of close contacts. |
| Samsami, M.; et.al. 51 | Iran | Adults | F/M | 49,7 y-o | 6 | CS | NR | NR |
| Lu; S.52 | China | Adults | F/M | No report | 3 | CS | NR | Use of mask for one week, at the end of the control test**. Pharmacological treatment. |
| Qian, G.; et.al. 53 | China | Both | F/M | 13 months - 60 y-o | 2 | CS | NR | Control test** after 15 days, identification of close contacts and testing **and PI. |
| Tian, S.; et.al.54 | China | Both | F/M | 47,5 y-o | 13 | CS | NR | Identification of close contacts and testing** and PI. |
| Hu, Z.; et.al.55 | China | Both | F/M | 14 y-o | 19 | CS | DM and HBP | Negative control test** up to 28 days. Education in healthy lifestyles. Identification of close contacts, testing and PI. |
| Du, W.; et.al. 56 | China | Children | F/M | 41,4 y-o | 8 | CS | NR | All the patients were in a hospital environment, so no specific control measures. |
| Park,S.J.; et.al.57 | SK | Adults | NR | 38 y-o | 4 | CS | NR | Control test** after 14 days, identification of close contacts, testing, telephone monitoring of close contacts and PI. |
| Luo, L.; et.al.58 | China | Both | F/M | 37 y-o | 5 | v | NR | Identification of close contacts and testing** and PI. All the contacts were in a hospital environment, so no specific control measures. |
| Mizumoto, K.; et.al. 59 | Japan | Both | F/M | 0 - 90 years old | 328 | CS | NR | Identification of close contacts, testing and PI. |
| Dong, Y.; et.al.60 | China | Children | F/M | 7 y-o | 94 | CS | NR | Identification of close contacts and testing **and PI. |
| Ki, M.61 | SK | Both | F/M | 42 y-o | 3 | CS | NR | Use of mask for two weeks. Identification of close contacts, testing** and PI. |
| Dong, X.; et.al.62 | China | Adults | M | 26 y-o | 1 | CS | NR | Control Test** after 14 days, identification of close contacts and testing **and PI. |
| Danis, K.; et.al.63 | France | Adults | M | NR | 1 | CS | NR | NR |
| Breslin, N.; et.al.64 | USA | Pregnancies | F | 29,7 y-o | 7 | CS | Obesity | Use of mask for two weeks. Control test**. Identification of close contacts, telephone monitoring and testing** |
| Yongchen, Z.; et.al. 65 | China | Both | F/M | 25 y-o | 5 | CS | NR | Control test**. |
* Number of participants;** Refers to (SARS-CoV2-RT-PCR).Source: Own construction of the team of researchers.
DISCUSSION
3 525 asymptomatic people were identified in this study (average 37,1 years old; age range: 0,5 to 82 years old). No mortality was reported. Except for one study that used serological tests 39, diagnosis in asymptomatic patients was made with positive reverse transcription polymerase chain reaction (SARS-COV2-RT-PCR) tests. Similarly, recovery was determined in most studies with one or two consecutive RT-PCR tests with negative results and normal chest tomography patterns. Positive control tests were observed after 7 to 24 days, with respect to the confirmatory diagnostic test.
Medical history of asymptomatic people diagnosed with COVID-19, less than 20% of the studies reported comorbidities such as high blood pressure, diabetes mellitus or obesity. An important group reported tomographic alterations presumptive of pneumonia, without symptoms and/or alterations evidenced by chest X-rays. The tomographic alterations coincide with that reported by some authors 66 who found that in 36 patients, of 4 studies in which the results of the chest tomography were available, 15 (41,7%) had abnormal findings with bilateral involvement and 14 (38,9%) unilateral.
Compared to the findings in immunological or blood chemistry tests, increased values of C-reactive protein, lactic dehydrogenase were evidenced; and lymphopenia or leukocytopenia; which is consistent with the findings of other researchers 17,6.
Although this review does not report results related to the effectiveness of the control measures, isolation and follow-up of cases and close contacts, there are diverse experiences and literature that records that where these measures have been applied, has led to a decrease in the transmission of the infectious agent within the susceptible population. Its non-application in a systematic and timely manner has shown the risks and consequences in the elderly and in people with comorbidities, as happened in the United States, Brazil, Mexico, India or Colombia.
On the contrary, its application in a continuous, systematic, early, and generalized way, even in densely populated countries such as Vietnam or Taiwan, has caused the transmission speed to decrease due to the detection and isolation of cases and contacts, and therefore the risks of transmission, morbidity and death have been reduced, as has also occurred in other countries such as Canada, New Zealand and some European countries 67-70.
Evidence also shows that, in asymptomatic patients, the viral load is similar to that of symptomatic patients. If these asymptomatic patients are not diagnosed, are not isolated, protection measures are not taken, they can potentially infect other people, so it is suggested to carry out tests that allow the detection of asymptomatic cases, to improve the strategies for control of COViD-197,53.
Limitations in the body of evidence
The studies selected in this review had several limitations. Many had a small sample size: 33 of 50 studies had between 1 and 20 participants, limited by the representativeness of the results presented. Additionally, the studies carried out in pregnant women had limitations related to the nonspecific reporting of findings in paraclinical tests and symptoms that could well be related to COVID-19 or infectious pathologies typical of pregnancy.
On the other hand, thirty of the fifty articles were case series, where most of them had basically descriptive analysis. Consequently, the measures and the results reports were heterogeneous, which limited the performance of a meta-analysis process of the information extracted.
Limitations of the review
The COVID-19 pandemic has represented a challenge for humanity, which has sought to seek alternatives for the prevention, control, mitigation and eradication of the disease. This has led to an 'explosion' of information from different parts of the world, which seeks to describe or propose alternatives for action in the face of the pandemic. Much of this information has a risk of bias or limitations of external validity, related to the availability of health technologies or the configuration of the health systems and services of the countries where the studies were carried out.
As a limitation of this study, a group of records was divided and screened by each of the reviewers independently. However, in order to overcome the risk of bias, in the eligibility and extraction phase, peer review and group consensus of the articles individually selected by the reviewers were favored.
Additionally, due to the fact that studies whose target population was asymptomatic were privileged, it led to the consolidated information having a high degree of heterogeneity, as stated previously, consequently, the possibility of analyzing aspects such as the basic reproduction of vi-ruses, the mean incubation time, infection rate, seroprevalence, and case fatality rate for COVID-19 in asymptomatic people; as happened with the study of certain researchers 71 who obtained such information from a group of studies that analyzed these aspects, but were directed to the symptomatic and asymptomatic population. On the other hand, this evidence synthesis did not report findings on the fecal-oral transmission 72 of the virus or on the average recovery time in the population object of this review. Nor did it identify the viral load of asymptomatic patients 73, nor how it was previously reported. Other variables of community transmission of COVID-19 were specifically recorded, such as screening strategies 74 and tracking strategies for this population 75 ♠