Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad Nacional de Agronomía Medellín
Print version ISSN 0304-2847
Rev. Fac. Nac. Agron. Medellín vol.64 no.2 Medellín July/Dec. 2011
Somatic Embryogenesis in Yam (Dioscorea rotundata)
Embriogenesis Somatica en Ñame (Dioscorea rotundata)
Isidro Elías Suárez Padrón1; Luz Angela Torres Arizal2 y Richard Litz3
1 Profesor Titular. Universidad de Córdoba - Facultad de Ciencias Agrícolas - Departamento de Ingeniería Agronómica y Desarrollo Rural. Carrera 6a No. 76-103, Montería. Córdoba, Colombia. <isidrosuarez@hotmail.com>
2 Bióloga. Universidad de Córdoba - Facultad de Ciencias Agrícolas - Laboratorio Biotecnología Vegetal. Carrera 6a No. 76-103. Montería, Córdoba, Colombia. <luz_torres23@hotmail.com>
3 Profesor Emeritus. University of Florida. Tropical Research and Education Center. 18905 SW 280st 33031 Homestead, Florida, United States of America. <relitz@ufl.edu>
Recibido: Noviembre 29 de 2010; aceptado: Noviembre 02 de 2011.
Abstract. Embryogenic yam (Dioscorea rotundata) cultures were induced from petioles of leaves of in vitro grown plants on medium supplemented with different 2.4-D concentrations. Cultures were maintained either on semisolid or in liquid MS medium supplemented with 4.52 µM 2.4-D. The effect of sucrose concentration on somatic embryo development was also evaluated and the effects of different BAP concentrations on somatic embryo conversion were determined. Treatments were distributed using a complete randomized design. The highest rate of induction occurred with 4.52 µM 2.4-D. Sucrose at 131.46 mM significantly enhanced somatic embryo development. The conversion rate was not affected by BAP.
Key words: Auxin, conversion, tropical species, food security.
Resumen. Cultivos embriogénicos de ñame (Dioscorea rotundata) fueron inducidos a partir de explantes consistentes de hojas con peciolos, aisladas de plantas establecidas en condiciones in vitro, en presencia de diferentes concentraciones de 2,4-D. Los cultivos inducidos fueron mantenidos en medio MS líquido o semisólido suplido con 4,52 µM 2,4-D. El efecto de las concentraciones de sacarosa sobre el desarrollo de embriones somáticos y el efecto de varias concentraciones de BAP sobre la tasa de conversión de embriones somáticos en plantas también fueron evaluados. Todos los tratamientos fueron distribuidos usando un diseño completamente al azar. El mayor porcentaje de inducción de tejidos embriogénicos ocurrió con 4,52 µM de 2,4-D. La adición de 131,46 mM de sacarosa incrementó significativamente el desarrollo de embriones somáticos. La tasa de conversión de embriones somáticos en plantas no fue afectada por las concentraciones de BAP.
Palabras clave: Auxina, conversión, especies tropicales, seguridad alimentaria.
White yam (Dioscorea rotundata) is a staple food for rural inhabitants of the Caribbean Coast of South America. It has great cultural value for people in tropical regions (Sanchez and Hernández, 2000). Yams are propagated from seeds and from tuber cuttings; however, flowering and seed production occur rarely for most cultivars in the tropics. Commercial crops are planted using tuber pieces as propagules. Vegetative propagation, and the poor flowering biology of the species, has eroded the genetic base of the species and breeding to overcome anthracnose, which is caused by the fungus, Colletotrichum gloeosporiodes, and the main limiting factor of yam production, has been very difficult. The disease attacks the entire plant, prevents tuber formation and causes plant death (Mignouna et al., 2001; Pérez et al., 2003).
Biotechnology technics can be used when traditional breeding is ineffective for crop improvement. One approach involves the in vitro selection of mutated cell lines in the presence of a selective agent (Royero et al., 2007). Embryogenic cultures have been utilized to obtain disease-resistant off-types following their challenge by the culture filtrates produced by Colletotrichum gloeosporioides and Elsinoe ampelina, respectively (Jayasankar et al., 1998; 2000). Plant regeneration from cells is necessary to apply in vitro selection or genetic transformation.
The objective of the present study has been to develop a protocol for somatic embryogenesis of Dioscorea rotundata as a plant regeneration technique from cellular tissues.
MATERIALS AND METHODS
Embryogenic culture induction and maintenance. Explants, consisting of leaf petioles (Espitia and Quintero, 1999), were isolated from in vitro-established 9811-090 Dioscorea rotundata plants growing on semi solid MS (Murashige and Skoog, 1962) medium supplemented with (in mg L-1) sucrose, (30000), myo-inositol (100), thiamine-HCl (0.1) and TC- Agar (6000) (Sigma®). Leaf petiole explants were inoculated into sterile 30 mL semi solid induction medium (IM) (De Wald et al., 1989; Witjaksono and Litz, 1999a) in (60 x 15 mm) Petri dishes. Induction medium consisted of B5 (Gamborg et al., 1968) major salts, MS minor salts and (in mg L-1) sucrose, (30000), myo-inositol (100), thiamine-HCl (0.1) and TC- Agar (6000). To evaluate the effect of 2,4-dichlorophenoxyacetic acid (2,4-D) on embryogenic culture induction, IM was independently supplemented with four (0.0; 4.52; 9.05 and 18.1 µM) 2,4-D concentrations. Treatments were distributed using a complete randomized design with 20 replicates per treatment. Every experimental unit consisted of a single Petri dish that was inoculated with three petiole explants. The Petri dishes were sealed with Parafilm® and were stored in darkness at 25 °C for 4-8 weeks. The number of induced explants as well as the type of embryogenic culture induced was registered weekly.
Embryogenic cultures were transferred onto semi solid maintenance medium (MM) (De Wald et al., 1989; Witjaksono and Litz, 1999a) that was supplemented with 4.52 µM 2.4-D and stored as indicated for induction with subculture to fresh medium at four-week intervals. Maintenance medium consisted of MS and (in mg L-1) sucrose (30000), myo-inositol (100), thiamine-HCl (0.1), TC- Agar (6000) and 4.52 µM 2.4-D. Approximately 200 mg of a two-week-old embryogenic culture that had been maintained on MM were inoculated into 250 mL Erlenmeyer flasks containing 50 mL of liquid maintenance medium (MM without TC- Agar). Erlenmeyer flasks were covered with aluminum foil, sealed with Parafilm® and maintained on a rotary shaker at 120 rpm in darkness at 25 °C. Cultures were transferred to fresh medium biweekly.
Somatic embryo development. Embryogenic cultures consisting of proembryonic masses (PEMs) from liquid medium were sieved through sterile 1.8 mm mesh nylon filtrate fabric. The smallest fraction was air-dried on several layers of sterile Kimwipes® to remove excess liquid medium, and the embryogenic tissue was inoculated into 20 mL semi solid somatic embryo development medium (SED) in sterile (100 x 20 mm) Petri dishes. SED consisted of MS medium with (in mg L-1) thiamine HCl, (0.4), myo-inositol (100) and Phytagel® (3000) (Sigma®). To evaluate the effect of sucrose concentration on somatic embryo development, SED also supplemented with three (87.64; 131.46 and 175.28 mM) sucrose levels. Approximately 100 mg of PEMs was evenly spread over the medium surface of each Petri dish and incubated for eight weeks in darkness at 25 °C. Treatments were distributed using a complete randomized design with 30 replicates per treatment. After eight weeks, the total number of opaque-white somatic embryos (>0.2 cm diam) that developed on each Petri dish was determined. Data were analyzed with ANOVA and means were separated by Duncan test.
Plant recovery. Opaque-white somatic embryos (>0.2 cm diam) were inoculated onto somatic embryo germination medium (SEG) supplemented with 2.89 µM GA3. SEG consisted of MS with (in mg L-1), thiamine HCl (4), myo-inositol (100), sucrose (30000) and Phytagel® (3000). In order to evaluate the effect of benzylamino purine (BAP) on somatic embryo conversion, four (0; 1.11; 2.22 and 4.44 µM) BAP levels were examined. The medium was autoclaved and dispensed in 25 mL aliquots into 125 mL sterile baby food jars. Seven white-opaque somatic embryos were inoculated into each jar (75 jars). Treatments were distributed using a complete randomized design with 20 replicates per treatment. The jars were covered with heavy duty aluminum foil, sealed with Parafilm® and were stored in translucent plastic boxes in a 16-h photoperiod provided by cool white fluorescent tubes (40-;50 µmol s-1 m-2) at 25 °C until roots and/or shoots were visible. Plants were recovered from opaque-white somatic embryos two-three months after inoculation onto SEG, and were maintained on MS basic medium. Data were analyzed with ANOVA and means were separated with Duncan test.
Medium sterilization. After all components were supplied, the pH of all media was adjusted to 5.7-;5.8 prior to addition of gelling agent. Media were sterilized by autoclaving at 121 °C at 1.1 kg cm-2 for 15 min.
RESULTS AND DISCUSSION
Embryogenic culture induction and maintenance. Embryogenic cultures consisted of PEMs (Figure 1A). Auxin (2.4-D) was necessary for induction and PEMs were visible after two weeks (Figure 2). The collected data showed that the lowest 2,4-D concentrations were sufficient for induction, while high concentrations of 2.4-D suppressed induction. Embryogenic cultures proliferated as PEMs on semisolid and in liquid medium (Figure 1B).
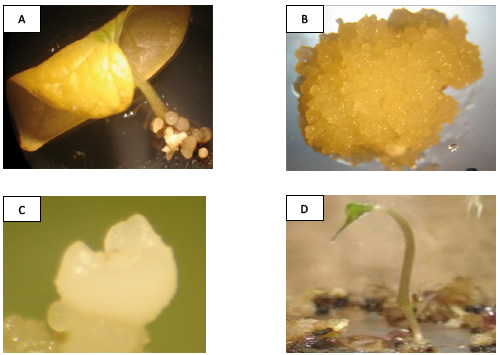
Figure 1. Dioscorea rotundata somatic embryogenesis. A: embryogenic culture induction, B: embryogenic culture maintenance, C: somatic embryo development and D: embryo conversion.
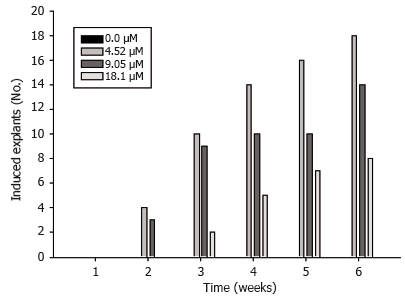
Figure 2. Effect of different auxin (2,4-D) µM on Dioscorea rotundata embryogenic tissue induction.
Somatic embryo development. Opaque-white somatic embryos developed from embryogenic cultures after transfer onto SED medium. The somatic embryos were opaque-white and round (Figure 1C). Results of ANOVA demonstrated that the number of developed somatic embryos was significantly affected (P< 0.0001) by sucrose concentration in the medium. Sucrose concentration at 131.46 mM resulted in more opaque-white somatic embryos per Petri dish (60) than sucrose at 87.64 mM (43). The lowest number of opaque-white somatic embryos (13) was observed with 175.28 mM sucrose.
Plant recovery. Opaque-white somatic embryos (≥0.3 mm diameter) germinated (i.e., root and shoot emergence) after four weeks on SEG medium. Recovered plants appeared to be normal with no evidence of morphological variation (Figure 1D). The analyzed data showed that BAP levels had no effect (P= 0.9399) on root and shoot development from somatic embryos (Figure 3).
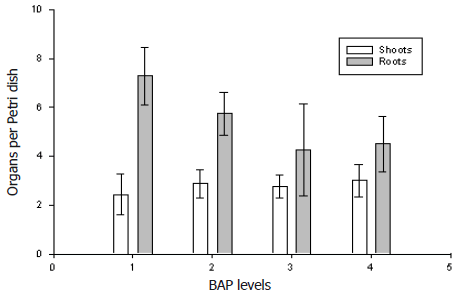
Figure 3. Effect of different BAP levels (1 = 0; 2 = 1.11; 3 = 2.22 and 4 = 4.44 µM) on shoots and root development means (bars=standard errors) from Dioscorea rotundata somatic embryos.
In vitro studies with Dioscorea species have included micropropagation from pre-existing meristems and organogenenesis of D. zingiberensis (Chen et al., 2003), shoot organogenesis form immature leaves of D. opposita (Kohmura et al., 1995), shoot culture and microtuber formation in D. composita (Alizadeh et al., 1998) and shoot multiplication and in vitro tuber formation from D. alata stem segments (John et al., 1993; Salazar and Hoyos, 2007). Somatic embryogenesis in D. floribunda, D. composita, D. alata and D. bulbifera has been previously reported (Ammirato, 1978; 1982). In the present study, embryogenic culture induction from D. rotundata leaf tissue was possible after two weeks culture on 2.4-D supplied medium in darkness, although low induction frequencies (<30%), indicates a strong recalcitrance of the species to induce embryogenic tissues. Differential effects of 2.4-D concentrations on embryogenic cell induction have been observed. Embryogenic cell masses from D. alata root explants were induced in liquid MS supplemented with 4.52 µM 2.4-D in the light (Twyford and Mantell, 1996), while the highest induction rate of induction of D. opposita embryogenic cultures was observed when stem segments were cultured in liquid medium supplied with 13.6 µM 2.4-D (Nagasawa and Finer, 1989). Strong auxins, such as 2.4-D and picloram, are normally used for embryogenic culture induction. They stimulate rapid cell division and induce a redetermination of cell type and function associated with differential changes in gene expression that is probably linked to demethylation of DNA (Kohlembach, 1978; LoSchiavo et al., 1989). In the current study, somatic embryo development from PEMs occurred on auxin-free medium; however, Ammirato (1982) reported somatic embryo development from D. bulbifera and D. floribunda embryogenic cultures on media supplied with either 0.1 µM zeatin or 0.1 µM ABA in the absence of 2.4-D, which indicates a possible genotype dependent response. Development of D. opposita and D. alata somatic embryos from PEMs was also observed following their transfer to auxin-free liquid media (Nagasawa and Finer, 1989; Twyford and Mantell, 1996). Somatic embryo formation can occur when low levels of weaker auxins are present in the medium; Shu et al. (2005) reported low frequency of D. zingiberensis somatic embryo development when PEMs were transferred onto semi solid medium supplied with 17.74 µM BA and 1.07 µM NAA.
A significantly larger number of somatic embryos occurred when development medium contained 4.5% sucrose while at 6.0% the value decreased indicating a possible overdose. Sucrose is the most suitable carbohydrate source for D. rotundata plant recovery from somatic embryos (Okezie et al., 1994). Higher sucrose levels increase medium osmolarity in order to stimulate somatic embryo maturation by lowering water content in the developing embryo, similar to the maturation stage of zygotic embryogenesis, allowing a more normal development process and protein accumulation (Compton and Gray, 1996; Gray, 1995). Additionally, higher sucrose level means more carbon nutrition for a better embryo development (Lee and Thomas, 1985; Carman, 1989). GA3 has been used to overcome somatic embryo dormancy and increase germination levels (Deng and Cornu, 1992). Twyford and Mantell (1996) observed enhanced D. alata plantlet recovery when somatic embryos were cultured on basal semisolid MS medium supplemented with 4.55 µM GA3. Likewise, cytokinine addition to the germination medium improved organization of the apical meristem of carrot somatic embryos (Fujimura and Komamine, 1975). In the present study, BAP had no effect on the recovered of plants. Similar results were reported for avocado somatic embryos (Witjaksono and Litz, 1999b).
CONCLUSIONS
Dioscorea rotundata can be regenerated via somatic embryogenesis. The highest frequency of embryogenic culture induction was observed on medium supplemented with 4.52 µM 2.4-;D. Sucrose at 131.46 mM significantly increased development of opaque-white somatic embryos, while BAP had no effect on somatic embryo conversion. This regeneration protocol can be utilized for non-traditional in vitro breeding techniques in yam.
BIBLIOGRAPHY
Alizadeh, S., S. Mantell and A. Viana. 1998. In vitro culture and microtuber induction in the steroid yam Dioscorea composita Hemsl. Plant Cell, Tissue and Organ Culture 53(2): 107-112. [ Links ]
Ammirato, P. 1978. Somatic embryogenesis and plantlet development in suspension cultures of the medicinal yam, Dioscorea floribunda. American Journal of Botany 65(27): 89-95. [ Links ]
Ammirato, P. 1982. Growth and morphogenesis in cultures of the monocot yam Dioscorea. pp. 169-170. In: Fujiwara, A. (ed.). Plant Tissue Culture. Proceedings 5th International Congress of Plant Tissue and Cell Culture. Japanese Association for Plant Tissue Culture Maruzen, Tokyo, Japan. [ Links ]
Carman, J. 1989. The in ovulo environmet and its relevance to cloning wheath via somatic embryogenesis. In Vitro Cellular and Developmental Biology 25: 1155-1162. [ Links ]
Chen, Y., J. Fan, F. Yi, Z. Lou and Y. Fu. 2003. Rapid clonal propagation of Dioscorea zingiberensis. Plant Cell, Tissue and Organ Culture 73(1): 75-80. [ Links ]
Compton, M. and D. Gray. 1996. Effects of sucrose and methylglyoxal bis-(guanylhydrazone) on controlling grape somatic embryogenesis. Vitis 35(1): 1-6. [ Links ]
Deng, M. and D. Cornu. 1992. Maturation and germination of walnut somatic embryos. Plant Cell, Tissue and Organ Culture 28(2): 195-202. [ Links ]
DeWald, S., R. Litz and G. Moore. 1989. Optimizing somatic embryo production in mango. Journal of American Society for Horticultural Sciences 114(4): 712-716. [ Links ]
Espitia, A. and I. Quintero, 1999. Estandarización de la técnica de micropropagación por embriogénesis somática en ñame (D. alata) var. Diamantes 22. Trabajo de grado Ingeniero Agrónomo. Facultad de Ciencias Agrícolas. Universidad de Córdoba, Montería. 84 p. [ Links ]
Fujimura, T. and A. Komamine. 1975. Effect of various growth regulators on the embryogenesis in a carrot cell suspension culture. Plant Science Letters 5(6): 359-364. [ Links ]
Gamborg, O., R. Miller and K. Ojima. 1968. Plant cell cultures I. Nutrient requirement of suspension cultures of soybean root cells. Experimental Cell Research 50(1): 151-158. [ Links ]
Gray, D. 1995. Somatic embryogenesis in grape. pp. 191-217. In: Jain, S., P. Gupta and R. Newton, (eds.). Somatic embryogenesis in woody plants. Kluwer Academic Publishers, Dordrecht, The Netherlands. 756 p. [ Links ]
Jayasankar, S. and R. Litz. 1998. Characterization of embryogenic mango cultures selected for resistance to Colletotrichum gloeosporioides culture filtrate and phytotoxin. Theoretical and Applied Genetics 96(6-7): 823-831. [ Links ]
Jayasankar, S., L. Zhijian and D. Gray 2000. In vitro selection of Vitis vinifera ''Chardonnay'' with Elsinoe ampelina culture filtrate is accompanied by fungal resistance and enhanced secretion of chitinase. Planta 211(2): 200-208. [ Links ]
John, J.L., W.H. Courtney and D.R. Decoteau. 1993. The influence of plant growth regulators and light on microtuber induction and formation in Discorea alata L. cultures. Plant Cell Tissue and Organ Culture 34: 245-252. [ Links ]
Kohmura, H., H. Araki and M. Imoto. 1995. Micropropagation of Yamaimoto Chinese yam (Dioscorea opposita) from inmature leaves. Plant Cell, Tissue and Organ Culture 40: 271-276. [ Links ]
Kohlembach, H. 1978. Regulation of embryogenesis in vitro. In: Schutte, H. and D. Gross (eds.). Regulation of developmental processes in plants. Proceedings of a conference held at Halle, Germany. 20 p. [ Links ]
Lee, C. and J. Thomas. 1985. Jojoba embryo culture and oil production. HortScience 20(4): 762-764. [ Links ]
LoSchiavo, F., L. Pitto, G. Giuliano, G. Torti, V. Nuti-Ronchi, D. Marazziti, R. Vergara, S. Orselli, and M. Terzi. 1989. DNA methylation of embryogenic carrot cell cultures and its variations as caused by mutation, differentiation, hormones and hypomethylating drugs. Theoretical and Applied Genetics 77(3): 325-331. [ Links ]
Mignouna, H., M. Abang, K. Green and R. Asiedu. 2001. Inheritance of resistance in water yam (Dioscorea alata) to anthracnose (Colletotrichum gloeosporioides). Theoretical and Applied Genetics 103(1): 52-55. [ Links ]
Murashige, T. and F. Skoog. 1962. A revised medium for rapid growt and bioassays whith tobacco tissue cultures. Physiologia Plantarum 15: 473-497. [ Links ]
Nagasawa, A. and J. Finer. 1989. Plant regeneration from embryogenic suspension cultures of Chinese yam (Dioscorea opposita Thunb.). Plant Science 60: 263-271. [ Links ]
Okezie, C., S. Okonkwo and F. Nwoke. 1994. Carbon source requiremen for the culture of white yam (Dioscorea rotundata) embryos in vitro. Acta Horticulturae 380: 329-334. [ Links ]
Pérez, L., M. Baquero, and J. Beltrán. 2003. Caracterización morfológica y patogénica de Colletotrichum spp. como agente causal de la antracnosis en ñame Dioscorea sp. Revista Colombiana de Biotecnología 5(1): 24-35. [ Links ]
Royero, M., T. Vargas and M. Oropeza. 2007. Micropropagación y organogénesis de Dioscorea alata. INCI 32(4): 247-252. [ Links ]
Salazar, R. y R. Hoyos. 2007. Multiplicación y tuberización de ñame (Dioscorea alata L.) en sistema de inmersión. Revista Facultad Nacional de Agronomía, Medellín 60(2): 3907-3921. [ Links ]
Sánchez, C. and L. Hernández. 2000. Descripción de aspectos productivos, de postcosecha y de comercialización del ñame en Córdoba, Sucre y Bolívar, http://www.turipana.org.co/name.htm; consulta: diciembre 2007. [ Links ]
Shu, Y., Ying-Cai, Y. and L. Hong-Hui. 2005. Plant regeneration through somatic embryogenesis from callus cultures of Dioscorea zingiberensis. Plant Cell, Tissue and Organ Culture 80(2): 157-161. [ Links ]
Twyford, C. and S. Mantell. 1996. Production of somatic embryos and plantlets from root cells of the Greater Yam. Plant Cell, Tissue and Organ Culture 46(1): 17-26. [ Links ]
Witjaksono, L. and R.E. Litz. 1999a. Induction and growth characteristics of embryogenic avocado cultures. Plant Cell, Tissue and Organ Culture 58(1): 19-29. [ Links ]
Witjaksono, L. and R.E. Litz. 1999b. Maturation of avocado somatic embryos and plant recovery. Plant Cell, Tissue and Organ Culture 58(2): 141-148. [ Links ]














