Introduction
Breast cancer is the most common tumor in the female population, affecting one in eight women, and the second leading cause of female death after lung cancer1. Axillary lymph node involvement is the main prognostic factor because it is the primary route of dissemination of cancer. Lymph node removal (known as LA) is also the primary cause of morbidity, leading to physical complications, including lymphedema and seroma, and the psychological distress that they cause.
These complications have discouraged the use of lymphadenectomy (LA) in patients with negative sentinel lymph node biopsy (SLNB), isolated tumor cells (ITCs), or micrometastases as well as those with positive SLNB who underwent conservative surgery (CS) according to the American College of Surgeons Oncology Group (ACOSOG) Z0011 study criteria2-3. However, LA in mastectomized patients with micrometastatic SLN is common despite the trial After Mapping of the Axilla: Radiotherapy or Surgery? (AMAROS)4-5.
Therefore, debate persists about whether LA instead of axillary radiotherapy (ART) (as proposed by the AMAROS trial) is ethical in patients with cT1-T2 cN0 breast cancer who underwent CS and radiotherapy (RT) or mastectomy in cases in which the probability of recurrence and overall survival is similar between LA and ART according to the evidence of this trial, but the morbidity of ART is lower than that of LA4-5. cT1-T2 patients are those whose tumor size is less than 2 cm or 5 cm, respectively. cN0 are patients that clinical axilla is negative1.
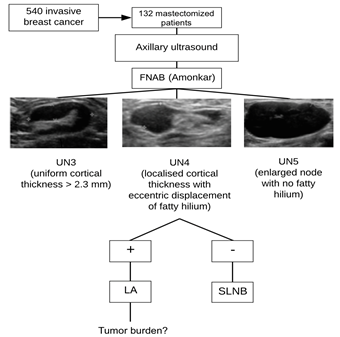
Amonkar et al6. classified the degree of suspicion of lymph node involvement into six categories based on ultrasonography findings as follows (from lowest to highest): UN2, normal (peripheral and uniform cortex, thickening <2.3 mm, and a fatty central hilum); UN3, indeterminate (uniform cortical thickening >2.3 mm); UN4, suspicious (focal cortical thickening >2.3 mm, eccentric, with displacement of the fatty hilum and cortical vascularization on color Doppler); and UN5, replaced (generalized cortical thickening without a fatty hilum)6. In the presence of signs of suspicion (nodes UN3, UN4, UN5), ultrasound-guided FNAB is indicated to assess the presence of metastatic infiltration.
In a previous case series, combined ultrasonography and preoperative FNAB identified 50% of the axillary metastases with high specificity and a low false-positive rate, allowing the execution of LA in cases in which lymph node involvement was confirmed7.
The intraoperative diagnosis of SLNs (also known as SLNB) in patients with clinically or radiologically negative axillary lymph nodes dictates axillary management.
SLNs were initially detected by hematoxylin-eosin staining until the development of the One Step Nucleic Acid Amplification (OSNA) method, which allowed analysis of the CK19 mRNA in 30 min, reducing the need for secondary surgeries1.
The OSNA coined the term “total tumor burden”, which is measured by the sum of the number of copies of the CK19 mRNA in each analyzed SLN and is considered low and high risk when the number of copies is <15,000 and >15,000, respectively1.
Micrometastases are those whose size ranges between 0.2 and 2 mm (equivalent to 250-5000 copies of CK19 mRNA/μl, based on the OSNA method), whereas isolated tumor cells (CTAs) are those with a smaller size (less than 250 copies of CK19 mRNA/μl). In contrast, macrometastases are greater than 2 mm in diameter (>5000 copies of CK19 mRNA/μl)1.
Several clinical trials were performed to optimize axillary management in breast cancer. The Z0011 study of the ACOSOG led by Giuliano2-3 revolutionized the treatment of breast cancer. This multicenter study evaluated the impact of LA on the survival of patients with breast cancer metastases using SLNB. The non-inferiority study was conducted in patients with invasive (cT1-T2) breast cancer without clinically palpable lymph node metastases (cN0), and with indication for CS and subsequent systemic therapy (adjuvant chemotherapy and radiotherapy). One or two metastatic SLNs on biopsy (considered a low tumor burden) were recruited and randomized into eligible or not eligible for LA. SLNs with ITCs and micrometastases were considered negative on biopsy. The study concluded that there were no significant differences in survival or axillary recurrence between SLNB and LA in this patient group2.
Subsequently, the European Organization of Research and Treatment of Cancer launched the 19801 AMAROS trial, which evaluated the non-inferiority of LA to ART in terms of 5-year recurrence of patients with cT1-T2cN0 breast cancer and positive SLNB (one or two positive SLNs). The trial concluded that LA and RTA with positive SLNB provided excellent and comparable axillary management in this patient group; the morbidity of RTA was lower than that of LA; and the rate of lymphedema in the first and second groups was 13% and 6%, respectively4-5.
The inclusion criteria were cT1-T2cN0 breast cancer treated by CS and RT or mastectomy (this group was not included in the ACOSOG Z0011 study) with or without RT of the chest wall. The results indicated that: axillary recurrence occurred in 0.43% and 1.19% of the LA and ART group patients respectively, there were no statistically significant differences in overall survival and disease-free period between the two groups, and the 5-year incidence of lymphedema was significantly higher in patients treated by LA (13%) than in patients treated by ATR (6%)4-5.
The second principle is beneficence8, which is based on the search for the maximum benefit to the patient and the reduction in potential damage according to the non-maleficence principle (primum non nocere) of the Hippocratic Oath. Our study is based on this principle. The application of the AMAROS trial criteria intends to reduce morbidity secondary to LA compared to RTA without increasing mortality and long-term axillary recurrence.
The third principle is justice8 and states that all individuals should be treated equally.
Thus, the objective of the present study is to demonstrate the ability of FNAB to detect non-metastatic or micrometastatic SLNs (cN0) in patients with indications for mastectomy and avoid LA based on evidence from the AMAROS trial.
Materials and methods
Patients who underwent breast cancer surgery in our unit were retrospectively evaluated from June 2011 to December 2014. Patient data were collected until this date and not until 2018 because the criteria from the ACOSOG Z0011 trial were adopted in our center. The execution of LA was interrupted in cases involving fewer than two positive SLNs since the patients met the trial criteria. Nodes with ITCs and micrometastases were considered negative according to St Gallen criteria (2009)9.
The inclusion criteria were: a total of 132 consecutive patients with a diagnosis of infiltrating breast in whom mastectomy was indicated cancer were selected in our unit.
The exclusion criteria were:
Conservative surgical treatment.
Patients with extranodal metastatic cancer at baseline (M1).
Patients diagnosed and/or treated at another health center.
Patients who received neoadjuvant chemotherapy (NC) (patients at stages cT3-T4/cN2-N3 or cT2cN0-N1 who could not undergo surgery because of small breast volume or the limited benefit of NC because of their molecular profile).
63 out of the 132 patients who met the AMAROS trial criteria were selected:
The study population was recruited consecutively at the indicated time periods. These patients were referred to our center by the Breast Cancer Screening Unit of the region of Murcia, Spain, and included: (i) women with suspected lymph node metastases, (ii) symptomatic women referred by other hospitals or other health centers (patients with palpable breast masses, nipple retraction, or skin inflammation), (iii) patients evaluated in our center because of a family history of breast cancer, or (iv) patients observed in our center for other reasons.
Each patient underwent a complete mammographic examination and ultrasonography of each breast. Ultrasonography was performed bilaterally in the breast and axilla with evaluation of the axillary levels I, II, and III (Berg levels) previously identified using a 12-Mhz linear probe. Ultrasound is the best technique to assess the axilla because it allows determining the relationship of the nodes with the pectoralis minor.
In axillary ultrasound, the signs of lymph node involvement were assessed according to the Amonkar criteria6 and their Berg levels were determined. Using a 17G needle, FNAB of the most distal lymph node metastases with the highest degree of suspicion was performed by a pathologist, in accordance to these criteria. Then, the pathologist confirmed whether the biopsied material was sufficient and the biopsy was repeated otherwise. Axillary FNABs were performed in clinically suspicious lymph nodes (UN3, UN4, and UN5) (Figure 1).
After tumor confirmation, bilateral breast magnetic resonance imaging was performed systematically to complete the staging (optimization of the cT parameter and assessment of multifocal involvement or multicentric involvement were applied to evaluate metastases in the contralateral axilla and internal mammary node chains).
Based on these observations, LA and SLNB were indicated in cases of positive and negative FNAB, respectively. The tumor burden (number of affected lymph nodes) was evaluated after LA in patients with negative FNAB and positive SLNB. The presence of two or more positive SLNs on SLNB was considered as high tumor burden.
A descriptive study was performed by assessing the internal validity or diagnostic accuracy (sensitivity [sn] and specificity [sp]) and external validity (positive predictive value [PPV] and negative predictive value [NPV]) of positive FNAB for detecting metastasis in SLNs.
The characteristics of the total mastectomized and AMAROS populations were analyzed using qualitative and quantitative variables (Tables 1 and 2).
Ethical considerations
The study was approved by the Research Ethics Committee of the General University Hospital Morales Meseguer in Murcia, Spain. In reference to bioethical implications in axillary management in breast cancer, the first ethical principle is individual respect8, which is justified by individual autonomy and the protection of subjects with limited autonomy. Both multicenter clinical trials (ACOSOG Z0011 and AMAROS) and the trial performed at our center were approved by our hospital’s Research Ethics Committee.
Table 1 Characteristics of mastectomized patients
FNAB fine needle aspiration biopsy.
SLNB sentinel lymph node biopsy.
SLN sentinel lymph node.
OSNA One Step Nucleic Acid Amplification.
IDC invasive ductal carcinoma.
ILC invasive lobular carcinoma.
DCIS ductal carcinoma in situ.
Table 2 Characteristics of AMAROS patients.
FNAB fine needle aspiration biopsy.
SLNB sentinel lymph node biopsy.
SLN sentinel lymph node.
OSNA One Step Nucleic Acid Amplification.
IDC invasive ductal carcinoma.
ILC invasive lobular carcinoma.
DCIS ductal carcinoma in situ.
Results
The sensitivity of FNAB for detecting positive lymph nodes in patients with a high tumor burden was 93% in our series, whereas specificity was 84%, PPV was 60%, and NPV was 79% (Table 3).
Discussion
Lymphedema is the most serious complication of breast cancer, affecting one in five patients. The clinical manifestations include swelling of the breast, trunk, and upper limb in the affected side as well as the impairment and deterioration of limb function, potentially leading to fibrosis. Psychological sequelae include stressful memories related to the disease, depression, and anxiety10. We found that the incidence of lymphedema was four times higher in patients treated by LA (19.9%) than in those who underwent SLNB only (5.6%)11. Some preventive measures include skin care and compression in the affected limb dressing; nonetheless, these measures do not significantly reduce the risk of limphedema development10. These comorbidities have discouraged the use of LA in patients with negative SLNB, ITCs, or micrometastases as well as in those with positive SLNB treated by CS according to the ACOSOG Z0011 study criteria. LA in mastectomized patients with micrometastatic SLNs is common despite evidence from the AMAROS trial showing axillary recurrence and overall survival being similar between LA and ART. However, the 5-year incidence of lymphedema is comparatively lower with ART.
Several factors may affect treatment choice, the first being the physician-patient relationship. Paternalism is developed by physicians who choose the medical procedure that will be performed because they believe they have the necessary expertise and know what is best for the patient according to the principle of beneficence. However, the principle of non-maleficence-primum non nocere-may be unintentionally disregarded, and the choice of LA versus RTA in the patients of this study is a good example. In the case of axillary treatment for mastectomized patients, the usual and established procedure before the publication of the AMAROS trial was LA in cases of axillary SLN involvement in patients with cN0 breast cancer and positive SLNB and patients with cN1. In many cases, the choice of the most aggressive treatment involves the use of defensive medicine. Therefore, by choosing LA, the physician ensures that the disease will be cured, even if it involves overtreatment. This situation involves a bioethical dilemma because physicians choose to safeguard their reputation without considering the consequences of the most aggressive treatment on the patient.
In addition, there is a conflict regarding the principle of autonomy. The patient is clarified via informed consent about the impending procedure. However, the physician does not always offer the possibility of RTA versus LA to patients who meet the AMAROS trial criteria considering that the most aggressive treatment is the most appropriate.
Our study evaluated the consequences of using the AMAROS trial criteria in patients undergoing mastectomy between June 2011 and December 2014. If we had recruited patients after that date (when lymphadenectomy began to be avoided due to the application of these studies), we would not have had lymphadenectomy specimens with which to compare the results and show that they could be unnecessary. These dates were chosen because, based on these principles, the ACOSOG Z0011 trial criteria were adopted in our center, and LA was not performed in patients with fewer than two positive SLNs provided that the trial criteria were fulfilled. If the patients had been recruited at a later date, we would not have had a histopathological result because these nodes would not have been resected.
In our center, mastectomy was indicated for 132 of the 405 patients diagnosed with breast cancer (32.5%), and LA was performed in 58 (44%) patients with metastatic SLNs. 43 (74.1%) of them, were diagnosed by FNAB, while 15 (25.9%) were diagnosed by SLNB after negative FNAB. Among the patients who underwent LA, 28 presented a high tumor burden; of them, 26 (92.9%) were diagnosed by FNAB, while two (7.1%) were diagnosed by SLNB. In one of the patients diagnosed by SLNB, three lymph nodes were positive in the postoperative histopathological analysis (two nodes plus one SLN), while six lymph nodes were positive in the other patient (five nodes plus one SLN). Of the 30 low tumor burden patients treated by LA, 17 (56.7%) were diagnosed by FNAB and 13 (43.3%) were diagnosed by SLNB. The AMAROS criteria were not applied to this general population. Nonetheless, if these criteria had been adopted, the 13 LA procedures could have been avoided in low tumor burden patients diagnosed by SLNB.
Therefore, the sensitivity of FNAB for detecting positive lymph nodes in the population of mastectomized patients in our series was 93%, while the specificity was 84%. With respect to the external validity variables, the probability of a high tumor burden in patients with positive FNAB was 60% (PPV), whereas the probability of a high tumor burden in patients with negative FNAB was 79% (NPV) (Table 3).
Several studies have shown the ability of ultrasound to diagnose axillary involvement compared to SLNB. They evaluated tumor characteristics and survival in patients with axillary involvement diagnosed by FNAB or SLNB12. The patients diagnosed by FNAB were older and presented palpable masses, larger tumors, higher tumor grade, lymphovascular invasion, negative hormone receptors, positive HER2, and a higher proportion of mastectomies compared to the group diagnosed by SLNB. They concluded that patients diagnosed by FNAB had fewer favorable characteristics and a worse prognosis12. Similar conclusions were made by Boone13, who assessed the tumor burden and disease stage using FNAB or SLNB and demonstrated that these parameters were worse in patients diagnosed by FNAB. The results of these two studies are consistent with those of our study, in which the diagnosis of positive SLNs by FNAB and SLNB in patients with a high tumor burden was 92.9% and 7.1%, respectively.
With regard to the ultrasound criteria used to identify clinical signs and described by Amonkar6 (FNAB before the diagnoses of nodes UN3, UN4, and UN5 in the present study), Ying Zhu et al.14 also obtained a cut-off point greater than 3.5 mm in cortical lymph node thickness for detecting metastatic infiltration with a sensitivity of 75.6% and specificity of 82.7%, which, when combined with FNAB, represented a sensitivity of 64.2% and specificity of 94.5% for detecting metastatic SLNs.
These results were obtained in the total mastectomized population without use of the AMAROS criteria but can be extrapolated to the population that meets these criteria. Of the 63 selected patients, SLNB was performed in 61; 51 and 10 of them had negative and positive SLNB, respectively. Nine of the positive cases presented a low tumor burden and one presented a high tumor burden; the latter had three positive lymph nodes in LA (one SLN and two other nodes). Therefore, if AMAROS criteria had been applied to these patients, nine of the 10 (90%) LA surgeries performed in patients with a low tumor burden could have been avoided.
Most LA procedures performed in the total mastectomized population13 could have been avoided in patients who met the AMAROS trial criteria9.
In our study, we did not assess the long-term implications of the choice of LA versus ART because it was retrospective. However, Moossdorff (2018)15 assessed the impact of the AMAROS trial on axillary management in mastectomized cT1-T2cN0 patients in 2005-2015 in their center and found that 87% of the patients underwent LA, of whom 61% were also treated by RT after mastectomy. Therefore, ART could have been used instead of LA in a considerable number of patients.
A prospective study assessed the possibility of replacing LA with ART in mastectomized cT1-T2cN0 patients16. Nonetheless, SLNB was not performed in patients who underwent post-mastectomy RT. The study concluded that LA could be replaced with post-mastectomy RT and ART in most patients with fewer than two positive SLNs16.
The morbidities of different axillary management strategies has been analyzed previously17 by comparing the function and mobility of the arm 18 months after surgery in patients treated with LA or RT. The authors found that morbidity was higher in the group treated with LA17.
Our study has some limitations. First, nodes with ITCs and micrometastases were considered negative on SLNB (according to the consensus of St Gallen (2009)9), whereas SLNs with micrometastases were considered positive using the AMAROS trial criteria. However, if the presented data are re-analyzed in the total population of patients who underwent mastectomy considering these criteria, of the 89 who performed SLNBs, 55 would be considered negative and 34 would be considered positive; of them, 32 patients would have a low tumor burden and two would have a high tumor burden. The number of patients with a high tumor burden did not change in this study, whereas the number of patients with a low tumor burden increased (32 patients whose SLNs contained micrometastases versus 13 patients whose SLNs did not contain micrometastases). These results support the AMAROS trial criteria because 32 LA procedures could have been avoided. Nodes with micrometastases were considered positive in the patients who met the AMAROS trial selection criteria. Of the 61 performed SLNBs, 41 biopsies were negative and 20 were positive; of the positive cases, 19 patients had a low tumor burden and one had a high tumor burden. Considering the positive cases, those with a low tumor burden would increase from nine to 19; therefore, 19 LA procedures could have been avoided. There were no significant differences in the number of patients with a high tumor burden.
Another limitation of the AMAROS trial is the lack of consensus regarding in which type of cT1-T2cN0 patient postmastectomy RT is more indicated. Although the meta-analysis of the Early Breast Cancer Trialist Cooperative Group showed that this procedure increased overall survival15, the American Society of Clinical Oncology/American Society of Radiation Oncology guidelines indicate that the decision regarding postmastectomy RT should be made on an individual basis15,16,18.
The AMAROS trial excluded multifocal involvement until 2008. They determined19 the SLN detection rate in patients from the AMAROS trial with multifocal and unifocal involvement. The rate of detection in the first and second groups was 96% and 98%, respectively, and metastatic SLNs were present in 51% and 28% of the patients in the first and second groups, respectively. However, the involvement of other lymph nodes was observed in 40% and 39% of the patients from these two groups, respectively, demonstrating that SLNB is safe in both patient groups. In our population, 68.2% of patients (both in the total group and the AMAROS trial group) had unifocal involvement.
Another limitation of the AMAROS and ACOSOG Z0011 trials is that most of the enrolled patients presented positive estrogen receptors and were aged >50 years (with a better prognosis in both populations). These results were confirmed in our study, in which the mean age of the patients was 59.7 years in both groups, and the most prevalent molecular pattern was luminal A (32% and 57% in the AMAROS and total patients, respectively), followed by luminal B HER 2- (11% and 30% in the AMAROS and total patients, respectively). These results violate the ethical principle of justice because these patients had an improved prognosis. However, belonging to a molecular group of higher-risk breast cancer and the presence of estrogen receptors were not exclusion criteria; these results were confirmed in our series (Tables 1 and 2). However, others have evaluated20 patients with triple-negative (the most aggressive) breast cancer and found that the 5-year local recurrence rate was low in this population and the main complication was distant recurrence.
Another factor that affects prognosis is lymphovascular involvement (involvement of the vessels and lymphatics adjacent to the tumor; therefore, an indicator of poor prognosis). In our series, this complication did not occur in 71.4% of the women who met the AMAROS criteria or 60.6% of the total analyzed population.
Another relevant factor is ART-associated morbidity. The side effects included limited shoulder motion (which was similar between LA and ART2 according to the AMAROS trial results), cardiac and pulmonary toxicity, and the possibility of secondary tumors with prolonged usage21. In this study, proton therapy was proposed as an alternative.
In contrast, the majority (77%) of patients from the AMAROS trial had a single positive SLN, while 40% had SLNs containing micrometastases or ITCs. The patients who underwent NC were excluded22, and these findings agree with our study (Table 2).
These results together with patient characteristics and the strict selection of patients who followed the AMAROS trial recommendations provide excellent evidence that LA should be avoided. First, only patients with small tumors (cT1-T2) were evaluated and, among them, those who underwent NC (usually those with a worse molecular prognosis) and had clinically negative axillary lymph nodes (cN0) were excluded. Therefore, axillary ultrasound plays a fundamental role in this patient group because it indicates disease prognosis and ultrasound can detect the clinical stage N0, indicating the importance of this examination for identifying patients with non-metastatic and micrometastatic SLNs.
However, despite this evidence, there is no consensus regarding axillary surgical management. For instance, a group evaluated23 compliance with the clinical guidelines of the ACOSOG Z0011 trial using a survey of 488 surgeons who treated 5,080 early-stage breast cancers between 2013 and 2015. Their study concluded that there were considerable variations in compliance with significant overtreatment (49% and 63% of surgeons would recommend LA in the presence of one and two micrometastatic SLNs, respectively). This study is a clear example of the adoption of paternalism. Most surgeons prefer overtreating over performing a lower-morbidity technique such as ART24,25.
Conclusions
Lymph node involvement is the main prognostic factor and primary cause of morbidity in breast cancer.
LA has been the standard treatment for axillary involvement in patients who undergo mastectomy. However, patients with cT1-T2cN0 breast cancer with an indication for mastectomy who do not meet the criteria for NC and with clinically negative axillary lymph nodes on FNAB can substitute LA with RT according to the AMAROS trial evidence with a high NPV (79%) to exclude patients with a high tumor burden.
From the ethical point of view, 13 LA procedures could have been avoided in the total mastectomized population (nine of these patients met the AMAROS trial criteria) in our series because of their low tumor burden, which represents a 90% reduction in the number of LA surgeries in this group of patients. This approach decreases morbidity and causes fewer physical and psychological sequelae (principle of beneficence) without reducing the overall survival and disease-free period (principle of non-maleficence) according to AMAROS trial evidence.
The sensitivity of FNAB for diagnosing positive SLNs in breast cancer patients with a high tumor burden was 93% in our series, which demonstrates the high relevance of this minimally invasive technique.