Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad Nacional de Agronomía Medellín
Print version ISSN 0304-2847
Rev. Fac. Nac. Agron. Medellín vol.65 no.2 Medellín June/Dec. 2012
POLYCLONAL ANTIBODIES TO DETECT THE CP-RT PROTEIN OF Potato Mop-Top VIRUS
ANTICUERPOS POLICLONALES PARA LA DETECCIÓN DE LA PROTEÍNA CP-RT DEL Potato Mop-Top VIRUS
Derly Andrade Molina1; Yuliana Gallo García2; Pablo Gutiérrez Sánchez3 and Mauricio Marín Montoya4
1 Biologist. Universidad de Nariño - Departament of Biology. Ciudad Universitaria Torobajo, Pasto, Colombia <derlyan11@gmail.com>
2 Biological Engineer. M.Sc. Biochemistry. Universidad Nacional de Colombia - Sede Medellín - Faculty of Sciences - Laboratory of Industrial Microbiology. A.A. 3840, Medellín, Colombia <ymgallo@gmail.com>
3 Associate Professor. Universidad Nacional de Colombia - Sede Medellín - Faculty of Sciences - School of Biosciences - Laboratory of Industrial Microbiology. A.A. 3840, Medellín, Colombia <paguties@unal.edu.co>
4 Associate Professor. Universidad Nacional de Colombia - Sede Medellín - Faculty of Sciences - School of Biosciences - Laboratory of Cell and Molecular Biology. AA. 3840, Medellín, Colombia <mamarinm@unal.edu.co>
Recibido: Junio 20 de 2012; aceptado: Septiembre 29 de 2012.
Abstract. Potato mop-top virus (PMTV) causes an important re-emerging disease in potato crops in Colombia due to the increased incidence of its protozoan vector Spongospora subterranea f.sp. subterranea (Sss), the causing agent of Powdery scab disease. For an accurate detection of PMTV it is recommended to combine different diagnostic tests and evaluate multiple samples per plant or tissue. In order to increase the number of available tools for detection of PMTV, antibodies targeting the RT domain of the CP-RT protein were developed in this work. Sequencing of the RT domain from the colombian strain R25 was achieved and using bioinformatic analysis, a potential antigenic region was identified. A peptide mimicking the antigenic region was inoculated in rabbits for the production of polyclonal antibodies. The antibodies were tested by ELISA using Nicotiana benthamiana bait-plants infected with Sss cystosori and potato plants collected in La Unión (Antioquia). The validity of serological tests was confirmed by RT-PCR. A complete sequence of the RT domain and 441 nt of the CP gene were obtained. Phylogenetic analysis identified strain R25 as closely associated to the PMTV lineage distributed worldwide. A total of 19.26 mg of anti-CP-RT polyclonal antibodies useful in detecting PMTV in infected plants were obtained. As CP-RT is involved in transmission of PMTV by Sss, these antibodies will be useful for supporting not only diagnostic programs but also basic and epidemiologic studies aimed at understanding interactions between PMTV and Sss.
Key words: ELISA, Pomovirus, RT-PCR, Solanum tuberosum.
Resumen. El Potato mop-top virus (PMTV) es uno de los virus re-emergentes en los cultivos de papa de Colombia, como resultado del aumento de la incidencia de su vector natural, el protozoo Spongospora subterranea f.sp. mamarinm@unal.edu.co (Sss), agente causal de la Sarna polvosa de la papa. Para la detección del virus se recomienda la realización simultánea de diferentes pruebas, así como la evaluación de múltiples submuestras por tejido bajo análisis. Con el fin de ampliar el rango de herramientas para la detección de PMTV, se obtuvieron anticuerpos dirigidos al dominio RT de la proteína CP-RT. Se secuenció dicho dominio en el aislamiento colombiano R25 de PMTV y se realizaron análisis bioinformáticos para ubicar regiones peptídicas con alto potencial inmunogénico. El péptido seleccionado fue inoculado en conejos para obtener anticuerpos, cuya utilidad fue evaluada a partir de pruebas de ELISA indirecto en plantas señuelo de Nicotiana benthamiana inoculadas con quistosoros de Sss y en plantas de papa obtenidas en La Unión (Antioquia). Estas pruebas estuvieron acompañadas con evaluaciones de RT-PCR. Se obtuvo la totalidad del dominio RT, así como 441 nt del gen CP. Los análisis filogenéticos identificaron la cepa R25 como asociada al linaje de PMTV mundialmente distribuido. Se obtuvieron 19,26 mg de anticuerpos, los que resultaron efectivos para detectar PMTV. Ya que CP-RT es responsable de la transmisión del PMTV por Sss, estos anticuerpos permitirán no sólo apoyar los programas de diagnóstico, sino también estudios básicos y epidemiológicos tendientes a evaluar las interacciones del PMTV y Sss.
Palabras clave: ELISA, Pomovirus, RT-PCR, Solanum tuberosum.
Potato (Solanum tuberosum L.) is a very important crop in Colombia, comprising 128,701 ha distributed in 14 provinces and supporting 69,000 families (MADR, 2009). The provinces of Antioquia, Boyacá, Cundinamarca and Nariño are responsible for 85% of the total production (approximately 2.3 million t/year). In recent years, Colombia has experienced a dramatic increase in Powdery scab disease, caused by the protozoan Spongospora subterranea (Wallroth) Lagerheim f.sp. subterranea Tomlinson (Sss). Sss not only causes significant reductions in tuber quality and production but is also the natural vector of Potato mop-top virus (PMTV). PMTV is one of the most prevalent viruses in the Andes and is under quarantine surveillance in several countries around the world (Salazar, 2006; Santala et al., 2010); in Colombia, its presence has been confirmed by RT-PCR and DNA sequencing (Vélez, 2007; Gil et al., 2011).
PMTV has a tripartite genome organization composed of positive single-stranded RNAs (Savenkov et al., 1999) and is the type species of the genus Pomovirus (Virgaviridae) (Adams et al., 2009). RNA1 encodes a methyltransferase and helicase protein (ORF1) and a readthrough protein with RNA-dependent RNA polymerase motifs (RdRp) (Savenkov et al., 1999, 2003). RNA2 encodes the 20 kDa coat protein and a 91 kDa readthrough protein, CP-RT. This protein has been shown to be located at the ends of viral particles and seems to be involved in PMTV transmission by Sss (Cowan et al., 1997). RNA3 encodes four proteins of 51, 21, 13 and 8 kDa, respectively. The first three proteins, also known as the Triple Gene Block or TGB, are involved in cell to cell movement, while the 8 kDa protein is a cysteine-rich polypeptide involved in systemic movement and development of necrotic symptoms (Savenkov et al., 1999, 2003). Interestingly, all three RNAs have a conserved tRNA-like structure at their 3'ends (Savenkov et al., 1999).
Symptoms caused by PMTV in potato varieties cultivated in Colombia and the Andean region are unknown (Salazar, 2006; Gil et al., 2011); however, it is reasonable to assume that parallel to the Sss increase (Carreño, 2009; Gilchrist et al., 2011; Osorio, 2012) the incidence of PMTV is also on the rise. Therefore, it is important to implement diagnostic tests that help to reduce the incidence of PMTV, support quarantine measures and aid in the development of resistant plants to Sss and/or PMTV. Unfortunately, detection of PMTV can be difficult due to low titer levels, symptom variability, erratic distribution in infected plants and systemic infections with naked RNA (Xu et al., 2004; Santala et al., 2010; Shemyakina et al., 2011). Current methods to detect PMTV include the use of bait plants such as Chenopodium amaranticolor and Nicotiana benthamiana (Jeffries, 1998); ELISA tests, using mono and polyclonal antibodies (Arif and Torrance, 1996; Nielsen and Mølgaard, 1997; Cerovska et al., 2003); RT-PCR, using CP and TGB2 specific primers (MacKenzie, 1996; Xu et al., 2004); real-time PCR, using Taqman probes (Mumford et al., 2000); RT-PCR-microplate hybridization (RT-PCR-MPH) (Nakayama et al., 2010); macro (Maoka et al., 2010) and microarrays (Nicolaisen, 2011).
The use of these techniques requires an adaptation of all sequence-dependent tests (such as antibodies, primers and probes) to the predominant genotypes in the region and their variations. In Colombia, RT-PCR detection of PMTV using CP and TGB2 specific primers has been satisfactory; however, amplification problems due enzyme inhibition are common (Vélez, 2007; Gil et al., 2011; Osorio, 2012). Gil (2010) performed a comparison between detections levels using ELISA (with commercial antibodies) and the qRT-PCR developed by Mumford et al. (2000), with mixed results. Using a total of 40 samples, 45% tested positive using qRT-PCR, while only 25 and 28% tested positive using ELISA and conventional RT-PCR, respectively. Interestingly, some samples tested positive for two out of three tests and in some cases PMTV was detected only in the ELISA test. These results corroborate the importance of combining several diagnostic tools in PMTV detection. Using synthetic peptides, Gallo (2012) obtained polyclonal antibodies targeting the N-terminal end of the CP protein of colombian isolates. These antibodies were able to detect PMTV in bait N. benthamiana plants and potato fields in eastern Antioquia.
The use of recombinant proteins and synthetic peptides derived from structural studies is an interesting alternative for the production of antibodies against viruses that are difficult to purify or present at very low titers, such as PMTV (Vaira et al., 1996; Helias et al., 2003; Cerovska et al., 2006). Due to its external location in the viral particle (Cowan et al., 1997) and the presence of highly hydrophylic segments, CP-RT seems to be a good candidate for the production of PMTV-specific antibodies. This protein is involved in transmission by Sss (Cowan et al., 1997; Reavy et al., 1997; Sandgren et al., 2001; Latvala-Kilby et al., 2009) and its detection could be used not only in diagnostics but also in epidemiological studies aimed at understanding the interaction of PMTV with its natural vector. This work describes the production of CP-RT antibodies using synthetic peptides mimicking potential surface-exposed regions of colombian PMTV strains from the municipality of La Unión (Antioquia). The antibodies were tested in bait-plants and field samples. The presence of PMTV was confirmed by RT-PCR and sequencing.
MATERIALS AND METHODS
Samples. This research was performed on PMTV strains inoculated in N. benthamiana bait-plants using Sss cystosori (Gil, 2010; Osorio, 2012), in a greenhouse at Paysandú experimental station of the Universidad Nacional de Colombia, located in the Santa Elena district at the municipality of Medellín (6º12'37'' N and 75º30'11'' W). Potato root and leaf samples were collected in the municipality of La Unión (Antioquia), (5°58'38'' N and 75°24'54'' W).
Partial sequencing of RNA2 from PMTV. Sequencing of the RT region of PMTV was performed on isolate R25 from La Unión (Antioquia). Total RNA was extracted from leaves and roots of infected N. benthamiana baits using the RNeasy plant mini (Qiagen®). 100 mg of tissue were resuspended in 450 mL of either RLT (leaf tissue) or RLC (root tissue) buffer, supplemented with 4.5 mL of ß-mercaptoethanol following manufacturer's recommendations. Extracted RNA was resuspended in 40 mL DEPC-treated water.
cDNA was obtained in two steps. During the retrotranscription step, primer 123-end (5' GTG AAC CAC GGT TTA RCC CTG KAA GC 3') was used. This primer binds to a conserved site at the 3' end of the three RNA segments of PMTV (Savenkov et al., 1999). This reaction was carried out in a total volumen of 15 mL containing 1.5 mL distilled water, 4 mL RT buffer (5X), 4 mL MgCl2 (25 mM); 2 mL dNTP's (10 mM), 1 mL of primer solution (10 mM), 0.5 mL RNase inhibitors (40U/mL), 2 mL M-MuLV Reverse Transcriptase (20 U/mL) (Fermentas®) and 5 mL of template RNA. The amplification cycle consisted of an incubation step at 37 °C por 60 min followed by 16 min of inactivation at 75 °C. Samples were preserved at 4 °C until further use. PCR reactions were carried out in a total volume of 25 mL containing 17.8 mL ddH20, 2.5 enzyme buffer (10X), 1.8 mL MgCl2 (25 mM), 0.5 mL dNTPs (10 mM), 0.5 mL of each primer (10 mM) (Table 1), 0.4 mL of Taq polymerase (5 U/mL) (Fermentas®) and 1 mL of cDNA. The PCR program consisted of a denaturation step at 95 °C for 30 s, and 40 cycles of 95 °C for 30 s, 54-62 °C (Tabla 1) for 45 s, 72 °C for 1 min and a final extension at 72 °C for 5 min. Amplicons were purified using the QIAquick Gel Extraction and QIAquick PCR Purification (Qiagen®) kits following the manufacturer's recommendations.
DNA was sequenced using the RT-PCR primers in an ABI Prism 3730xl (PE Biosystems®) at Macrogen®. Sequences were edited with BioEdit 6.0.6 (Hall, 1999) and their identity confirmed with BLASTn (http://www.ncbi.nlm.nih.gov/BLAST/Blast.cgi). RNA2 was assembled with CAP3 (Huang and Madan, 1999); the reading frame was confirmed at the EXPASY server (http://www.expasy.ch/tools/dna.html). DNA and protein similarity between PMTV strains was analyzed using Mega 5.0 (Tamura et al., 2011).
Production of CP-RT specific antibodies. Potential antigenic peptides in the RT domain of PMTV were identified with a hydrophilicity analysis using the Hopps-Woods method (Hopps and Woods, 1981) implemented in BioEdit 6.0.6 (Hall, 1999) and ProtScale(http://expasy.org/tools/protscale.html). Peptide Pep_CP_RT was selected as it combined high sequence conservation and antigenicity. Pep_CP_RT was synthesized at GenScript® using solid-phase synthesis with a 2-Cl-Trityl resin. The crude peptide was dissolved in 10% acetonitrile and analyzed by reverse-phase HPLC on a Shimazu 20A system using a X-bridge column (Waters, i.d.4.6 mm x 250 mm). The peptides were purified by semi-preparative HPLC. Peptide Pep_CP_RT was coupled to the carrier protein keyhole limpet hemocyanin (KLH) and inoculated into two New Zealand rabbits with reinforcements at 14, 35 and 56 dpi. Polyclonal antibodies, Anti_CP_RT, were affinity-purified, resuspended in PBS buffer at pH 7.4 (4 g NaCl, 0.1 g KCl, 0.288 g Na2HPO4, 0.048 g KH2PO4 per liter) and lyophilized until further use.
Specificity of the Anti_CP_RT antibodies against Pep_CP_RT was evaluated using dot-blot. Pep_CP_RT was fixed in a nitrocellulose membrane and blocked with 5% skim milk in PBS (pH 7.4) for 1 h at room temperature. Afterwards, the membrane was incubated during three hours in a 1:1000 dilution of Anti_CP_RT. The blot was incubated with anti-rabbit alkaline phosphatase conjugated secondary antibody (Bio-Rad®) for 1 h, washed three times with PBS and developed with BCIP/NBT (5-bromo-4-chloro-3-indolyl phosphate/Nitroblue Tetrazolium). The minimum detectable amount of peptide was determined by dot-blot using dilutions of Pep_CP_RT in the de 5 mM to 600 mM range. The minimum amount of antibodies was determined using dilutions of Anti_CP_RT in the 1:1000 a 1:8500 range at a 0.2 mM concentration of Pep_CP_RT.
Detection of PMTV by indirect ELISA. Efficacy of the Anti_CP_RT antibodies was evaluated by indirect ELISA using 86 samples from N. benthamiana bait plants inoculated with Sss cystosori isolated in Antioquia, Boyacá, Cundinamarca and Nariño (Gil, 2010; Osorio, 2012). 44 samples were collected from roots and 42 from leaves. 100 mg of tissue were macerated in extraction buffer (Bioreba®) supplemented with 10 g/L egg albumin for root samples and in PBS (pH 7.4) in the case of leaf samples. 200 mL of homogenate were added into wells of a polystyrene plate and incubated in a wet chamber for 1 h. After incubation, the plate was washed four times with PBS-T (PBS supplemented with 0.05% Tween 20) and 200 mL of Anti_CP_RT (1:1000) were added. After 2 h of incubation at room temperature, the plate was washed again with PBS-T and 200 mL of anti-rabbit alkaline phosphatase conjugated antibodies (1:1000) (Bio-Rad®) were added. After 1 h of incubation, the plate was developed using 200 mL 25X Bio-Rad developing solution and incubated in the dark for 30 min. Absorbance was measured in a Multiscan plate reader at 405 nm (Thermo®). Readings two-times higher that the negative control were considered positive (Matthews, 1993).
Detection of PMTV by RT-PCR. The presence of PMTV was confirmed in 17 ELISA-positive samples by RT-PCR using the procedures described above with primers targeting the CP (H360: 5' CAT GAA GGC TGC CGT GAG GAA GT 3' and C819: 5'CTA TGC ACC AGC CCA GCG TAA CC 3') and TGB2 (PMTVF4: 5' CAG CAA CCA CAA ACA GAC AGG-3' and PMTVR4: 5' AGC CAC TAA CAA AAC ATA CTG C-3') genes described in previous studies (MacKenzie, 1996; Xu et al., 2004; Vélez, 2007; Gil et al., 2011).
Detection of PMTV in field samples. The usefulness of Anti_CP_RT antibodies was tested in potato samples collected in the municipality of La Unión (Antioquia) using indirect ELISA as described above. Three root samples and two from leaves were collected in each of four selected potato plots. Results were compared with a commercial DAS-ELISA kit targeting the CP protein of PMTV (Bioreba®). Root samples were also evaluated by RT-PCR using primers H360 and C819 targeting the CP gene. Finally, two random amplicons were sequenced to confirm their identity.
RESULTS AND DISCUSSION
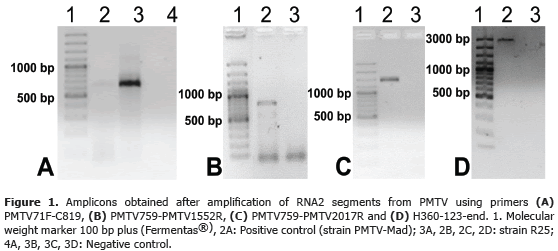
Partial sequencing of the RNA2 from PMTV. With the exception of PMTV71F-C819, all RT-PCR primers (H360-C819, PMTV759-PMTV2017R, PMTV759-PMTV1552R and H360-123-end) amplified fragments of the expected size (Figure 1). In this way, a 2,566 nt contig was assembled ranging from position 398 to 2,963 relative to the PMTV-Sw from Sweeden (Accession AJ243719) (Sandgren et al., 2001). This contig comprises a fragment of the CP gene, the complete RT domain and a segment of the 3' UTR. In summary, a 81.8% coverage of the RNA2 from the colombian isolate R25 of PMTV was obtained, comprising 147 residues (out of 176) of the CP protein and the complete 649 residues of the RT domain. It is important to note that CP-RT is translated as a 825 amino acids protein including the CP domain.
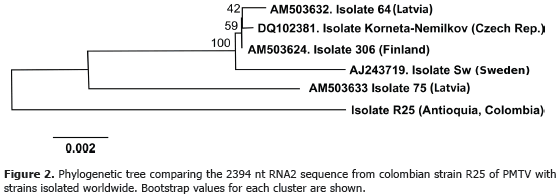
A phylogenetic analysis was performed comparing 2,394 nt of isolate R25, and isolates from Latvia (64: AM503632 and 75: AM503633), Czech Republic (Korneta-Nemilkov: DQ102381), Finland (306: AM503624) and Sweeden (Sw: AJ243719). The dendrogram shows a single clade and two individual branches. The clade was supported by a 100% bootstrap and included all european isolates with the exception of isolate 15 from Latvia, which was segregated as an individual branch. Isolate R25 was also in an individual branch (Figure 2). A similar topology was obtained using amino acid sequences although with lower bootstrap support (64%) (data not shown).
All isolates share at least 97% identity at both nucleotide and amino acid level, resulting in a very low genetic distances for the whole population (d=0.0122; SD=0.0014). This result suggests that the R25 isolate is part of the PMTV lineage distributed worldwide. Previously, Gil et al. (2011) found two PMTV lineages in Colombia, using CP sequencing of isolates from different potato-growing provinces. The first one was highly similar to the worldwide strain (>97% genome identity). The second lineage shared less than 76% identity with the first group and may correspond to a novel pomovirus species according to the criteria proposed by Adams et al. (2009) for the Virgaviridae family.
Despite the high levels of identity found in this study, 24 variable sites were detected in the RT domain region of RNA2, nine of them correspond to amino acid substitutions only present in the R25 strain (K194E, S276R, V330M, T484S, E509G, S708P, R747S, S790G and G792V). Future studies should investigate the biological importance of these mutations and their effect on transmission by Sss. Interestingly, Carreño (2009) and Osorio (2012) have found a higher level of variation in Colombian Sss strains as compared to temperate zones.
Several studies on PMTV performed in Colombia (Gil et al., 2011), Europe (Latvala-Kilby et al., 2009; Santala et al., 2010) and USA (Xu et al., 2004; Davis et al., 2010) concluded that development of detection methods is a key factor in controlling PMTV in potato crops. This is a complex issue due to the erratic distribution of PMTV in plants and tubers, the wide variation of symptoms that it causes in different cultivars, the low titers that reaches in the plant tissues and the ability of this virus to cause systemic infections in absence of assembled particles (Wright et al., 2010). For this reason, this study aims at the obtention of antibodies useful in the serological detection of PMTV using the sequence information obtained for the RNA2 of strain R25.
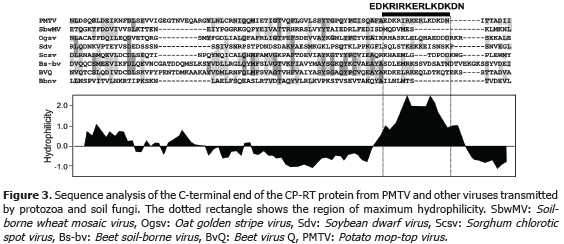
Production of peptides and CP-RT specific antibodies. The search of antigenic regions was based on the method of Hopp and Woods (1981), which identifies clusters of hydrophilic residues as potential epitopes. Figure 3 shows a sequence analysis of the CP-RT protein of viruses transmitted by fungi and protozoa, including PMTV. This comparison reveals that the most variable region correlates well with a highly hydrophilic segment. In addition, this region is very well conserved between different PMTV strains and was chosen to synthesize an immunogenic peptide with sequence EDKRIRKERLKDKDN. The CP-RT protein is involved in PMTV transmission by Sss as demonstrated by Cowan et al. (1997) and Sandgren et al. (2001) and its detection should complement serological tests based on the CP protein (Arif and Torrance, 1996, Nielsen and Mølgaard, 1997; Gallo, 2012). Moreover, detection of CP-RT will allow the detection of virions in their transmissible form by Sss.
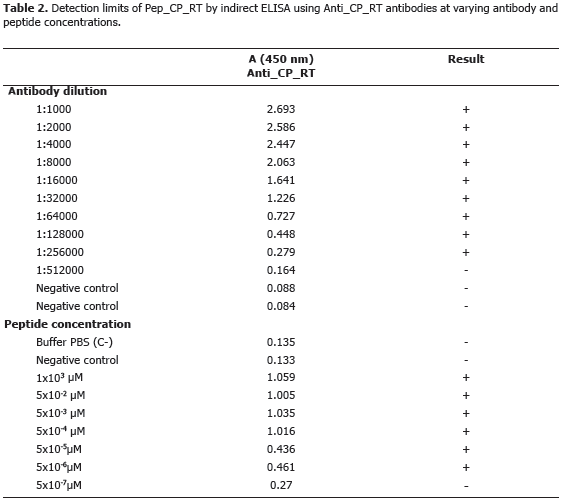
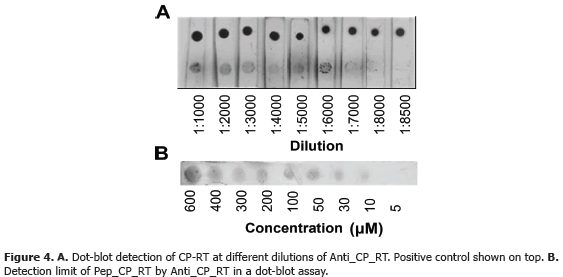
A total of 19.26 mg of polyclonal antibodies were obtained after rabbit inoculation with 0.5 mg of peptide. Serial dilutions showed a titer of 1:256000 for indirect ELISA (Table 2) and 1:8500 for dot-blot (Figure 4A). Sensitivity tests revealead threshold detection concentrations of 5 mM (Figura 4B) and 5x10-7 mM (Table 2) for dot-blot and ELISA, respectively.
This result shows a ten million-fold sentivity of ELISA as compared to dot-blot. This sentivity values are only valid for alkaline-phosphatase detection and could be significantly improved using alternative detection systems such as chemiluminiscense. ELISA was chosen as the most appropriate technique for subsequent analysis.
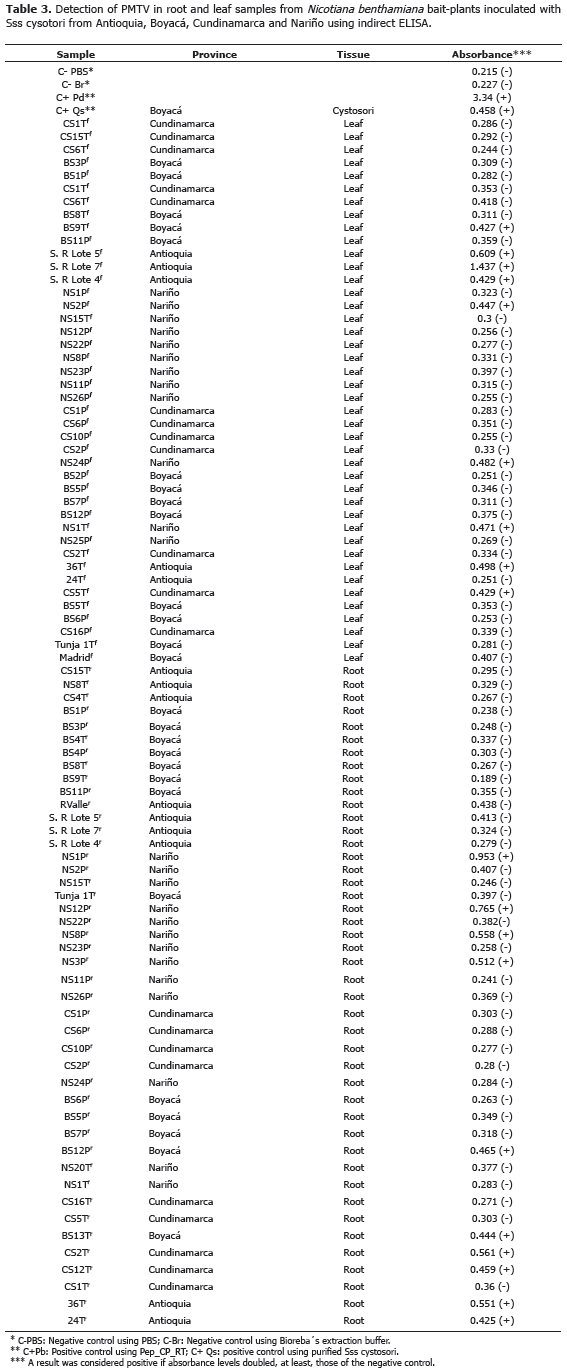
Detection of PMTV using indirect ELISA. Anti_CP_RT antibodies were first tested in root and leaf samples from N. benthamiana bait-plants inoculated with Sss strains from the provinces of Antioquia, Cundinamarca, Boyacá and Nariño. From a total of 42 leaf samples, 21.4% tested positive by indirect ELISA with absorbance values in the 0.427-1.437 range. Ten root samples (22.7%) out of 44 were positive with absorbance values in the 0.425-0.953 range. Absorbance values for the negative control were 0.215 and 0.227 for leaf and root samples, respectively (Table 3).
RT-PCR detection of PMTV. RT-PCR amplified fragments of ~460 bp of the CP gene in 15 out of 17 positive ELISA samples. On the other hand, primers PMTVF4 and PMTVR4 targeting the TGB2 gene amplified a ~417 bp fragment in only 6 of all evaluated samples.
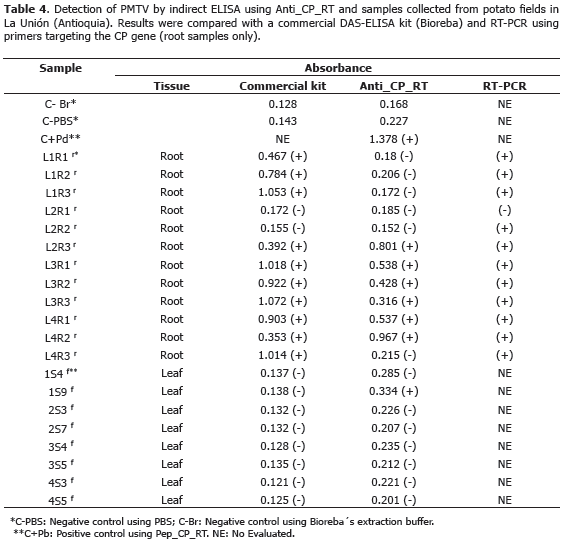
Pilot field test for PMTV detection. Selected plots had a previous report of Sss which was confirmed in collected samples with typical diagnostics traits such as root galls. Indirect ELISA detected PMTV in one leaf and six root samples. None of the leaf samples tested positive using a commercial DAS-ELISA kit (Bioreba®); however, 10 out 12 root samples were positive using the same kit. Commercial antibodies resulted in absorbance readings in the 0.353-1.072 range for positive samples while Anti_CP_RT antibodies gave readings in the 0.316-0.967 range (Table 4). RT-PCR resulted in ~460 bp amplicons of the CP genes and a total of 11 positive root samples out of 12 (Table 4). The higher proportion of positive samples in roots as compared to leaf samples can be explained by the role of CP-RT in virus transmission. CP-RT is expected to be more abundant in the basal portion of plants where the Sss vector is present in contrast to aerial plant parts where PMTV can be found in naked form together with movement proteins (Wright et al., 2010). Differences in the number of positive samples detected by the two antibodies used in this study confirms the importance of combined diagnostic methods, as previously suggested by Xu et al. (2004) and Santala et al. (2010) who demonstrated several inconsistencies in detection methods targeting PMTV. Our results suggest the generalized presence of PMTV virions together with CP-RT proteins in field plants, a fact that can be explained by the high incidence levels of Sss in potato fields of La Unión (Jaramillo and Botero, 2007; Osorio, 2012). Two RT-PCR amplicons were randomly selected to confirm their identity by DNA sequencing. BLAST analysis revealed 99% identity (E-Value=0.0 and 99% coverage) relative to PMTV isolates worldwide, confirming the presence of PMTV in the selected plants.
The antibodies hereby presented can be used for the specific detection of CP-RT in diagnostic tools used in conjunction with complementary PMTV tests such as conventional RT-PCR (Arif et al., 1994; Xu et al., 2004) and Real-time RT-PCR (Mumford et al., 2000). Additionally, these antibodies could be useful in epidemiological studies aimed at understanding the interaction of PMTV with different components of Sss life cycle. Kirk (2008) underlined the importance of performing studies that elucidate the physical location of PMTV inside zoospores and other cellular structures of Sss as well as persistence of PMTV in the resistant structures of Sss. These questions could be addressed with the developed antibodies using several serological tests such as immunodetection and the use of fluorescent probes.
Currently, there are several aspects on the basic biology of PMTV that could be addressed using RT-specific antibodies, in particular issues related to the different stages of transmission by Sss. It is of great importance to determine the rates of suppression at the amber stop codon of RNA2 in different plant tissues and at different Sss infestation levels. Cowan et al. (1997), reported a suppression frequency of 17% but the effect of the vector on this suppression was not addressed.
Sss has become an increasing problem for the potato industry in Colombia worsen by the high incidence levels of PMTV (Gil, 2010; Osorio, 2012). It is of the utmost importance to implement diagnostic tools against both pathogens as the ones described in this work and previous studies (Vélez, 2007; Carreño, 2009; Gil et al., 2011; Gallo, 2012; Osorio, 2012). Reliable diagnostics are the foundations of successful tuber certification programs, genetic improvement projects and in the determination of quarantine measures.
CONCLUSIONS
In this work, we present 81.8% of the RNA2 sequence from a local PMTV strain isolated from a potato field in eastern Antioquia. This sequence comprises 147 residues of the C-terminal end of the CP protein and the complete 649 residues of the RT domain. This is valuable information to improve our understanding of the molecular characteristics of PMTV in Colombia.
Using bioinformatic analysis of the RT domain of the CP-RT protein, a conserved and potentially antigenic region was identified. A synthetic peptide mimicking this hydrophilic segment was synthesized and served as antigen in the production of polyclonal antibodies useful in diagnosis and epidemiological studies of PMTV.
The efficacy of the Anti_CP_RT antibodies was compared against CP-specific antibodies. In spite of higher detection levels with the latter (83% vs. 50%), Anti_CP_RT can complement traditional diagnostic tests and would allow the study of the interaction of PMTV with its host Sss thanks to the role of CP-RT in virus transmission.
ACKNOWLEDGEMENTS
This research was possible thanks to financial and technical support by Ministerio de Agricultura y Desarrollo Rural (proyecto 090-2007S4527-87-08), Universidad Nacional de Colombia, Sede Medellín and Fedepapa. We also thank José Fernando Gil and Inés Osorio for providing Sss inoculated N. benthamiana plants.
BIBLIOGRAPHY
Adams M.J., J.F. Antoniw and J. Kreuze. 2009. Virgaviridae: a new family of rod-shaped plant viruses. Archives of Virology 154(12): 1967-1972. [ Links ]
Arif, M., L. Torrance and B. Reavy. 1994. Improved efficiency of detection of Potato mop-top furovirus in potato tubers and in the roots and leaves of soil-bait plants. Potato Research 37(4): 373-381. [ Links ]
Arif, M. and L. Torrance. 1996. Detection of Potato mop-top virus in potato tubers. Proceedings Crop Protection in Northern Britain 1:325-330. [ Links ]
Carreño A.J. 2009. Evaluación de la variabilidad genética de Spongospora subterranea f. sp. subterranea mediante la comparación de regiones ITS del ADN ribosomal de cepas procedentes de las regiones productoras de papa en Colombia. Tesis Magíster en Ciencias Agrarias. Facultad de Agronomía. Universidad Nacional de Colombia. Bogotá. 104 p. [ Links ]
Cerovska, N., T. Moravec, P. Rosecka, M. Filigarova and T. Pecenkova. 2003. Nucleotide sequences of coat protein coding regions of six Potato mop-top virus isolates. Acta Virológica 47(1): 37-40. [ Links ]
Cerovska N, M. Filigarová and T. Pecenkeva. 2006. Production of polyclonal antibodies to a recombinant Potato mop-top virus non-structural triple gene block protein 1. Journal of Phytopathology 154(7): 422-427 [ Links ]
Cowan, G.H., L. Torrance and B. Reavy. 1997. Detection of Potato mop-top virus capsid readthrough protein in virus particles. Journal of General Virology 78(7): 1779-1783. [ Links ]
Davis, N., I. Mallik, J.M. Crosslin and N.C. Gudmestad. 2010. First report of Potato mop-top virus in North Dakota. Plant Disease 94(12):1506. [ Links ]
Gallo, Y. 2012. Generación de antígenos derivados de la proteína de la cápside de PVY, TaLMV y PMTV, para la producción de anticuerpos útiles en el desarrollo de pruebas serológicas. Tesis Magíster en Bioquímica. Facultad de Medicina. Universidad Nacional de Colombia. Bogotá. Colombia. 148 p. [ Links ]
Gil, J.F. 2010. Diagnóstico y caracterización molecular de virus asociados al cultivo de la papa en Colombia, con énfasis en el virus Mop Top (PMTV, Pomovirus). Tesis Magíster en Biotecnología. Facultad de Ciencias. Universidad Nacional de Colombia. Medellín. 228 p. [ Links ]
Gil, J.F, P.A. Gutiérrez, J.M. Cotes, E.P. González y M. Marín. 2011. Caracterización genotípica de aislamientos colombianos del Potato mop-top virus (PMTV, Pomovirus). Actualidades Biológicas 33(94): 69-84. [ Links ]
Gilchrist, E., J. Soler, U. Merz and S. Reynaldi. 2011. Powdery scab effect on the potato Solanum tuberosum ssp. andigena growth and yield. Tropical Plant Pathology 36(6): 350-355. [ Links ]
Hall, T.A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41:95-98. [ Links ]
Helias, V., E. Jacquo, M. Guillet, Y. Hingrat and D. Giblot-Ducray. 2003. Production of recombinant Potato mop-top virus coat protein in Escherichia coli and generation of antisera recognising native virus protein. Journal of Virological Methods 110(1): 91-97. [ Links ]
Hoop, T.P. and R.K. Woods. 1981. Predictions of protein antigenic determinants from amino acid sequences. Proceedings of the National Academy of Sciences of the United States of America 78 (6): 3824-3828. [ Links ]
Huang, X. and A. Madan. 1999. CAP3: A DNA sequence assembly program. Genome Research 9(9): 868-877. [ Links ]
Jaramillo, S. y J.M. Botero. 2007. Respuesta de diferentes poblaciones de Spongospora subterranea f. sp. subterranea a la rotación entre dos cultivares de papa (Solanum tuberosum ssp. andigena). Revista de la Facultad Nacional de Agronomía Medellín 60(2): 3859-3876. [ Links ]
Jeffries, C.J. 1998. Potato FAO/IPGRI. Technical guidelines for the safe movement of germplasm 19: 62-63. [ Links ]
Kimura, M. 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution 16(2):111-120. [ Links ]
Kirk, H.G. 2008. Mop-top virus, relationship to its vector. American Journal of Potato Research 85(4): 261-265. [ Links ]
Latvala-Kilby S., J.M. Aura, N. Pupola, A. Hannukkala and J.P. Valkonen. 2009. Detection of Potato mop-top virus in potato tubers and sprouts: combinations of RNA2 and RNA3 variants and incidence of symptomless infections. Phytopathology 99(5):519-531. [ Links ]
MacKenzie, D.J. 1996. Detection of Potato mop-top virus in leaf or tuber tissue by reverse transcription-polymerase chain reaction. Document CPHBT96K03, Centre for Plant Health, Agriculture and Agri-Food Canada, Sidney, B.C., Canada. [ Links ]
MADR (Ministerio de Agricultura y Desarrollo Rural) - ENA (Encuesta Nacional Agropecuaria). 2009. Oferta Agropecuaria, cifras 2009. En: http://www.agronet.gov.co/www/docs_agronet/201046112648_RESULTADOS_ENA_2009.pdf. 194 p.; consulta: noviembre 2011. [ Links ]
Maoka, T., S. Sugiyama, Y. Maruta and T. Hataya. 2010. Application of cDNA macroarray for simultaneous detection of 12 potato viruses. Plant Disease 94(10): 1248-1254. [ Links ]
Matthews, R.E.F. 1993. Diagnosis of plant virus diseases. CRC Press, Canadá. 374 p. [ Links ]
Mumford, R.A., K. Walsh, I. Barker and N. Boonham. 2000. Detection of Potato mop top virus and Tobacco rattle virus using a multiplex real-time fluorescent reverse transcription polymerase chain reaction assay. Phytopathology 90(5):448-453. [ Links ]
Nakayama T., T. Maoka, T. Hataya, S. Motoshige, S. Fuwa and M. Mor. 2010. Diagnosis of Potato mop-top virus in soil using bait plant bioassay and RT-PCR-microplate hybridization. American Journal Potato Research 87(2): 218-285. [ Links ]
Nicolaisen, M. 2011. An oligonucleotide-based microarray for detection of plant RNA viruses. Journal of Virological Methods 173(1): 137-143. [ Links ]
Nielsen, S.L. and J.P. Mølgaard. 1997. Incidence, appearance and development of potato mop-top furovirus-induced sprain in potato cultivars and the influence on yield, distribution in Denmark and detection of the virus in tubers by ELISA. Potato Research 40(1):101-110. [ Links ]
Osorio, I. 2012. Variabilidad genética de Spongospora subterranea y su virus asociado PMTV en Colombia. Tesis Magíster en Geomorfología y Suelos. Facultad de Ciencias, Universidad Nacional de Colombia. Medellín. 139 p. [ Links ]
Reavy, B., M. Sandgren, H. Barrer, P. Heino and P. Oxelfelt, P. 1997. A coat protein transgene from a Scottish isolate of Potato mop-top virus mediates strong resistance against Scandinavian isolates which have similar coat protein genes. European Journal of Plant Pathology 103(9): 829-834. [ Links ]
Salazar, LF. 2006. Emerging and re-emerging potato diseases in the Andes. Potato Research 49(1): 43-47. [ Links ]
Sandgren, M., E. Savenkov and J.P. Valkonen. 2001. The readthrough region of Potato mop-top virus (PMTV) coat protein enconding RNA, the second largest RNA of PMTV genome, undergoes structural changes in naturally infected and experimentally inoculated plants. Archives of Virology 146(3): 467-477. [ Links ]
Santala J., O. Samuilova, A. Hannukkala, S. Latvala, H. Kortemaa, U. Beuch, A. Kvarnheden, P. Persson, K. Topp, K. Ørstad, C. Spetz, S.L. Nielsen, H.G. Kirk, M. Budziszewska, P. Wieczorek, A. Obrepalska, H. Pospieszny, A. Kryszczuk, J. Sztangret, Z. Yin, M. Chrzanowska, E. Zimnoch, E. Jackeviciene, L. Taluntyte, N. Pupola, J. Mihailova, I. Lielmane, L. Järvekülg, K. Kotkas, E. Rogozina, A. Sozonov, I. Tikhonovich, P. Horn, I. Broer, S. Kuusiene, J. Staniulis, J.G. Uth, G. Adam and J.P. Valkonen. 2010. Detection, distribution and control of Potato mop-top virus, a soil-borne virus, in northern Europe. Annals of Applied Biology 157(1): 163-178. [ Links ]
Savenkov, E.I., M. Sandgren and J.P. Valkonen. 1999. Complete sequence of RNA 1 and the presence of tRNA-like structures in all RNAs of Potato moptop virus, genus Pomovirus. Journal of General Virology 80(10): 2779-2784. [ Links ]
Savenkov, E.I., A. Germundsson, A.A. Zamyathin, M. Sandgren and J.P.T. Valkonen. 2003. Potato mop-top virus: The coat protein-encoding RNA and the gene for cysteine-rich protein are dispensable for systemic virus movement in Nicotiana benthamiana. Journal of General Virology 84(4): 1001-1005. [ Links ]
Shemyakina, E.A., A.G. Sclovyev, O.G. Leonova, V.I. Popenko, J. Schiemann and S.Y. Morozov. 2011. The role of microtubule association in plasmodesmal targeting of Potato mop-top virus movement protein TGBp1. The open virology Journal 5(1): 1-11. [ Links ]
Tamura K., D. Peterson, N. Peterson, G. Stecher, M. Nei M and S. Kumar. 2011. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28(10): 2731-2739. [ Links ]
Vaira, A.M., M. Vecchiati, V. Masenga and G.P. Accotto. 1996. A polyclonal antiserum against recombinant viral protein combines speci?city and versatility. Journal of Virological Methods 56(2): 209-219. [ Links ]
Vélez, B. 2007. Detección e identificación del Potato mop- top virus (PMTV) en áreas de producción de papa donde se encuentra Spongospora subterranea en dos departamentos de Colombia. Tesis Magíster en Ciencias Agrarias. Facultad de Agronomía. Universidad Nacional de Colombia. Bogotá. 115 p. [ Links ]
Wright K.M., G.H. Cowan, N.I. Lukhovitskaya, J. Tilsner, A.G. Roberts, E.I. Savenkov and L. Torrance. 2010. The N-terminal domain of PMTV TGB1 movement protein is required for nucleolar localization, microtubule association, and long-distance movement. Molecular Plant Microbe Interaction 23(11): 1486-1497. [ Links ]
Xu H., T.L. Dehaan and S.H. De Boer. 2004. Detection and confirmation of Potato mop-top virus in potatoes produced in the United States and Canada. Plant Disease 88(4): 363-367. [ Links ]