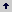Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad Nacional de Agronomía Medellín
Print version ISSN 0304-2847
Rev. Fac. Nac. Agron. Medellín vol.60 no.1 Medellín Jan./June 2007
A REVIEW ON BENEFICIAL EFFECTS OF RHIZOSPHERE BACTERIA ON SOIL NUTRIENT AVAILABILITY AND PLANT NUTRIENT UPTAKE
EFECTOS BENEFICOS DE BACTERIAS RIZOSFÉRICAS EN LA DISPONIBILIDAD DE NUTRIENTES EN EL SUELO Y LA ABSORCIÓN DE NUTRIENTES POR LAS PLANTAS
Nelson Walter Osorio Vega1
1 Profesor Asociado. Universidad Nacional de Colombia, Sede Medellín. Facultad de Ciencias. A.A. 3840, Medellín, Colombia. <nwosorio@unalmed.edu.co>
Recibido: Octubre 26 de 2006; aceptado: mayo 8 de 2007.
ABSTRACT
This paper is a review of the benefits of rhizosphere bacteria on plant nutrition. The interaction between plant and phosphate-solubilizing- bacteria is explained in more detail and used as model to illustrate the role that rhizosphere bacteria play on soil nutrient availability. Environmental conditions of rhizosphere and mycorrhizosphere are also discussed. Plants can release carbohydrates, aminoacids, lipids, and vitamins trough their roots to stimulate microorganisms in the soil. The soil volume affected by these root exudates, aproximately 2 mm from the root surface, is termed rhizosphere. Rhizosphere bacteria participate in the geochemical cycling of nutrients and determine their availability for plants and soil microbial community. For instance, in the rhizosphere there are organisms able to fix N2 forming specialized structures (e.g., Rhizobium and related genera) or simply establishing associative relationships (e.g. Azospirillium, Acetobacter). On the other hand, bacterial ammonifiers and nitrifiers are responsible for the conversion of organic N compounds into inorganic forms (NH4+ and NO3-) which are available for plants. Rhizosphere bacteria can also enhance the solubility of insoluble minerals that control the availability of phosphorus (native or applied) using for that organic acids or producing phosphatases that act on organic phosphorus pools. The availability of sulfur, iron and manganese are also affected by redox reactions carried out by rhizosphere bacteria. Likewise, chelating agents can control the availability of micronutrients and participate in mechanisms of biocontrol of plant pathogens. Due to these and other benefits on plant growth, some rhizosphere bacteria have been called Plant Growth Promoting Rhizobacteria (PGPR). The benefits of PGPR have also been obtained, and even enhanced, in presence of mycorrhizal fungi. Some authors have employed the term mycorrhizosphere to describe the part of the soil affected by these interactions.
Key words: rhizosphere, plant growth promoting rhizobacteria, phosphate solubilizing microorganisms, nutrient cycling.
RESUMEN
Este artículo se constituye en una revisión de los beneficios de bacterias rizosféricas sobre la nutrición vegetal. La interacción entre planta y bacterias solubilizadoras de fosfato es explicada en mayor detalle y usada como modelo para ilustrar el rol que algunas bacterias de la rizosfera juegan en la disponibilidad de nutrientes en el suelo. Las condiciones ambientales de la rizosfera también se discuten con detalle. Los beneficios de estas bacterias han sido obtenidos, y mejorados, en presencia de hongos formadores de micorrizas. Algunos autores han acuñado el termino micorrizosfera para describir la parte del suelo afectada por estas interacciones. Las plantas pueden liberar carbohidratos, aminoácidos, lípidos y vitaminas, entre otros, a través de sus raíces y estimular con ello la actividad y el número de microorganismos del suelo que las rodea. Este volumen de suelo afectado por tales exudados, aproximadamente 2 mm desde la superficie de la raíz, es llamado rizosfera. Las bacterias rizosfericas participan en el ciclo geoquímico de nutrientes y determinan su disponibilidad para las plantas y la comunidad microbial del suelo. Por ejemplo, en la rizosfera algunas bacterias fijan N2 simbiótica o asociativamente, otras son importantes en la conversión del nitrógeno de compuestos orgánicos a formas inorgánicas (NH4+ y NO3-) disponibles para las plantas. También es relevante la habilidad de algunas bacterias rizosféricas para disolver fosfatos insolubles (nativo y aplicado) a través de ácidos orgánicos, mientras que otras son más activas en la liberación de fosfato de compuestos orgánicos mediante enzimas fosfatasas. Por otro lado, la disponibilidad del azufre, hierro, manganeso es afectada por reacciones bioquímicas de oxido-reducción llevadas a cabo por bacterias de la rizosfera. De la misma manera, agentes quelatantes liberados por estas bacterias controlan la disponibilidad y absorción de micronutrientes y participan en el biocontrol de patógenos de plantas. Debido a estos beneficios sobre la nutrición y el crecimiento vegetal estas bacterias rizosfericas han sido llamadas rizobacterias promotoras del crecimiento vegetal (PGPR, por sus siglas en inglés).
Palabras claves: rizosfera, rizobacterias promotoras del crecimiento vegetal, microorganismos solubilizadores de fosfato, ciclo de nutrientes.
 CONCLUSIONS
CONCLUSIONS
 ACKNOWLEDGEMENTS
ACKNOWLEDGEMENTS
 REFERENCES
REFERENCES
Rhizosphere. The rhizosphere is the region of soil that is immediately near to the root surface and that is affected by root exudates (Kennedy 1999); it was described for first time by Lorenz Hiltner 1904. There are different types of substances that diffuse from the roots and that stimulate the microbial activity, such as carbohydrates (sugars and oligo-saccharides), organic acids, vitamins, nucleotides, flavonoids, enzymes, hormones, and volatile compounds (Prescott, Harley and Klein 1999).
The result is a dense and active microbial population that interacts with the roots and within it. The rhizosphere effect on the soil microbial population can be measured comparing the population density (colonies forming units, CFU) between the rhizosphere soil (R) and the bulk soil (S), for which the R/S ratio is employed (Atlas and Bartha 1997). The rhizosphere effect is higher for bacteria followed by fungi (Table 1) and even higher for some functional groups of bacteria (e.g., ammonifiers, denitrifiers). By contrast algae exhibit more number in the bulk soil than in the rhizosphere. The type of plant can also affect the R/S ratio, which can be associated with the amount and type of root exudates (Table 2).
Table 1. Number of microorganisms (CFU g-1 soil) in the rhizosphere (R) of wheat (Triticum aestivum L.) and bulk soil (S) and their R/S ratio.
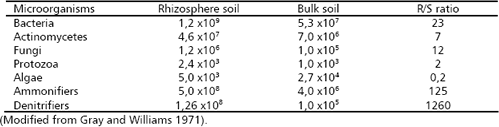
Table 2. Number of bacteria (CFUx106 g-1 soil or root dry mass) in the rhizoplane and rhizosphere of different plants, and in the bulk soil (S) and their R/S ratio
There are also differences between the population density at the root surface (rhizoplane) and the rhizosphere. Although on the rhizoplane there are numerous microorganisms only 4-10 % of its total surface area is in physical contact with soil microorganisms (Bowen 1980). Differences in the type of soil were not found in the literature, but some soils or media that exhibit severe constrains for microbial growth (e.g., acidic and Alrich soils that abundant in the tropics) can exhibit R/S ratio higher for bacteria and other microorganisms. On the other hand, Gilbert, Handelsman, and Parke 1994 pointed out that a lower R/S effect is associated with suppressive soils for root pathogens, in that sense the microbial activity of non-rhizosphere microorganisms can also play an important role in plant disease control.
This lower R/S effect seem to be involved in the experiments of Zhang, Dick, and Hoitink 1996 who found less severity of root rot (caused by Pythium ultimum and P. aphanidermatum) and leaf-anthracnose (Colletotrichum orbiculare) of cucumber plants grown in compostrich medium (suppressive) than those grown in sphagnum peat (conducive). The extent of the rhizosphere varies with the plant and the soil, but it is widely accepted that it covers at least 2 mm from the rhizoplane. Some authors have shown that the influence can be at least up to 10 mm (Table 3). The diversity of microorganisms is also variable, close to the rhizoplane there is a diverse community but as the distance from the rhizoplane increases the diversity is lower. Papavizas and Davey 1961 found similar effects on rhizosphere actinomycetes and fungi, this seems to be associated with the concentration of carbon in the soil solution (root exudates), which decreases from the rhizoplane (Yeates and Darrah 1991).
Table 3. Number of bacteria at increasing distances from the root surface (Paul and Clark, 1996).

The release of root exudates can be affected by several factors in the plant, soil and environment. According to Bowen and Rovira 1999, plants can released between 10-30 % of photosynthates through the root system. Whipps and Lynch 1986 reviewed this subject and found that a same factor (e.g., water stress, low soil pH, chemical applied to foliage) produced increase or decrease in the release of organic compounds in different plants. Roots also secret polysaccharides mucilage and loses cap cells detached from the root tip when it grows through the soil (McCully 1999), releasing thus more carbonaceous compounds into the rhizosphere.
The physical-chemical conditions that predominant in the rhizosphere are useful to understand the role that plays microorganisms, particularly bacteria on soil nutrient availability. The concentration of oxygen in the rhizosphere is very low due to the high demand of oxygen required for the respiration of carbonaceous compounds and the highly dense microbial population. Consequently, the concentration of CO2 is high. These conditions create an ambient anaerobic, and reduction reactions are favored. It is evident from the Table 1 where the denitrifiers (anaerobic bacteria) had a higher R/S ratio (1260) facilitating the reduction of some elements such as nitrogen, sulfur, iron and manganese.
The rhizosphere pH is usually lower than the bulk soil in 1-2 units. Several mechanisms are responsible of this effect: (i) production of CO2 by respiration processes, (ii) pump of H+ in nutrient uptake by plant and microbes, (iii) release of organic acids by roots and microbes, (iv) Organic matter decomposition, and (v) N2 fixation by the symbiosis Rhizobiumlegume (Marschner 1997). The effects can also vary with the soil buffer capacity and the type of plant involved. Acid conditions favor the solubilization of soil minerals (e.g., calcium phosphates) (Bowen and Rovira 1999). The characteristics of the rhizosphere vary with plant species and soil conditions. The rhizosphere of flooded rice exhibits an environment more aerobic than the bulk soil. The aerenquima tissue of rice plants permits the transport of O2 to the roots and its release into the rhizosphere (Marschner 1997). This facilitates the oxidation of Fe and Mn that given the reductive conditions of flooded soils tend to increase their availability up to levels that become toxic for plants.
Mycorrhizosphere. Most land plants form a symbiotic association with soil fungi called mycorrhiza (myco= fungus, rhiza= root) (Sylvia 1999). The mycorrhizal association favors water and nutrient uptake, particularly P, Cu and Zn; soil structure development and stability, and biological control of plant pathogens (Marschner and Dell 1994). It is recognized that the mycorrhizal association is a natural strategy that most plants have developed in their evolution process since their establishment on the earths surface (Paul and Clark 1996).
The fungal hypha is practically an extension of the root system that increases the volume of soil explored (Brady and Weil 1999). The mycorrhizal hypha also release carbonaceous compounds into the surrounding soil forming a niche called mycorrhizosphere (Rambelli 1973, Linderman 1988). It is important differentiate between two niches. Usually, the benefits of rhizosphere microorganisms are increase in presence of the mycorrhizal symbiosis.
Plant growth promoting rhizobacteria (PGPR). Rhizosphere bacteria can enhance the plant growth and crop yield by different ways. The acronym PGPR has been widely used to group these microbes (Bowen and Rovira 1999). Recently, Bashan and Holguin 1998 proposed the division of PGPR in two classifications: Biocontrol-Plant Growth Promoting Bacteria (Biocontrol-PGPB) and PGPB. These authors affirm that this separation is important in order to differentiate the mechanisms employed by these bacteria to promote the growth of plants. Biocontrol-PGPB are strictly those bacteria that participate in the biocontrol of plant pathogens while PGPB are bacteria that has other functions different to biocontrol (e.g., nutritional, hormonal). Also they suggested replace the term rhizobacteria for simply bacteria, because some bacteria can promote the plant growth but they are not inhabitants of the rhizosphere.
This paper deals with rhizosphere bacteria whose effects are associated with plant nutrition. Although Bashan and Holguins proposal is interesting, it is very difficult to separate the effects of both categories.
Nitrogen fixation. Nitrogen is one of the most limiting plant nutrients for plant growth (Havlin et al. 1999). Some rhizosphere bacteria have the ability to fix N2 into organic forms that can then be used by plants. The rhizosphere conditions favor the N2 fixation because it is carried out by heterotrophic bacteria that use organic compounds as source of electrons for the reduction of N2. Prominent among these microorganisms are the N2 fixers of the genera Rhizobium, Bradyrrhizobium, Mesorhizobium, Allorhizobium, Sinorhizobium, and Mesorhizobium that form symbiosis with legumes. In this case the concentration of O2 is regulated by hemoglobin and the supply of carbonaceous compounds occurs in the interior of nodules avoiding thus competition of other microorganisms (Graham 1999). They are perhaps the most studied interaction between plant and bacteria.
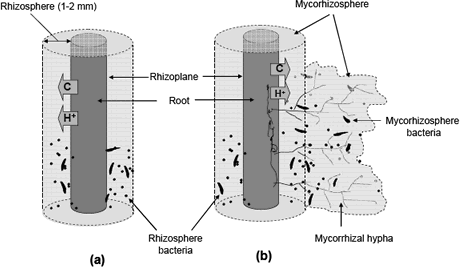
Figure 1. Schematic illustration of rhizosphere (a) and mycorhizosphere (b).
Another N2 fixer is Azotobacter paspali, which grows in the rhizosphere of tropical grasses, such as Paspalum notatum c.v. batatais and Digitaria species, with which exhibit certain degree of specificity (Zuberer 1999). Although fixation of 5- 25 kg N ha-1 year-1 are widely accepted, values as high as 90 kg N ha-1 year–1 have been reported. Acetobacter diazotrophicus is a N2 fixer that can grow inside of the root tissue (endorhizosphere) of sugarcane, including vascular tissues where can achieve number of 10 6 cells g-1 of these tissues. For its particular location, A. diazotrophicus has the advantage of a supply of carbon without microbial competition and apparently can tolerate high concentration of O2 than other bacteria. Sugarcane can derive as much as 100- 150 kg N ha-1 from this association.
One of the most studied associative symbioses is that formed by Azospirillum spp. and roots of numerous grasses, including important cereal crops (Okon 1994, Chanway 1997). Increases in the plant growth and yield by 5-30 % have been reported. The benefits seem to be due to the stimulation of nutrient uptake, production of plant growth regulators (auxins, giberrellins, and cytokinins), rather than to N2 fixation. Bashan, Rojas and Puente 1999 and Carillo-Garcia et al. 2000, have reported that cactus species inoculated with A. brasilense improved their establishment and development in desert soils.
Other non symbiotic N2 fixing bacteria Azotobacter chrococcum, Bacillus polymyxa, and Clostridium pasteurianum had increased seedling vigor of corn, wheat, and tomato and promoted earlier flowering of tomato (Rovira 1963). Perhaps the response was also due to hormonal effects and not to N2 fixation.
Positive responses in plant growth with N2 fixers can be expected in soils where N supply is limited. For instance, desert soils (Aridisols in the U.S. soil taxonomy; Buol et al. 1997) have very low organic matter and lack available water that restricts plant growth. The positive results of Bashan, Rojas, and Puente 1999 and Carillo-Garcia et al. 2000, support this affirmation. Other types of soils (e.g., ash volcanic soils) with low N supply could be conducive for N2 fixers. Similarly, eroded soils that have lost the soil organic matter from their surface or that have been burned soils can be rehabilitated for plant growth using rhizosphere N2 fixers. When legumes are employed the inoculation with their symbiotic partners (Rhizobium species or related genera) can improve the establishment of plants. In the cases of nonlegumes, the results of the inoculation with freeliving N2 fixers, such as Azospirillium and Azotobacter, are uncertain. Successful results have been obtained when these rhizosphere bacteria are combined with plants that have high efficiency in the photosynthesis (C4 plants), thus the C supply for these heterotrophic bacteria might be satisfactory.
Manganese. The availability of manganese (Mn) in the rhizosphere is affected by two major factors: redox condition and pH (Bohn, McNeal and OConnor 1985). In oxidized soils manganese is present in its oxidized form, Mn4+, in the lowsoluble mineral Pryolusite. Some rhizosphere bacteria (Bacillus, Pseudomonas, and Geobacter) can reduce oxidized Mn4+ to Mn2+, which is the chemical form that is metabolically useful for plants. The reaction is as follows:
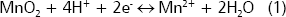
In this reaction two points are important, the reduction of Mn requires electrons and protons. Electrons are supplied by the decomposition of carbonaceous compounds and protons can be supplied by the proton excretion system of root cells (Marschner 1997). Consequently, the activity of Mn-reducers is highly favored in the rhizosphere. Applications of organic matter also can favor the reduction of Mn ( Hue, Vega and Silva 2001). In alkaline soils where Mn usually is insoluble the rhizosphere effect is beneficial, but in acidic soils with abundance of Mn-minerals (e.g., Wahiawa soil in the Oahu Island, Hue et al. 1998) excessive reduction of Mn can induce Mn toxicity in sensitive plants. Arines, Porto and Vilarino 1992, found that the mycorrhizosphere can reduce the activity of Mn-reducers and favor the Mn oxidation, which can be favorable for the management of Mn-rich soils.
Mn plays an important role in the resistance of plants to plant disease. Mn, as well as Cu, is required for the synthesis of lignin, which increase the resistance of the root tissues to the penetration of pathogens, consequently Mn-deficient plants are more susceptible to the attack of plant pathogens. Gaeumannomyces graminis, like many other soilborne pathogenic fungi, is a powerful oxidizer of Mn that impairs the lignification of root at infection sites (Graham and Webb 1991).
Effective rhizosphere Mn-reducers (e.g., Pseudomonas sp.) could have beneficial effects not only on plant nutrition but also on biocontrol of pathogens (Marschner 1997).
In addition, roots and rhizosphere bacteria can produce chelating-agents (phenolic compounds, organic acids) that form soluble complex with Mn and other elements avoiding the reprecipitation of Mn (Marschner 1997).
In contrast, in flooded soils where the availability of Mn2+ can be high, the Mn-oxidization by rhizosphere bacteria would favor plant growth. Rice roots release O2 in the rhizosphere avoiding Mn-toxic effects. On the other hand, Mn-oxidizing bacteria in the rhizosphere of plants grown in M-deficient soils can play an important role in plant disease control (Gilbert, Handelsman and Parke 1994). By reversing reaction (1), these bacteria reduce the availability of Mn2+ for fungal pathogens limiting its ability to attack roots.
Iron. The dynamics of iron in the rhizosphere is very similar to that of manganese (Bohn, McNeal, and OConnor 1985). Soil Fe is present in oxidized forms Fe3+ as a component of the structure of insoluble minerals Goethite (FeOOH) or hematite (Fe2O3) (Lindsay 1979). Rhizosphere bacteria (Bacillus, Pseudomonas, Geobacter, Alcaligenes, Clostridium, and Entero-bacter) can reduce Fe3+ to Fe2+, the form required by plants. Electrons and protons are available in the rhizosphere and consequently iron is reduced, however it can be reprecipitated (Mullen 1999). The reactions of reduction are as follows:
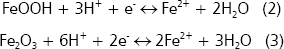
Under Fe-deficiency, rhizosphere bacteria, particularly fluorescent Pseudomonas, produce chelating agents (side-rophores) that form soluble complexes with Fe2+ and that are available for these bacteria (Marschner 1997). Scher 1986, found in Fusarium-suppressive soils that Pseudomonas putida produced a siderophore that sequestered iron. The complex siderophore-Fe can only be used by P. putida but no by Fusarium, which requires iron to synthesize enzymes that degrade the plant cell walls. However, when Fe-EDTA (an iron fertilizer) was applied, the biocontrol was lost because Fusarium could use this fertilizer.
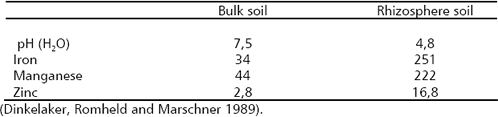
A strong Fe-chelating agent, EDDA enhanced the effect of P. putida. Van Peer et al. (1990) found similar effects with EDDHA. Again, mechanisms related with nutritional effects participate in the biocontrol of plant pathogens. On the other hand, iron is a component of heme groups in catalase and peroxidaes enzymes, which are required in the synthesis of lignin (Marschner 1997). Lignification of wall cells is a common response of plants when are challenged by plant pathogens. Iron deficiency plants can be more vulnerable to plant pathogens. In Table 4 the rhizosphere effects on pH and the availability of Fe, Mn and Zn are presented.
Table 4. Soil pH and micronutrient availability (DTPA-extractable, mmol kg-1 soil) in bulk soil and rhizosphere of white lupin (Lupinus albus).
Solubilization of phosphates by rhizosphere bacteria. In recent years, great attention has been dedicated to study the role that soil microorganisms play in the dynamics of phosphate (P), particularly those able to solubilize insoluble P forms (Rao 1992). These microorganisms are bacteria and fungi that inhabitant the rhizosphere (Barea and Azcon 1975, Bowen and Rovira 1999). Most soil bacteria can solubilize insoluble phosphates, particularly active are those that belong to the genera Pseudomonas, Enterobacter and Bacillus as well as some soil fungi, Penicillium and Aspergillus (Domey and Lipmann 1988, Patgiri and Bezbaruah 1990, Rao 1992, Rokade and Patil 1993, Whitelaw 2000). Some researchers prefer to use fungal P-solubilizers arguing that bacterial strains can lost their ability to solubilize P after several cycles of in vitro culture (Whitelaw, 2000), but this point is quite controversial.
The mechanisms involved in the microbial solubilization of P are the production of organic acids and the release of protons to the soil solution (Kim, McDonald and Jordan 1997). Inoculation with phosphate solubilizing rhizosphere bacteria (PSRB) and other soil microorganisms, such as arbuscular mycorrhizal fungi (AMF), might enhance even more the benefits of this P solubilization.
Why to study PSRB?. One of the most important problems in tropical agriculture is the low-soil-phosphate (P) availability. Many of the tropical soils are highly weathered and have a high P fixation capacity that makes their management more difficult (Sanchez 1976). Sanchez and Logan 1992, estimated that 1018 million ha in the tropics have a high P fixation capacity. In tropical America there are 659 million ha affected, 210 in Africa, and 199 in Asia (Figure 2). The term P-fixation is used in reference to a series of complex reactions that remove bioavailable soil P from the soil solution, where roots directly take up plant nutrients (Barber 1995). Such reactions consist in the sorption of phosphates on the solid surface of soil colloids and in the precipitation of phosphates with some cations in the soil solution (Havlin et al. 1999).
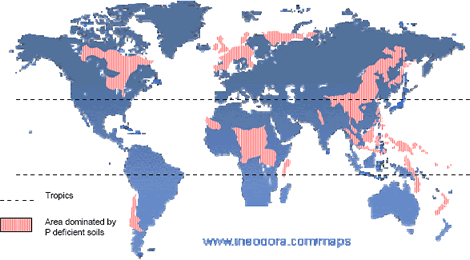
Figure 2. Phosphate-deficient soils in the world (adapted from Van Wambeke 1976).
Phosphate sorption is caused mainly by the presence of crystalline or non-crystalline hydrous-oxides of iron and aluminum in highly weathered soils of humid regions and acid savannas (Mattlingly 1975). Allophane (a non-crystalline aluminum-silicate) and humus-Al/Fe complexes are the responsible of the P sorption in soils derived from volcanic parent materials (Schwertmann and Herbillon 1992, Shonji, Nanzyo and Dahlgren 1993).
The precipitation of P in acidic soils occur with active forms of aluminum (Al3+, Al(OH)2+, Al(OH)2+) and iron (Fe2,,3+). In calcareous soils, P is sorbed on the surface of calcium carbonate (Mattingly 1975) or precipitated with calcium (Ca2+) (Bohn, McNeal and OConnor 1985). The predominance of these mechanisms depends on the degree of soil weathering and soil pH. In past decades, several strategies have been employed to reduce the P fixation. These consist of use of high rates of P fertilizers, selection of fertilizers, time and method of application, combination with amendments and other fertilizers, use of soil tests, etc. (Engelstad and Terman, 1980). However, the efficiency of P fertilizers is still low (5-10 %) (Havlin et al. 1999). Currently, there are environmental concerns in regard to the high levels of P fertilization (Brady and Weil 1997). Rock phosphates (apatite) are fertilizers amply recommended for soils with high P fixation capacity because other more soluble sources are quickly fixed. However, rock phosphates are extremely insoluble, particularly in alkaline soils, and a little more reactivity is always desired (Hammond and Leon 1992, Chien and Hammond 1978).
Mechanisms of P solubilization by rhizosphere bacteria. Several mechanisms have been proposed to explain the P solubilization by PSRB, they are associated with the release of organic and inorganic acids, and the excretion of protons that accompanies to the NH4+ assimilation (Kucey 1983, Roos and Luckner1984, AbdAlla 1994, Illmer, Barbato and Schinner 1995, Asea, Kucey and Stewart 1988, Whitelaw 2000). In addition, the release of phosphatase enzymes that mineralize organic P compounds has been also suggested as another mechanism involved (Stevenson 1986). Azam and Memon 1996, affirm that Nitrosomonas and Thiobacillus mobilized inorganic phosphates by producing nitric and sulfuric acid. Equally, phosphates may be released from solid compounds by carbonic acid formed as a result of the decomposition of organic residues (Memon 1996).
Many organic acids are effective in solubilizing soil phosphates, these acids are produced by rhizosphere microorgansims (Marschner 1997). Bolan et al. 1994, studied the influence of the addition of organic acids on high P-fixing soils. These acids decreased the P sorption on the clay surfaces, favored the solubilization of rock phosphate, and increased dry matter of ryegrass (Lolium rigidium) and plant P uptake. Hue 1991, found similar results in the availability of P when added organic acids on tropical soils in Hawaii and concluded that the efficiency of P fertilizers might be enhanced if these are added with organic acids or, more practically with green manures or animal wastes.
Kim, McDonald and Jordan 1997, point out that the production of organic acid was the major mechanism involved in the solubilization of hydroxyapatite (rock phosphate) by the PSRB Enterobacter agglomerans, but other mechanisms might be involved. Under in vitro conditions, the pH of the growth medium has decreased as a result of the release of organic acids by PSRB. Some of the organic acids commonly found are gluconic acid (Di-Simine, Sayer and Gadd 1998, Bar-Yosef et al. 1999), oxalic acid, citric acid (Kim, McDonald and Jordan 1997), lactic acid, tartaric acid, aspartic acid (Venkateswarlu et al. 1984). These acids are the product of the microbial metabolism, mostly by oxidative respiration or by fermentation of organic carbon sources (e.g., glucose) (Atlas and Bartha 1997, Prescott, Harley and Klein 1999). Such biological reactions occur in the rhizosphere where carbonaceous compounds are used by PSRB and the phosphate released is taken up by the roots or mycorrhiza symbiosis.
When PSRB are inoculated to neutral or alkaline soils, the acid production decreases the rhizosphere pH, favoring thus the solubility of calcium phosphates and apatites. If the activity of H+ increases in the reactants of the reactions (4) and (5), these reactions proceed. In addition, the sequestering of Ca by organic anions favors the reactions.
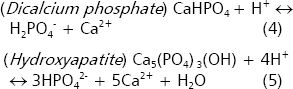
In acid soils, the minerals variscite and strengite control the solubility of phosphate (Lindsay 1979). The presence of organic acids propitiates the formation of complexes with Al and Fe ions, which in turn facilitates the dissolution of these minerals. If Fe3+ and Al3+ are sequestered via chelation with organic anions the reactions 6 and 7 proceed to the right. However, this point is controversial because the reduction in soil pH might also solubilize other iron and aluminum minerals that would reprecipitate again phosphates to form newly strengite and variscite (Lindsay 1979).
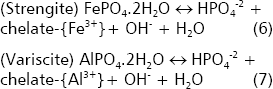
On the other hand, organic anions produced by PSRB can also compete with phosphates for fixation sites on the surface of soil colloids. He and Zhu 1997, 1998 demonstrated that sorbed phosphates on the surfaces of kaolynite, goethite, montmorillonite and amorphous Aloxides were displaced by microbial activity presumably using organic acids.
Experiences with PSRB. The inoculation with Bacillus megatherium var. phosphaticum in Russian soils (Mollisols) has been the best known reference of massive use of PSRB (Stevenson 1986). However, trials carried out in many locations demonstrated little consistency, which it is not surprising due to the diversity of factors involved. In fact, similar contradictions may be found in the response of crops where P fertilizers have been applied (Sumner 1987). In some cases, the inoculation with known PSRB has enhanced the plant growth without affecting plant P uptake. Freitas, Banerjee and Germida 1997, found that the inoculation with the PSRBs Bacillus thuringiensis, B. brevis, B. megatherium, B. polymyxa, B. sphaericus and Xanthomonas maltophila increased the growth and yield of canola (Brassica napus), but they did not increase the plant P uptake. PSRB can also release substances that promote root growth such as hormones, enzymes, antibiotics; enhance availability of other nutrients (e.g. Mn and Fe), and exert biocontrol of plant pathogens (Rao 1992, Premono et al. 1994, Toro et al. 1996, Bashan and Holguin 1998, Azcon and Barea 1996, Kopler, Lifshitz and Schroth 1988, Frankenberg and Arshad 1995). The efficiency of PSRB has been questioned because: (i) organic substances required for these microorganisms are scarce in nonrhizosphere microsites, (ii) antagonism and competition with other microorganisms in the rhizosphere, and (iii) low translocation of solubilized phosphates through soil because they can be again refixed by soil components (Tinker 1980, Bolan 1991, Azcon and Barea 1996).
Mycorrhizosphere and PSRB. There are several advantages with the combined use of arbuscular mycorrhizal fungi (AMF) and PSRB. First, mycorrhizal plants can release a higher amount of carbonaceous substances into their rhizosphere (mycorrhizosphere) than nonmycorrhizal plants (Rambelli 1973, Linderman 1988). Second, the extensive net formed around the roots by the mycorrhizal hyphae can efficiently facilitate the uptake of phosphate released by PSRB, avoiding thus its refixation. As long as the PSRB remain in the rhizosphere (or mycorrhizosphere), there is a great opportunity to satisfy their C requirement and deliver phosphates into the soil solution (Figure 3). Kim, Jordan and McDonald 1998 a, b, studied the effect of individual and dual inoculation of Enterobacter agglomerans (PSRB) and Glomus etunicatum (AMF) on tomato growth and P uptake. They found that there was a synergistic effect when both microorganisms were inoculated (Table 5).
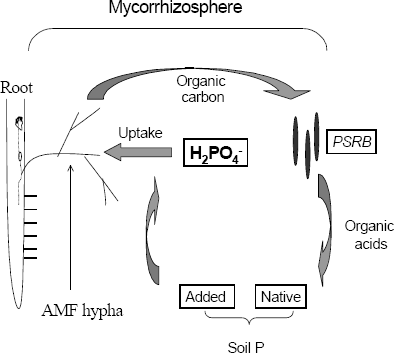
Figure 3. Diagram presentation of the solubilization of phosphates in the mycorrhizosphere and the mycorrhizal P uptake.
Table 5. Effects of E. agglomerans (PSRB) and G. etunicatum (AMF) inoculation on tomato plant growth and P uptake (75 days after inoculation).
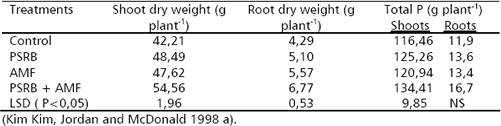
Alkaline phosphatase activity was higher in the treatment with G. etunicatum as well as the combination G. etunicatum + E. agglomerans. There was higher P concentration in the rhizosphere and higher oxalic acid production when both microorganisms were concurrently inoculated. In this experiment glucose was applied as an energy source to increase the release of organic acids by PSRB.
Similar synergistic effects have been found in sunflower (Helianthus annuus) with the triple inoculation of Azotobacter chroococcum, Penicillium glaucum and Glomus fasciculatum (Gururaj and Mallikarjunaiah 1995); in cotton with the inoculation of Pseudomonas striata and Azospirillum sp. (Prathiba, Alagawadi and Sreenivasa 1995); in rice favorable effects were also reported with P. striata and Bacillus polymyxa (Mohod, Gupta and Chavan 1991); in chili (Capsicum annuum) with G. fasciculatum or G. macrocarpum and P. striata (Sreenivasa and Krishnaraj 1992); in wheat with P. putida, P. aeruginosa and P. fluorescens in combination with G. clarum. Gaur et al. 1990 found the same type of response in wheat with P. striata and G. fasciculatum. In other experiment with wheat, Gaur et al. 1990 obtained positive results with the combination of two PSRB, P. striata and Agrobacterium radiobacter, with G. fasciculatum and Gigaspora margarita, the greatest plant growth was obtained when these microbes and fertilizers were added.
PSRB-AMF and rhizobia. Kopler, Lifshitz and Schroth 1988 found more legume-Rhizobium nodulation when also added Pseudomonas spp. Sturz et al. 1997 found that the nodulation of Rhizobium leguminosarum b.v. trifolii was promoted on red clover (Trifolium pratense) when it was coinoculated with Bacillus insolitus, B. brevis or Agrobacterium rhizogenes. Similar results were obtained with the inoculation of G. mosseae and Azorhizobium caulinodans in Sesbania rostrata (Rahman and Parsons 1997).
In soybean the combination of Bradyrhizobium japonicum, P. fluorescens and G. mosseae have given equally good results (Shabayey, Smolin and Mudrick 1996). Such results are likely due to a higher P uptake promoted by the PSRB and AMF, which may satisfy the high P requirements of the symbiotic N2 fixing process (Azcon and Barea 1996, Young, Chen and Chao 1990).
Apparently, there is a certain degree of specificity among the PSRB, AMF and P source. Toro, Azcon and Herrera 1996 studied the effect of the combination of AMF (Glomus spp.) and eight PSRB on the growth and P nutrition of a tropical legume, kudzu (Pueraria phaseoloides). PSRB were isolated from an Oxisol and were characterized by their ability to solubilize rock phosphate, iron phosphate and aluminum phosphate. In general, when kudzu-Rhizobium-AMF were coinoculated with PSRB there was an increase in the plant growth, yield and nutritional status. However, such synergism was not observed in all combinations. For instance, Azospirillum sp., Bacillus sp., and Enterobacter sp. had a higher effect when were coinoculated with G. Mosseae. Pseudomonas sp. and an unidentified isolate had a better performance when were combined with G. fasciculatum. On the other hand, Fe-phosphate solubilizers were more effective alone, while Al- and rock phosphate-solubilizers performed better when were concurrently inoculated with AMF.
Germide and Walley 1996, pointed out that is also possible to observe no effects or even unfavorable effects with PSRB inoculation. This seems to be caused by alteration in the rooting patterns (root distribution and root length), reduction in the AMF colonization of roots. Baas 1990 affirms that multiple inoculation of microorganisms might cause competition among them for rhizosphere exudates and with the host plant for the uptake of available P.
Prospective research on PSRB. In a series of elegant experiments, De la Fuente and Herrera 1999, isolated the gene that codes the overproduction of citrate synthetase in the TCA cycle of a strain of Pseudomonas aeruginosa (a known PSRB). This gene was then transferred to tobacco cells of plants that not exhibit Al tolerance. Transgenic plants were produced high amounts of citric acid and citrate and grew in solutions with high concentration of Al. The process was successfully replicated with papaya plants. Although these experiments were oriented to enhance the Al tolerance of these plants, it is directly associated with the mechanisms proposed for the solubilization of soil phosphates.
Currently, the fungal inoculum Penicillium bilaii is commercially available in North America with the name of ProvideTM, which has been successfully tested to enhance plant P uptake of several plants (Whitelaw 2000). Little research on phosphate solubilizers has been carried in tropical soils that usually exhibit a higher P fixation capacity than temperate soils. Recently, Osorio and Habte (unpublished) isolated several phosphate solubilizers including bacteria from the rhizosphere of L. leucocephala naturally grown in three Hawaiian soils (Tantalus, Wahiawa and Kaena soil series). The most effective P solubilizer was a fungus identified as Mortierella sp., which in turn was the most efficient producer of acidity in an in vitro test, several effective PSRB were also isolated (not yet identified).
It is uncertain if many of the mechanisms proposed for the PSRB operate at the same level of effectiveness in diverse soils with variable mineralogy. PSRB have also been used in the industry of P fertilizers. Usually rock phosphates are slightly acidulated with inorganic acids to increase its reactivity (Chien and Hammond 1978), or used as raw material to produce more soluble fertilizers for which strong acids are added (Young and Davies 1980). It is an expensive process due to the high cost of inorganic acids. Bar-Yosef et al. 1999 found that Pseudomonas cepacia, a known PSRB, was very efficient to oxide glucose and to produce gluconic and 2-keto-gluconic acids in a reactor containing rock phosphate. Once the acids were dissociated, protons reacted with rock phosphate and released phosphate ions that were then precipitates with Ca to form soluble fertilizers (super-phosphates). Thus, PSRB yield benefits not only in their natural niche, the rhizosphere, but also in other environments.
Phosphate solubilizing rhizosphere bacteria has a high potential to be used in the management of P deficient soils. PSRB may be coinoculated with AMF generating synergistic effects on plant growth and P uptake. The compatibility between PSRB and AMF seems to have certain degree of specificity, for which is recommended to investigate what are the more effective combinations. The mechanisms of P solubilization by PSRB are associated with the production of organic and inorganic acids, proton excretion, and phosphatase activity. Organic acids are produced by the oxidation of carbonaceous originated in the rhizosphere, from the soil organic matter or added as manure. Organic acids decrease the rhizosphere pH favoring the solubility of precipitated P forms. Organic anions can also compete or even replace phosphate sorbed on the surfaces of soil clays, they also can chelate Al and Fe avoiding thus the precipitation of phosphate.
The author thanks Dr. Anne Alvarez and Dr. Francoise Robert from the University of Hawaii, HI, USA , and Dr. Mauricio Marin Montoya from the Universidad Nacional de Colombia, Sede Medellín for reading the manuscript.
Abd-Alla, M. H. 1994. Use of organic phosphorus by Rhizobium legumino-sarum biovar viceae phosphatases. In: Biology and Fertility of Soils. Vol. 18, no. 3; p. 216-218. [ Links ]
Arines, J.; Porto, M. E. and Vilariño, A. 1992. Effect of manganese on vesicular-arbuscular mycorrhizal development in red clover plant and soil Mn-oxidizing bacteria. In: Mycorrhiza, Vol. 1, no. 3; p. 127-131. [ Links ]
Asea, P. E. A.; Kucey, R. M. N., and Stewart, J. W. B. 1988. Inorganic phosphate solubilisation by 2 Penicillium species in solution culture and soil. In: Soil Biology and Biochemistry. Vol. 20; p. 459-464. [ Links ]
Atlas, R. and Bartha, R. 1997. Microbial ecology. New York: Addison Wesley Longman. 694 p. [ Links ]
Azam, F. and Memom, G. H. 1996. Soil organisms. p. 200-232. In: Bashir, E. and Bantel, R., eds. Soil Science. Islamabad: National Book Foundation. [ Links ]
Azcon, C. and Barea, J. M. 1996. Interactions of arbuscular mycorrhiza with rhizosphere microorganisms. p. 47-68 p. In: Guerrero, E., ed. Mycorrhiza. biological soil resource. Santafé de Bogotá, Colombia : FEN. [ Links ]
Barber, S. A. 1995. Soil nutrient bio-availability. New York: John Wiley and Sons. 414 p. [ Links ]
Barea, J. M. and Azcon, R. 1975. Possible synergistic interactions between endogone and phosphate-solubilizing bacteria in low-phosphate soils. p. 409-417 p. In: Mosse, B. and Tinker, P. B., eds. Endomycorrhizas. London: Academic Press. [ Links ]
Bar-Yosef, B.; Rogers, R. D.; Wolfram, J. H. and Richman, E. 1999. Pseudomonas cepacia-mediated rock phosphate solubilization in kaolinite and montmorillonite suspensions. In: Soil Science Society of America Journal. Vol. 63; p. 1703-1708. [ Links ]
Bashan, Y. and Holguin, G. 1998. Proposal for the division of plant growth promoting rhizobacteria into two classifications: Biocontrol-PGPB and PGPB. In: Soil Biology and Biochemistry. Vol. 30, no. 8; p. 1225-1228. [ Links ]
________; Rojas, A. and Puente, M. E. 1999. Improved establishment and development of three cactus species inoculated with Azospirillum brasilense transplanted into disturbed urban desert soils. In: Canadian Journal of Microbiology. Vol. 45, no. 12; p. 441-451. [ Links ]
Bass, R. 1990. Effects of Glomus fasciculatum and isolated rhizosphere microorganisms on growth and phosphate uptake of Plantago major spp. pleiosperma. In: Plant and Soil. Vol. 124, no. 2; p. 187-193. [ Links ]
Bohn, H.; McNeal, B., and OConnor, G. 1985. Soil chemistry. New York: John Wiley and Sons. 341 p. [ Links ]
Bolan, N. S. 1991. A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. In: Plant and Soil. Vol. 134, no. 2; p. 189-207. [ Links ]
________; Naidu, R.; Mahimairaja, S. and Baskaran, S. 1994. Influence of low-molecular-weight organic acids on the solubilization of phosphates. In: Biology and Fertility of Soils. Vol. 18, no. 4; p. 311-319. [ Links ]
Bowen, G. D. 1980. Misconceptions, concepts, and approaches in rhizosphere biology, 283-304 p. In: Ellwood, D. C.; Hedger, J. N.; Lathan. M. J.; Lynch, J. M., and Slater, J. H., eds. Contemporary microbial ecology. London: Academic Press. [ Links ]
________ and Rovira, A. D. 1999. The rhizosphere and its management to improve plant growth. In: Advances in Agronomy. Vol. 66; p. 1-102. [ Links ]
Brady, N. C. and Weil, R. R. 1999. The nature and properties of soils. 12th ed. Upper Saddle River, N.J.: Prentice Hall, 881 p. [ Links ]
Buol, S.; Hole, F. D.; McCraken, R. J. and Southard, R. J. 1997. Soil genesis and classification. Iowa State University Press, Ames. 527 p. [ Links ]
Carillo-Garcia, A.; Bashan, Y.; Diaz, E., and Bethlenfalvay, G. J. 2000. Effects of resource-island soils, competition, and inoculation with Azospirillium on survival and growth of Pachycereus pringlei, the giant cactus of the Sonoran desert. In: Restoration Ecology. Vol. 8, no. 1; p. 65-73. [ Links ]
Chanway, C.P. 1997. Inoculation of tree roots with plant promoting soil bacteria: an emerging technology for reforestation. In: Forest Science. Vol. 43, no. 1; p. 99-112. [ Links ]
Chien, S. H. and Hammond, L. L. 1978. A comparison of various laboratory methods for predicting the agronomic potential of phosphate rocks for direct application. In: Soil Science Society of America Journal. Vol. 42; p. 935-939. [ Links ]
De la Fuente, J. M. and Herrera, L. 1999. Advances in the understanding of aluminum toxicity and the development of aluminum tolerant transgenic plants. In: Advances in Agronomy. Vol. 66; p. 103-121. [ Links ]
Dinkelaker, B.; Romheld, V. and Marschner, H. 1989. Citric acid excretion and precipitation of calcium citrate in the rhizosphere of white lupin (Lupinus albus). In: Plant Cell Environment. Vol. 12, no. 3; p. 285-292. [ Links ]
Di-Simine, C. D.; Sayer, J. A., and Gadd., G. M. 1998. Solubilization of zinc phosphate by a strain of Pseudomonas fluorescens isolated from a forest soil. In: Biology and Fertility of Soils. Vol. 28, no. 1; p. 87-94. [ Links ]
Domey, S. and Lipmann, G. 1988. Stimulation of plant growth by phosphate, solubilizing bacteria. In: Vancura, V. and Kunc, F., eds. Interrelationships between microorganisms and plants in soil. In: Developments in Agricultural and Managed Forest Ecology. Vol. 17; p. 457-461. [ Links ]
Engelstad, O. P. and Terman, G. L. 1980. Agronomic effectiveness of phosphate fertilizers. p. 311-332. In: Khasawneh, F. E.; Sample, E. and Kamprath, E., eds. The role of phosphorus in agriculture. Madison, WI: American Society of Agronomy. [ Links ]
Frankenberg, W. T. and Arshad, M. 1995. Phytohormones in soils: microbial production and function. New York: Dekker. 503 p. [ Links ]
Freitas, J. R.; Banerjee, M. R., and Germida, J. J. 1997. Phosphate solubilizing rhizobacteria enhance the growth and yield but not phosphorus uptake of canola (Brassica napus L.). In: Biology and Fertility of Soils. Vol. 24, no. 4; p. 358-364. [ Links ]
Gaur, A.; Rana, J.; Jalali, B. and Chand, H. 1990. Role of VA mycorrhizae, phosphate solubilizing bacteria and their interactions on growth and up-take of nutrients by wheat crops. p. 105-106. In: The National Conference on Mycorrhizae (1990: Hisar, India ) Proceeding. Hisar, India : Trends in Mycorrhizal Research. [ Links ]
Germida, J. J. and Walley, F. L. 1996. Plant growth promoting rhizobacteria alter rooting patterns and arbuscular mycorrhizal fungi colonization of fields-grown spring wheat. In: Biology and Fertility of Soils. Vol. 23, no.2; p. 113-120. [ Links ]
Gilbert, G. S.; Handelsman, J. and Parke, J. L. 1994. Root camouflage and disease control. In: Phytopathology. Vol. 84; p. 222-225. [ Links ]
Graham, P. H. 1999. Biological dinitrogen fixation: symbiotic. p. 322-368. In: Sylvia, D.; Fuhrmann J.; Hartel, P. and Zuberer, D., eds. Principles and applications of soil microbiology. Upper Saddle River, N.J: Prentice Hall. 550 p. [ Links ]
Graham, R. D. and Webb, M. J. 1991. Micronutrients and plant disease resistance and tolerance in plants. p. 329-370. In: Morvedt, J.; Cox, F. R.; Shuman, L. M. and Welch, R. M., eds. Micronutrients in agriculture. Madison, WI: Soil Science Society of America, (Books series No. 4). [ Links ]
Gray , T. R. G. and Williams, S. T. 1971. Soil microorganisms. Edinburgh: Oliver and Boyd. 240 p. [ Links ]
Gururaj, R. and Mallikarjunaiah, R. 1995. Interactions among Azotobacter chroococcum, Penicillium glaucum and Glomus fasciculatum and their effect on the growth and yield of sunflower. In: Helia. Vol. 18, no. 23; p. 73-84. [ Links ]
Hammond, L. and Leon, L. 1992. Evaluation of the North Caroline natural phosphate as a phosphoric fertilizer. In: Suelos Ecuatoriales. Vol. 22, no.1; p. 143-150. [ Links ]
Havlin, J.; Beaton, J.; Tisdale, S. L. and Osorio, N. W. 1999. Soil fertility and fertilizers. Upper Saddle River, NJ: Prentice Hall. 499 p. [ Links ]
He, Z. L. and Zhu, J. 1997. Transformation and bioavailability of specifically sorbed phosphate on variable charge mineral soils. In: Biology and Fertility of Soils. Vol. 25, no. 2; p. 175-181. [ Links ]
________ and Zhu, J. 1998. Microbial utilization and transformation of phosphate adsorbed by variable charge minerals. In: Soil Biology and Biochemistry. Vol. 30, no. 7; p. 917-923. [ Links ]
Hiltner, L. 1904. Uber neure Erfahrungen und probleme auf dem gebiet der bockenbakteriologie und unter besonderer bercksichtigung der grundungung un brache. In: Arbeiten Deutscher Landwirtschafts Gesellschaft. Vol. 98; p. 59-78. [ Links ]
Hue. N. V. 1991. Effects of organic acids/anions on P sorption and phytoavailability in soils with different mineralogies. In: Soil Science. Vol. 152; p. 463-471. [ Links ]
________; Silva, J.; Uehara, G.; Hamasaki, R. T.; Uchida, R. and Bunn, P. 1998. Managing manganese toxicity in former sugarcane soils of Oahu. Honolulu: University of Hawaii, Cooperative Extension Service. 7 p. [ Links ]
Hue. N. V.; Vega, S., and Silva, J. 2001. Manganese toxicity in a Hawaiian Oxisol affected by soil pH and organic amendments. In: Soil Science Society of America Journal. Vol. 65, no. 1; p. 153-160. [ Links ]
Ilmer, P.; Barbato, A. and Schinner. F. 1995. Solubilization of hardly soluble ALPO4 with P-solubilizing microorganisms. In: Soil Biology and Bio-chemistry. Vol. 27, no. 3; p. 265-270. [ Links ]
Kenedy, A. 1999. The rhizosphere and spermosphere, 389-407 p. In: Sylvia, D.; Fuhrmann, J.; Hartel, P., and Zuberer, D., eds. Principles and applications of soil microbiology. Upper Saddle River, NJ: Prentice Hall. 499 p. [ Links ]
Kim, K. Y.; McDonald, G. A., and Jordan, D. 1997. Solubilization of hydroxyapatite by Enterobacter agglomerans and cloned Escherichia coli in culture medium. In: Biology and Fertility of Soils. Vol. 24, no. 4; p. 347-352. [ Links ]
Kim, K. Y.; Jordan, D., and McDonald, G. A. 1998a. Effect of phosphate solubilizing bacteria and vesicular-arbuscular mycorrhizae on tomato growth and soil microbial activity. In: Biology and Fertility of Soils. Vol. 26, no. 2; p. 79-87. [ Links ]
________; ________ and ________. 1998b. Enterobacter agglomerans, phosphate solubilizing bacteria and microbial activity in soil. Effect of carbon sources. Soil Biology and Biochemistry. Vol. 30; no. 8; p. 995-1003. [ Links ]
Kopler, J.; Lifshitz, R., and Schroth, M. 1988. Pseudomonas inoculants to benefit plant production. In: ISI. Atlas of Sciences: Animal and Plant Sciences. Vol. 1, no.1; p. 60-64. [ Links ]
Kucey, R. M. N. 1983. Phosphate solubilising bacteria and fungi in various cultivated and virgin Alberta soils. In: Canadian Journal of Soil Science, Vol. 63; p. 671-678. [ Links ]
Linderman, R. G. 1988. Mycorrhizal interaction with the rhizosphere microflora: The mycorhizosphere effect. In: Phytopathology, 78, no. 3; p. 366-371. [ Links ]
Lindsay, W. 1979. Chemical equilibria in soils. New York: John Wiley and Sons. 449 p. [ Links ]
Marschner, H. 1997. Mineral nutrition of higher plants. London: Academic Press. 889 p. [ Links ]
Marschner, H. and Dell, B. 1994. Nutrient uptake in mycorrhizal symbiosis. p. 89-102. In: Robson, A. D.; Abott, L. K., and Malaccjuk, N., eds. Management of mycorrhizas in agriculture, horticulture and forestry. Netherlands : Academic Publishers. 252 p. [ Links ]
Mattingly, G. E. G. 1975. Labile phosphate in soils. In: Soil Science. Vol. 119; p. 369-375. [ Links ]
McCully, M. 1999. Roots in soil: unearthing the complexities of roots and their rhizospheres. In: Annual Review of Plant Physiology and Plant Molecular Biology. Vol. 50; p. 695-718. [ Links ]
Memon, K. S. 1996. Soil and fertilizer phosphorus. p. 291-316. In: Bashir, E. and Bantel, R., eds. Soil science. Islamabad: National Book Foundation. [ Links ]
Mohod, S.; Gupta, D. N., and Chavan, A. S. 1991. Effects of P solubilizing organisms on yield and N uptake by rice. In: Journal of Maharashtra Agricultural Universities. Vol. 16, no. 2; p. 229-231. [ Links ]
Mullen, M. 1999. Transformation of other elements. p. 369-386. In: Sylvia, D.; Fuhrmann, J.; Hartel, P. and Zuberer, D. Principles and applications of soil microbiology. Upper Saddle River, NJ: Prentice Hall. [ Links ]
Okon, Y. 1994. Azospirillium/plant associations. Boca Raton, Fl.: CRC Press, 192 p. [ Links ]
Papavizas, G. C. and Davey, C. B. 1961. Extent and nature of the rhizosphere of Lupinus. In: Plant and Soil. Vol. 14; p. 215-236. [ Links ]
Patgiri, I. and Bezbaruah, B. 1990. Strains contributing to phosphorus mobilization in acid soils. In: Indian Journal of Agricultural Sciences. Vol. 60, no. 3; p. 197-200. [ Links ]
Paul, E. A. and Clark, F. E. 1996. Soil microbiology and biochemistry. San Diego, CA: Academic Press. 340 p. [ Links ]
Prathibha, C. K.; Alagawadi, A. and Sreenivasa, M. 1995. Establishment of inoculated organisms in rhizosphere and their influence on nutrient uptake and yield cotton. In: Journal of Agricultural Sciences. Vol. 8, no.1; p. 22-27. [ Links ]
Premono, E.; Anas, I.; Soepardi, G.; Hadioetomo, R.; Saono, S., and Sisworo, W. 1994. Isolation and selection of phosphate-solubilizing microorganisms from a sugarcane plantation. In: Majalah Perusahaan Gula. Vol. 30, no. 3-4; p. 25-29. [ Links ]
Prescott, L., Harley, J. and Klein, D. A. 1999. Microbiology. Boston: Mc Graw-Hill. 962 p. [ Links ]
Rahman, M. K. and Parsons, J. W. 1997. Effects of inoculation with Glomus mosseae, Azorhizobium caulinodans and rock phosphate on the growth of and nitrogen and phosphorus accumulation in Sesbania rostrata. In: Biology and Fertility of Soils. Vol. 25, no.1; p. 47-52. [ Links ]
Rambelli, A. 1973. The rhizosphere of mycorrhizae. p. 299-343 In: Marks, G. C. and Kozlowski, T. T., eds. Ectomycorrhyzae, their ecology and physiology. New York and London: Academic Press. [ Links ]
Rao, N. S. 1992. Biofertilizers in agriculture. Rotterdam: AA Balkema. 188 p. [ Links ]
Rokade, S. M. and Patil, P. 1993. Phosphate solubilizing microorganisms: a review. In: Journal of Maharashtra Agricultural Universities. Vol. 18, no. 1; p. 93-101. [ Links ]
Roos, W. and Luckner, M. 1984. Relationships between proton extrusion and fluxes of ammonium ions and organic acids in Penicillium cyclopium. In: Journal of General Microbiology. Vol. 130, no. 4; p. 1007-1014. [ Links ]
Rouat, J. W. and Katznelson, H. 1961. A study of the bacteria on the root surface and in the rhizosphere soil of crop plants. In: Journal of Applied Bacteriology. Vol. 24, no. 2; p. 164-171. [ Links ]
Rovira, A. D. 1963. Microbial inoculation of plants.
Salih, H. M.; Yahya, A. I; Abdul-Rahem, A. M. and Munam, B. H. 1989. Availability of phosphorus in a calcareous soil treated with rock phosphate or superphosphate as affected by phosphate-dissolving fungi. In: Plant and Soil. Vol. 120, no. 1; p. 181-185. [ Links ]
Sanchez, P. 1976. Properties and management of soils in the tropics. New York: John Wiley and Sons. 618 p. [ Links ]
________ and Logan, T. 1992. Myths and science about the chemistry and fertility of soils in the tropics. p. 35-46. In: Lal, R. and Sanchez, P., eds. Myths and science of soils of the tropics. Madison, WI: Soil Science Society of America . [ Links ]
Scher, F. M. 1986. Biological control of Fusarium wilts by Pseudomonas putida and its enhancement by EDDA. p. 109-117. In: Swinburne, T., ed. Iron, sidero-phores, and plant diseases. New York: Plenum. [ Links ]
Schwertmann, U. and Herbillon, A. J. 1992. Some aspects of fertility associated with the mineralogy of highly weathered tropical soils. p. 47-60. In: Lal, R. and Sanchez, P., eds. Myths and science of soils of the tropics. Madison, WI: Soil Science Society of America . [ Links ]
Shabayey, V. P.; Smolin, V. Y., and Mudrick. 1996. Nitrogen fixation and CO2 exchange in soybeans inoculated with mixed cultures of different microorganisms. In: Biology and Fertility of Soils. Vol. 23, no. 1; p. 425-430. [ Links ]
Shonji, S.; Nanzyo, M. and Dahlgren, R. 1993. Volcanic ash soils. Amsterdam: Elsevier. 288 p. [ Links ]
Sreenivasa, M. and Krishnaraj, M. 1992. Synergistic interaction between VA mycorrhizal fungi and a phosphate solubilizing bacterium in chili. In: Zentralblatt fur mikrobiologie. Vol. 147, no. 1-2; p. 126-130. [ Links ]
Stevenson, F. J. 1986. Cycles of soil. New York: John Wiley and Sons. 380 p. [ Links ]
Sturtz, A. V.; Christie, B. R.; Matheson, B. G. and Nowak, J. 1997. Biodiversity of endophytic bacteria which colonize red clover nodules, roots, stems and foliage and their influence on host growth. In: Biology and Fertility of Soils, 25, no. 1; 13-19. [ Links ]
Sumner, M. 1987. Field experimentation: changing to meet current and future needs. p. 119-132. In: Brown, J. R., ed. Soil testing: sampling, correlation, calibration and interpretation. Madison, WI: Soil Science Society of America . [ Links ]
Sylvia, D. 1999. Mycorrhizal symbioses. p. 408-426. In: Sylvia, D.; Fuhrmann, J.; Hartel, P. and Zuberer D., eds. Principles and applications of soil microbiology. Upper Saddle River, NJ: Prentice Hall. [ Links ]
Tinker, P.B. 1980. Role of rhizosphere microorganisms in phosphorus uptake by plants. p. 617-654. In: Khasawneh, F. E.; Sample, E. C. and Kamprath, E. J., eds. The role of phosphorus in agriculture. Madison, WI: Soil Science Society of America . [ Links ]
Toro, M.; Azcon, R. and Herrera, R. 1996. Effects on yield and nutrition of mycorrhizal and nodulated Pueraria phaseoloides exerted by P-solubilizing rhizobacteria. In: Biology and Fertility of Soils. Vol. 21, no. 1-2; p. 23-29. [ Links ]
Van Peer, R.; Van Kiuik, A. J.; Rattin, K. H. and Schippers, B. 1990. Control of Fusarium wilt in carnation grown on rockwool by Pseudomonas sp. strain WCS417a and by Fe-EDDA. In: Netherlands Journal of Plant Pathology. Vol. 96, no. 5; p. 119-132. [ Links ]
Van Wambeke, A. 1976. Formation, distribution, and consequences of acid soils in agricultural development. p. 15-24. In: Wright, M. J., ed. Plant adaptation to mineral stress in problem soils.Washington, D.C.: Technical Assistance Bureau, Agency for International Development. [ Links ]
Venkateswarlu, B.; Rao, A. V.; Raina P. and Ahmad, N. (1984). Evaluation of phosphorus solubilization by microorganisms isolated from arid soil. In: Journal of Indian Society of Soil Science Vol. 32, no. 2; p. 273–277 [ Links ]
Whipps, J. M. and Lynch, J. M. 1986. The influence of the rhizosphere on crop productivity. In: Advances in Microbial Ecology. Vol. 9; p. 187-244. [ Links ]
Whitelaw, M. A. 2000. Growth promotion of plants inoculated with phosphate solubilizing fungi. In: Advances in Agronomy. Vol. 69; p. 99-151. [ Links ]
Yeates, G. and Darrah, P. R. 1991. Microbial changes in a model rhizosphere. In: Soil Biology and Biochemistry. Vol. 23, no. 10; p. 963-971. [ Links ]
Young, C. C.; Chen, C. L., and Chao, C. C. 1990. Effect of Rhizobium, vesicular-arbuscular mycorrhiza and phosphate solubilizing bacteria on yield and mineral phosphorus uptake of crops in subtropical-tropical. In: Transactions of the 14th International Congress of Soil Science. Kyoto, Japan : ICSS. III. p. 55-60. [ Links ]
Young, R. and C. Davies. 1980. Phosphate fertilizers and process technology. p. 195-226. In: Khasawneh, F. E.; Sample, E. and Kamprath, E., eds. The role of phosphorus in agriculture. Madison, WI: Soil Science Society of America . [ Links ]
Zhang, W., Dick, W. A. and Hoitink, H. A. J. 1996. Compostinduced systemic acquired resistance in cucumber to Pythium root rot and anthracnose. In: Phytopathology. Vol. 86; p. 1066-1070. [ Links ]
Zuberer, D. A. 1999. Biological dinitrogen fixation: introduction and non-symbiotic. p. 295-321. In: Sylvia, D.; Fuhrmann, J.; Hartel, P. and Zuberer, D., eds. Principles and applications of soil microbiology. Upper Saddle River, NJ: Prentice Hall [ Links ]