Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad Nacional de Agronomía Medellín
Print version ISSN 0304-2847
Rev. Fac. Nac. Agron. Medellín vol.65 no.2 Medellín June/Dec. 2012
CHARACTERIZATION OF PHYTOPATHOGENIC FUNGI, BACTERIA, NEMATODES AND VIRUSES IN FOUR COMMERCIAL VARIETIES OF HELICONIA (Heliconia sp.)
CARACTERIZACIÓN DE HONGOS, BACTERIAS, NEMÁTODOS Y VIRUS FITOPATÓGENOS EN CUATRO VARIEDADES COMERCIALES DE HELICONIA (Heliconia sp. )
Nathali López Cardona1 y Jairo Castaño Zapata2
1 Agronomic Engineer. Universidad de Caldas - Faculty of Agricultural Sciences. Phytopathology Master's Program. Street 65 No. 26-10. Manizales, Colombia <nathali.lopez.cardona@gmail.com>
2 Titular Professor. Universidad de Caldas. Faculty of Agricultural Sciences. Phytopathology Program. Street 65 No. 26-10. Manizales, Colombia. <jairo.castano_z@ucaldas.edu.co>
Recibido: Abril 09 de 2012; aceptado: Octubre 22 de 2012.
Abstract. Analysis of 914 samples of roots, rhizomes, pseudostems, inflorescences and leaves of four commercial varieties of heliconia, cultivated at the municipality of Chinchiná-Caldas (Colombia), allowed to identify five genera of plant pathogenic fungi (Rhizoctonia, Fusarium, Colletotrichum, Helminthosporium and Curvularia), three genera of plant pathogenic bacteria (Ralstonia, Pseudomonas and Erwinia), two species of viruses (Banana streak virus (BSV, Badnavirus,) and Cucumber mosaic virus (CMV, Cucumovirus,)), and seven genera of plant parasitic nematodes (Helicotylenchus, Tylenchus, Meloidogyne, Ditylenchus, Aphelenchoides, Pratylenchus, and Radopholus). Of these, Fusarium sp., affecting pseudostems, Pseudomonas sp., affecting leaves and inflorescences, and the plant parasitic nematodes Ditylenchus sp., Aphelenchoides sp., Pratylenchus sp. and Radopholus sp., are new records in the heliconia production in Colombia. The most limiting diseases corresponded to leaf blight, caused by Helminthosporium sp.; the bacterioses, caused by Pseudomonas sp.; the spotted stems, caused by Fusarium sp.; and soft rot of the pseudostems, caused by Erwinia sp. The pathogenicity tests demonstrated that Colletotrichum sp. and Phoma sp. are not pathogenic in leaves; while Fusarium sp., inoculated in pseudostems, Helminthosporium sp. and Pseudomonas sp., inoculated in leaves, and Colletotrichum sp. and Pseudomonas sp., inoculated in inflorescences, had incidence values of 83.3, 86.6, 93.3, 100.0 and 100.0%, respectively.
Key words: Heliconiaceae, diagnosis, diseases, pathogenicity.
Resumen. El análisis de 914 muestras de raíces, rizomas, pseudotallos, inflorescencias y hojas de cuatro variedades comerciales de heliconia, cultivadas en el municipio de Chinchiná-Caldas (Colombia), permitieron identificar cinco géneros de hongos fitopatógenos (Rhizoctonia, Fusarium, Colletotrichum, Helminthosporium y Curvularia), tres géneros de bacterias fitopatógenas (Ralstonia, Pseudomonas y Erwinia), dos especies de virus (Banana streak virus (BSV, Badnavirus,) y Cucumber mosaic virus (CMV, Cucumovirus,)), y siete géneros de nematodos fitoparásitos (Helicotylenchus, Tylenchus, Meloidogyne, Ditylenchus, Aphelenchoides, Pratylenchus y Radopholus). De ellos, Fusarium sp., afectando pseudotallos, Pseudomonas sp., afectando hojas e inflorescencias, y los nematodos fitoparásitos Ditylenchus sp., Aphelenchoides sp., Pratylenchus sp. y Radopholus sp., son reportes nuevos en la producción de heliconias en Colombia. Las enfermedades más limitantes correspondieron al tizón foliar, causado por Helminthosporium sp.; la bacteriosis, causada por Pseudomonas sp.; el manchado de pseudotallos, causado por Fusarium sp.; la pudrición de calcetas, causada por Erwinia sp.; y el moko, causado por Ralstonia solanacaearum. Las pruebas de patogenicidad demostraron que Colletotrichum sp. y Phoma sp. no son patogénicos en hojas; mientras que Fusarium sp., inoculado en pseudotallos, Helminthosporium sp. y Pseudomonas sp., inoculados en hojas, y Colletotrichum sp. y Pseudomonas sp., inoculados en inflorescencias, presentaron valores de incidencia de 83,3, 86,6, 93,3, 100,0 y 100,0%, respectivamente.
Palabras clave: Heliconiaceae diagnóstico, enfermedades, patogenicidad.
Taking under consideration the importance of heliconias (Heliconia sp.) cultivation in Colombia as an export product and an additional alternative for job creation plans, research must be carried out in order to determine the biotic factors limiting their production. The country has an important competitive advantage for tropical flowers production; in more than 40 years, Colombia has increased its income going from quite a few annual thousand dollars to more than US $1,094 million dollars exported during 2008. Currently, Colombia has become the first flower provider for the United States, the first worldwide carnation producer-exporter and the second worldwide flower exporter country after Holland, thus generating 183,000 jobs (99,000 direct jobs and 84,000 indirect jobs) in a 7,500 ha sown field dedicated to exportation cultivation, contributing 9.9% to the gross domestic product (GDP) and putting in 25% of the rural female employment in Colombia (Asocolflores, 2009).
More than 250 species of the Heliconia genus have been described, of which 97 are registered in Colombia and 48 of these have been described as endemic species, placing the country as the biggest biodiversity center for this genus in the world. For this reason, Colombia has been ranked as a strong competitor in the international market of these flowers. It is estimated that in the Coffee Triangle and the Valle del Cauca there exist approximately 471 ha cultivated with heliconias and foliage, contributing 7% of the national export. In Risaralda, the place in which the greatest heliconia sown field area in the Coffee Triangle is concentrated, there are around 120 ha dedicated to this cultivation and the number of flower growers is higher than 150, which represent an annual income close to US $1,500,000 (Díaz, 2006; Asocolflores, 2009).
It is common to observe that when the sown field of cultivation becomes bigger, the presence of diseases caused by a diversity of phytopathogenic microorganisms also increases, which is known as the price of varietal popularity (Castaño, 2002). This situation is not foreign to the heliconias production in Colombia. In spite of this, most researchers on this species have focused in a variety of topics such as heliconia inventories, taxonomy, ecology, distribution and classification that have been found in the country, which has given up precise information and to research about the pathogenic agents affecting this genera species and, even more, the relation they have with climate elements such as precipitation, relative humidity, and temperature. The previous, has lead to the need to take measures of inappropriate handling which generate a series of environmental and economic consequences afterwards.
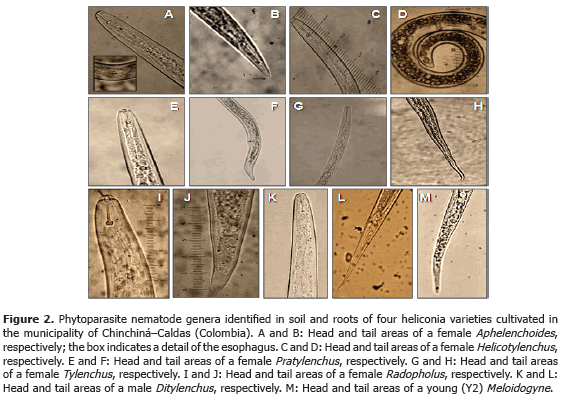
The only published work about the identification of diseases in Colombian heliconias has been developed by Villegas et al. (2005) and Alarcón (2007). The reports suggest the presence of fungi genera, such as Fusarium, Pestalotia, Helminthosporium and Colletotrichum; bacteria, such as Erwinia paradisiaca and Ralstonia solanacearum; phytoparasite nematodes of the Meloidogyne, Helicotylenchus, Rotylenchus and Tylenchus genera, without considering the Radopholus genus, which has been considered as the most aggressive genus in the plantain and banana cultivations in the world.
In Hawaii, Sewake and Uchida (no date) describe that diseases caused by Bipolaris incurvata, Exserohilum (=Helminthosporium) rostratum, Pyriculariopsis sp., Mahabalella sp., Mycosphaerella sp. and Rhizoctonia solani in H. bihai var. Lobster Claw One and H. caribaea, can become very limiting in heliconia production. Likewise, Nakati (no date) and Rabelo (2007) consider that Exserohilum sp. and Bipolaris sp. are the most destructive foliar pathogens of heliconia cultivation in that state.
In Venezuela, Madríz et al. (1991) report that Colletotrichum musae limits heliconias commercialization, because it produces brown with white center spots which demarcate the main leaves vein in Heliconia caribaea, but also affects inflorescence in which, in the initial disease stages, produces irregular and sunken brown-black spots in the bracts, and in the disease advanced stage causes widespread dry decay in the whole inflorescence.
In this research, some of the phytopathogens affecting the production in four commercial varieties of heliconias in the municipality of Chinchiná-Caldas (Colombia) were identified, as a contribution for the appropriate implementation of the cultivation management strategies. The study consisted of the identification, following Koch's postulates, of pathogens affecting the production of four heliconia commercial varieties (H. caribaea Lam. var. Kawachi, H. caribaea Lam. var. Salmon, H. lobster (L.) var. Orange Lobster and H. ortotricha (L.) var. Edge of Night). The species were selected because of their commercial importance in the national and international fields.
MATERIALS AND METHODS
Area of study. The research was carried out in el Rosario farm in the municipality of Chinchiná-Caldas (Colombia), at an altitude of 1,200 masl, 80% average relative humidity, an average temperature of 22 ºC, a 2,500 mm average annual precipitation, and with homogeneous soil characterized as Chinchiná unit. This region was selected because of its cultivated area (4.2 ha) in the species of interest for this research, and because it presents highly favorable conditions for the cultivation of heliconias in the region. For H. caribaea var. Kawachi and H. caribaea var. Salmon 1 ha sown was evaluated with the two species (0.5 ha per species), and for H. ortotricha var. Edge of the Night and H. orange lobster var. 0.72 and 2.51 ha were evaluated, respectively.
Recognition of diseases
Observation and description of symptoms in the field. In order to carry out and develop this activity, all possible combinations of symptoms of probable biotic origin, such as spots, necrosis, blockage, decay and abnormal growth, were described in the evaluated varieties.
Sample gathering. Between the months of March and December 2011 the collection of 914 samples was carried out, covering leaves, roots, corms, pseudostems, inflorescences and soil associated to plants which presented any characteristic symptom or any abnormality, in which the presence of phytopathogenic agents could be suspected. The samples with lesions from an apparently fungal origin were placed in plastic bags; for lesions apparently caused by bacteria and viruses, humid paper was used; and for the phytoparasite nematodes, soil and root samples were taken. The samples were processed in the Phytopathology Laboratory at Universidad de Caldas.
Isolation and identification of phytopathogenic agents.
Fungi: moist chambers, scrapes, cuts and dissections were prepared in order to observe the fungi characteristic structures; later, samples from the chambers were taken out, and based on French and Herbert's methodology (1980), superficial scrapes were performed in the affected areas using a scalpel, or the mycelium was removed using a sterilized dissection needle. The fungal material was observed in a LW Scientific® Revelation III light microscope, with a 40X lens, previous staining with lactophenol (20 g crystalline phenol, 20 mL lactic acid, 20 mL glycerin, 20 mL distilled water and cotton blue at 5% in water) (Castaño y del Rio, 1994). Another part of the scrape, was planted in Petri dishes with PDA (potato, dextrose and agar 39 g L-1 water) and then they were incubated at 25 ºC in an incubator (Precision Scientific®, Standard model 815) with the purpose of obtaining abundant sporulation in order to subsequently carry out the pathogenicity testing. In the symptoms reproduction, between four and five healthy experimental units per pathogen (inflorescences or leaves) were used, which were inoculated using a DeVilbiss® No. 15 sprinkler or a 5 mL syringe, with spores suspension adjusted to a 1.5 x 105 spores mL-1 of sterile distilled water (SDW), and using three repetitions for each inoculated pathogen. The experimental units were kept in field and laboratory conditions until the specific symptoms of each disease developed. Then, these pathogens were re-isolated again to verify the diagnosis and accomplish this way Robert Köch's postulates. The identification was based on specialized fungi taxonomic keys (Hanlin, 1990; Hanlin, 1998; Barnett and Hunter, 1998; Castaño and Salazar, 1998). All the pathogenic isolations were stored at 4 ºC in sterile bottles containing SDW, following the methodology proposed by Qiangqiang et al. (1998). The Rhizoctonia sp. and Curvularia sp. fungi had been subjected to pathogenicity tests by Villegas et al. (2005), and because of that they were not used to apply Köch's postulates in this study.
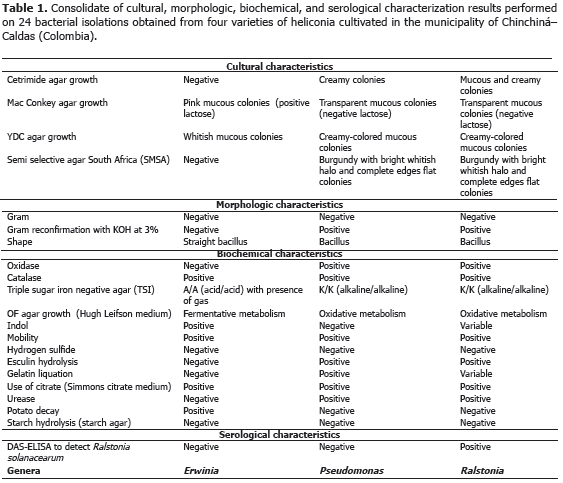
Bacteria: tests were carried out in different means of cultivation in order to observe cultural, morphologic and biochemical characteristics of bacterial cultures following the Schaad (1988) schema. For the cultural characteristics, the growth on Cetrimide agar, MacConkey agar, and semi-selective South Africa agar (SMSA) were observed; to identify the morphologic characteristics, Gram staining and Gram reconfirmation with KOH at 3% was performed; and the shape, was identified through an observation under the light microscope with a 100X objective lens with immersion oil. Finally, to identify the biochemical characteristics, the presence of oxidase, catalase, the change of color in triple sugar-iron (TSI) agar, the growth in the Hugh-Leifson medium (OF), the indol production, the mobility and the presence of sulfide acid in the SIM media, the urease production in urea agar and the starch hydrolysis, were analyzed. Additionally, DAS-ELISA testing was carried out to detect the Ralstonia solanacearum species. As in the methodology used for fungi diagnosis, with pure bacterial colonies isolated and cultivated in nutritive agar, a bacterial suspension adjusted to 9.0 x 108 UFC mL-1 of ADE was inoculated making wounds with an handmade inoculating device which was made from two fine sheet blades fixed on an 8 x 5 cm rectangular piece of wood. Inoculation was carried out submerging the inoculating device in the bacterial suspension, in order to subsequently cause wounds on healthy leaves or inflorescences in order to reproduce the disease symptoms. All the pathogenic isolations were stored at 4 ºC in a BHI (Brain - Heart- Infusion) medium with the purpose of carrying out their subsequent molecular characterization. Ralstonia solanacearum and Erwinia sp. microorganisms had been submitted to pathogenicity tests in the research work carried out by Villegas et al. (2005), reason why they are not included in this work.
Nematodes: in each sampling date, three repetitions per sample were carried out in each variety. Each sample consisted of roots, 30 g, and soil, 30 g, which contained each between eight and ten sub-samples, depending on the cultivated area. Each sample (soil and roots) was taken 25 cm around each plant and to a depth of 30 cm (Araya and Chaves, 1997). Phytonematodes extractions and quantifications were carried out using the liquidization, centrifugation and sugar flotation method (Araya, 1995; Guzmán and Castaño, 1997). This way, the number of nematodes per milliliter was obtained and the total population per analyzed sample was calculated. The specimens mounting were done in slide plates for their observation under the optical microscope. The results were expressed in number of nematodes per 100 g of soil and 100 g of roots. The identifications were made following Thorne (1961) and Mai et al. (1996) taxonomic keys.
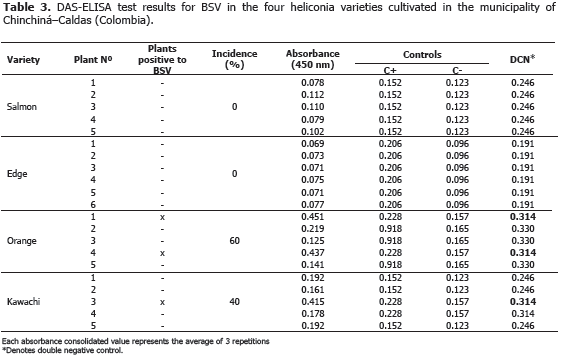
Viruses: the BSV y CMV viruses were identified using the ELISA (DAS-ELISA and TAS-ELISA) commercial serological tests and following the instructions given by the manufacturer (Agdia®, United States). In all cases, the parameter of classification for positive tests was assumed when the absorbance value was twice larger than the media of negative controls.
Variables to be evaluated. The incidence (%) of all diseases found in the production of four varieties of heliconias, was determined according to observed symptomatology in the field in each affected organ (rhizome, pseudostems, pedicel, inflorescence and leave). The incidence was determined using the following formula:
![]()
Statistical analysis. The obtained results in the pathogenicity tests were submitted to variance analysis using the Stat graphics Plus 5.1 program, and the averages of each evaluation were analyzed using Duncan's Multiple Range Contrast test with a 95% level of confidence.
RESULTS AND DISCUSSION
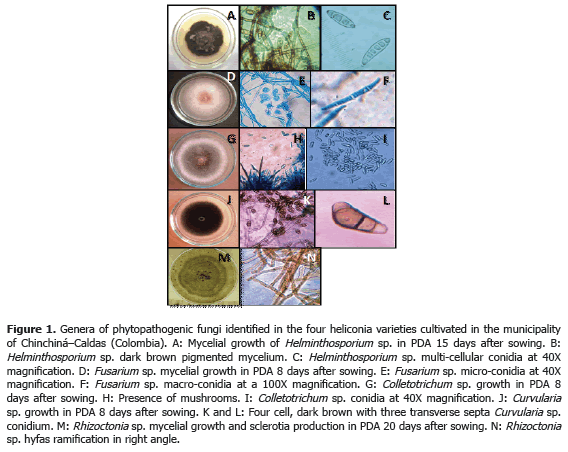
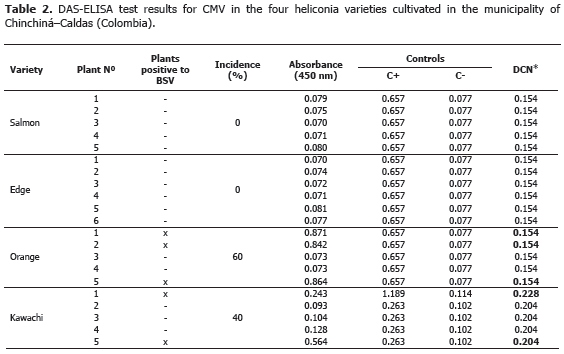
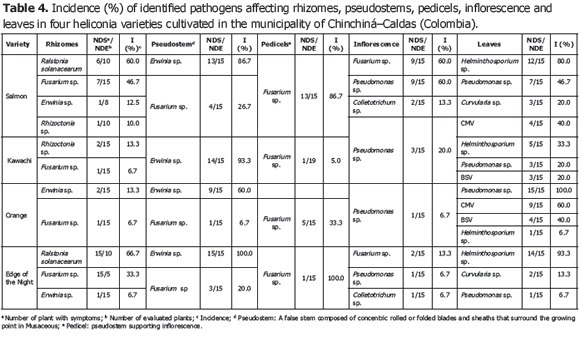
Analysis of 914 samples including roots, rhizomes, pseudostems, inflorescences and leaves, belonging to the four heliconia varieties studied allowed the identification of five genera of phytopathogenic fungi (Rhizoctonia, Fusarium, Colletotrichum, Helminthosporium and Curvularia) Figure 1; three genera of phytopathogenic bacteria (Ralstonia, Pseudomonas and Erwinia) Table 1; two species of viruses (Banana Streak Virus (BSV), Badnavirus and Cucumber Mosaic Virus (CMV), Cucumovirus) Tables 2 and 3; and seven phytoparasite nematodes genera (Helicotylenchus, Tylenchus, Meloidogyne, Ditylenchus, Aphelenchoides, Pratylenchus and Radopholus) Figure 2. These results coincide with Villegas et al. (2005) and Alarcón (2007) results, but they include six new pathogen records affecting heliconias production in Colombia which are: Fusarium sp., affecting pseudostems; Pseudomonas sp., affecting leaves and inflorescences; and nematodes of the genera Ditylenchus, Aphelenchoides, Pratylenchus, and Radopholus.
Madríz et al. (1991), mention that species H. caribaea, H. latispatha and H. psittacorum are very susceptible to phytopathogenic fungi attack. In Venezuela, these authors report the attack of Phyllostica musae, Glomerella cingulata, Alternaria alternata, Gloeosporium musarum, Colletotrichum musae, Guignardia musae, Curvalaria sp., Fusarium oxysporum, Mycosphaerella musicola, Drechslera musae-sapientum and Pestalotiopsis sp. to seven species of heliconia (H. caribaea, H. latispatha, H. psittacorum, H mariae, H. platystachys H. revoluta and H. rostrata).
A record of diseases associated to heliconias production in Hawaii, developed by the Vegetal Pathology Department at University of Hawaii and the United States Agricultural Diagnostic Service Center, indicated that pathogens isolated from roots and rhizomes, such as Calonectria spathiphylli, Phytophthora nicotianae, Pythium sp., Rhizoctonia solani, Ralstonia solanacearum, Radopholus similis, Meloidogyne sp., Pratylenchus sp., Rotylenchus reniformis, and Helicotylenchus sp., are important for decreasing heliconias production, followed by foliar diseases which can become very destructive and are caused by Bipolaris incurvata, Exserohilum (=Helminthosporium) rostratum, Pyriculariopsis sp., Mahabalella sp. and Mycosphaerella sp. (Sewake and Uchida, no date).
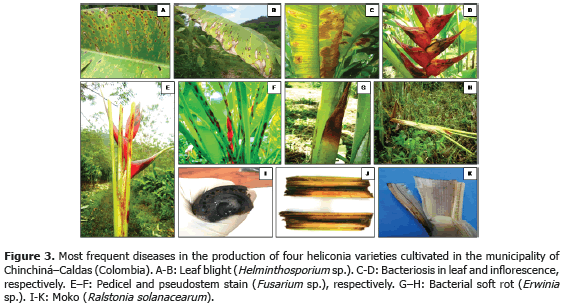
The most frequent diseases found in this investigation (Table 4; Figure 3) corresponded to:
1) Leaf blight caused by Helminthosporium sp. presenting an incidence of 93.3% (Table 3) in the Edge of the Night variety whose symptoms corresponded to irregular oval brown spots surrounded by an approximately 1.5 mm in diameter chlorotic halo which can coalesce causing the drying of the whole foliar plate (Figure 3B). These results coincide with Alarcón (2010), who reported identical symptoms in Heliconia ortotricha var. Red and Heliconia rostrata. A record of diseases associated with heliconia production in Hawaii, carried out by the Plant Pathology Department at University of Hawaii and the United States Agricultural Diagnostic Service Center, indicated that foliar diseases caused by Bipolaris incurvata, Exserohilum (=Helminthosporium) rostratum, Pyriculariopsis sp., Mahabalella sp. and Mycosphaerella sp. may become very limiting in heliconias production (Sewake and Uchida, no date). Similarly, Nakati (no date) and Rabelo (2007), in Brazil, consider that Exserohilum sp. and Bipolaris sp. are the most destructive foliar pathogens in heliconia cultivations in that country.
2) Bacteriosis in inflorescence and leaves caused by Pseudomonas sp., registering a 60% incidence in inflorescence of the Salmon variety (Table 3) and a 100% incidence in leaves in the Orange variety. Bacteria producing irregular watery spots which coalesce in order to destroy the whole foliar plate forming a big spot which advances from the foliar limb to the main leaf vein (Figure 3C). It is common to observe the presence of concentric rings in the lesions which usually confuse the evaluator with the presence of Cordona sp. or Alternaria sp. In the inflorescences, the pathogen produces brown watery lesions which can necrose the whole tissue. Usually the disease appears more frequently in the edges of inflorescence and advances to become a huge necrotic spot (Figure 3D).
3) Pseudostems and pedicels stained caused by Fusarium sp., presenting incidences from 20 to 100% in the Edge of the Night variety respectively (Table 3). The pathogen produces typical reddish spots with an elongated and rhomboid shape in pseudostems of the Edge of the Night variety (Figure 3F), and in the Orange, Kawachi and Salmon varieties. The fungus produces small, elongated, dotted and reddish spots in the pseudostems. Sick pedicels, which accompany the marketable flower, discredit their quality and represent economic losses for the flower grower (Figure 3E). In Brazil, Reis (2010) reported 31 isolations of Fusarium oxysporum f. sp. cubense affecting 88% of the tropical flowers producer pieces of land, including heliconias; the H. bihai, H. psittacorum cv. Golden Torch, H. psittacorum cv. Golden Torch Adrian, H. rostrata, H. stricta Capri, H. psittacorum cv. Sassy and H. caribaea species were considered as resistant to vascular wilting. The moderately resistant species were H. latispatha and H. wagneriana, while Heliconia psittacorum cv. Alan Carle and H. chartacea cv. Sexy Pink were susceptible, and H. stricta Fire Bird was highly susceptible. These results do not coincide with those observed in this study because the H. caribaea cv. Salmón species was the most susceptible to the disease. Mata et al. (no date) mention that from the four Fusarium oxysporum f. sp cubense existing races, race 4 is the most pathogenic in banana from the Cavendish, and Heliconias group.
4) Bacterial soft rot caused by Erwinia sp., reaching a 100% incidence in the Edge of Night variety (Table 3), whose symptoms are characterized by the presence of watery decay (Figure 3G), with pungent, putrid smell and with presence of bacterial exudates. At an advanced stage of the disease, the pathogen can completely necrose the pseudostem allowing the plant to bend where the lesion is located (Figure 3H). According to Belalcázar and Merchán (1991) the main cause for the disease is the plant nutritional disequilibrium, especially in boron and potassium. Among the factors increasing the disease severity are drought periods followed by heavy rain. Bacterial soft rot is a disease that must be taken into account for the integrated management of heliconia diseases, since tough the disease stays during the productive cycle affecting bacterial soft rot, it is very possible that as the bacterial inoculum concentration increases the symptoms of plants with bent pseudostems become more frequent which would change the disease into a limiting one for the production of the heliconias studied.
5) Moko caused by Ralstonia solanacearum, which was identified affecting rhizomes in the Salmon and Edge of the Night varieties with 60 and 66.7% incidence, respectively (Table 3), is characterized because it produces the plants death, marked wilting, mature leaves tanning, loss of swelling in the leaves, delay in growth and severe chlorosis in young leaves. At the internal level, pseudostems present decay and vascular bundles necrosis (Figures 3J and K). One of the most prominent characteristics of the disease could be observed in laboratory conditions when the affected plant organs produced abundant bacterial exudation known as Moko (Figure 3I). R. solanacearum is a highly aggressive phytopathogenic bacterium with a worldwide distribution and a wide range of host plants including around 50 botanic families comprising cultivations, such as potatoes (Solanum tuberosum L.), tomatoes (Solanum lycopersicum L.), tobacco (Nicotiana tabacum L.), bananas (Musa paradisiaca L.), heliconias (Heliconia sp. L.), anthuriums (Anthurium sp. Schott) and peanuts (Arachis hypogaea L.) (Denny and Hayward, 2001). Traditionally, members of R. solanacearum have been subdivided in five races based on their host range and five biovars depending on their metabolic capacity for the use of diverse carbon sources; race 1 (biovars 1, 3 or 4) affects a great amount of plants including potatoes, tomatoes and the solanaceae family in general; race 2 (biovars 1 or 3) affects plantains, bananas and heliconias; race 3 (biovars 2) is considered specific for potatoes and is associated with some solanaceae family plants; race 4 (biovars 4) attacks ginger; and race 5 affects blackberry plants (Rubus glaucus Bentham) (Hayward, 1991).
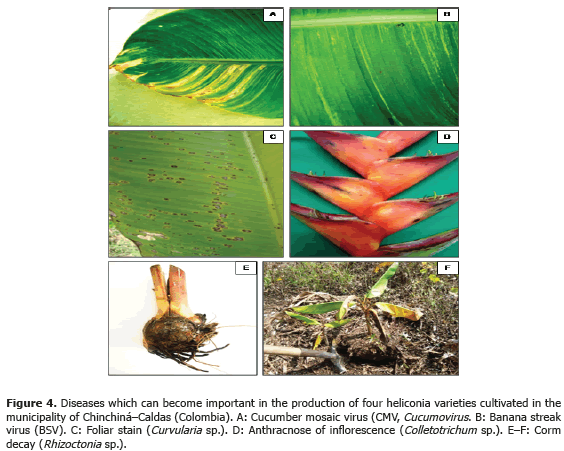
Less frequent diseases in this research (Table 4; Figure 4) corresponded to inflorescence Antracnose (Colletotrichum sp.) with a 13.3% and 20% incidence in the Salmon variety, respectively. In Venezuela, Madríz et al. (1991) reported that Colletotrichum musae produces brown with white center spots which demarcate the main rib in Heliconia caribaea leaves; but also affect inflorescences in which, in initial stage of the disease, produce irregular and sunken brown-black spots in the bracts and, in advanced stage of the disease, causes a dry decay widespread on all the inflorescence. The Curvularia lunata species has been reported in Brazil, causing foliar spots in Heliconia psittacorum (Assis et al., 2002; Lins and Coelho, 2004; Costa, 2007).
Corm decay (Rhizoctonia sp.) presented a 13.3% incidence in the Kawachi variety. Rhizoctonia solani Kühn is one of the most common plants pathogen in the world. Almost every cultivation can be affected by R. solani or other Rhizoctonia species; in Hawaii, R. solani causes decay in roots in H. bihai var. Lobster Claw One and H. caribaea (Sewake and Uchida, no date).
BSV and CMV viruses presented a 40% and 60% incidence in the Orange variety, respectively. Plants affected by the BSV virus did not manifest delay in growth or production. In the Edge of the Night and Salmon varieties, CMV virus was not detected maybe because of the severe attack of leaf blight, caused by Helminthosporium sp. in this variety. This virus is not limiting in heliconia production presently, but the symptoms associated to the pathology, as well as the foliar distortion and atrophy, can become production limiting factors while the disease incidence and the cultivation area increase.
BSV and CMV can act as a viral complex causing secondary veins necrosis, stripes and mosaics in the leaves. The complex was identified in a plant from the Orange variety (Tables 2 and 3). Similar results were obtained in Dominico-Hartón plantain by López (2009). In the Department of Caldas, Dominico-Hartón plantain plants infected with BSV can present reduction in the bunch size associated with a 35% production loss; while those infected with CMV, can reduce production to a 62% (López, 2009). Belalcázar (1996) report 50% or more loss in weight for bunches produced by infected plants with CMV in Valle del Cauca.
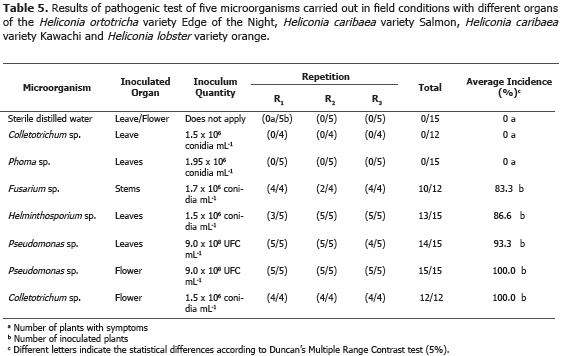
Pathogenicity Test. Köch's postulates application allowed to clarify the registered diseases etiology in the four heliconia varieties studied (Table 5).
The application of Duncan's multiple range test (5%) allowed to conclude that Colletotrichum sp. and Phoma sp. were not pathogenic in leaves; while Fusarium sp., inoculated in stems, Helminthosporium sp. and Pseudomonas sp., inoculated in leaves, and Colletotrichum sp. and Pseudomonas sp., inoculated in florescence, reached 83.3, 86.6, 93.3, 100.0 and 100.0 incidence percentages, respectively (Table 5). Pestalotia sp., Botrytis sp., Hendersonia sp., Nigrospora sp., Aspergillus sp., Diplodia sp., Rhizopus sp., Cladosporium sp., Coniotyrium sp., Cordana sp., Xanthomonas sp., Chaetomium sp., Mycosphaerella musicola, Alternaria sp. and Cercospora sp., reported by Villegas et al. (2005), were not reported in any of the four heliconia varieties evaluated, probably because they used different heliconia varieties in that investigation.
CONCLUSIONS
The results of this investigation allowed to identify, in an heliconia commercial cultivation located in Chinchiná, Caldas, the presence of Rhizoctonia sp., Fusarium sp., Colletotrichum sp., Helminthosporium sp. and Curvularia sp. phytopathogenic fungi; Ralstonia solanacearum, Pseudomonas sp. and Erwinia sp. bacteria; BSV and CMV viruses, and Helicotylenchus, Tylenchus, Meloidogyne, Ditylenchus, Aphelenchoides, Pratylenchus and Radopholus phytoparasite nematodes. New records of pathogens, which affect heliconia production in Colombia, are included which are: Fusarium sp., affecting pseudostems; Pseudomonas sp., affecting leaves and florescence; and phytoparasite nematodes, of the Ditylenchus, Aphelenchoides, Pratylenchus and Radopholus genera.
BIBLIOGRAPHY
Agdia. 2008. Compound direct ELISA, alkaline phosphatase label: User guide. Disponible en red: https://orders.agdia.com/Documents/m20.pdf. consulta: mayo 2011. [ Links ]
Alarcón, J. 2007. Enfermedades en la producción de heliconias en los departamentos de Caldas, Risaralda y Quindío. Agronomía 15(1): 45 - 61. [ Links ]
Alarcón, J. 2010. Manejo fitosanitario y productivo de heliconias. Instituto Colombiano Agropecuario, ICA y Asociación Colombiana de Exportadores de Flores, ASCOLFLORES. 106 p. [ Links ]
Assis, S.M., L. Mariano, M. Gondim, M. Menezes e R. Rosa. 2002. Doenças e pragas das helicônias. Diseases and pests of heliconias. Universidade Federal Rural de Pernambuco, Recife. Brasil. 102 p. [ Links ]
Araya, M. 1995. Efecto depresivo de ataques de Radopholus similis en banana (Musa AAA). CORBANA 20(43): 3-5. [ Links ]
Araya, M. y A. Chaves. 1997. Selección del tipo de planta para el muestreo de nematodos en banano (Musa AAA). INFOMUSA 7(1): 23-26. [ Links ]
Asocolflores (Asociacion Colombiana de Exportadores de Flores). 2009. Floricultura colombiana: un caso de colaboración exitosa en protección de cultivos. En: http://www.croplifela.org/pages_html/presentaciones/solano.pdf. 63 p.; consulta: abril 2012. [ Links ]
Barnett, H.L. and B.B. Hunter. 1998. Illustrated genera of imperfect fungi. Fourth edition. Burgess Publishing Company, Minnesota, USA. 218 p. [ Links ]
Belalcázar, S. y V. Merchán. 1991. Control de enfermedades. pp. 243-297. En: Belalcázar (ed.). El Cultivo del Plátano (Musa AAB Simmonds) en el Trópico. Instituto Colombiano Agropecuario, ICA, Armenia. 376 p. [ Links ]
Belalcázar, S. 1996. Plagas y enfermedades del plátano. Boletín de Sanidad Vegetal No. 4. Instituto Colombiano Agropecuario. 102 p. [ Links ]
Castaño, J. and H. Salazar. 1998. Illustrated guide for identification of plant pathogens. Universidad de Caldas. Manizales, Colombia. 108 p. [ Links ]
Castaño, J. 2002 Principios básicos de fitoepidemiología. Centro Editorial Universidad de Caldas. Manizales, Colombia. 396 p. [ Links ]
Castaño, J. y M.L. Del Río. 1994. Guía para el diagnóstico y control de enfermedades en cultivos de importancia económica. Tercera Edición. Zamorano Academic Press, Honduras. 290 p. [ Links ]
Costa, C. 2007. Fungos associados as plantas ornamentais tropicais no distrito federal. 2007. Dissertação de Mestrado em Fitopatologia. Universidade de Brasília, Brasília - DF. 114 p. [ Links ]
Denny, T. and A. Hayward. 2001. Ralstonia. pp. 151-166. In: Schaad, N., J. Jones. and W. Chun. (eds.). Laboratory guide for identification of plant pathogenic bacteria. Saint Paul MN, APS Press. 373 p. [ Links ]
Diaz, J.A. 2006. Diagnóstico de la cadena productiva de heliconias y follajes en los departamentos del eje cafetero y Valle del Cauca (Colombia). Organización de la Naciones Unidas, ONU. En: http://www.unctad.org/biotrade/National/Colombia/Colombia-docs/Sector_assessment_heliconias_Feb06.pdf. consulta: abril 2012. [ Links ]
French, E. y T. Hebert. 1980. Métodos de investigación fitopatológica. Instituto Interamericano de Ciencias Agrícolas IICA. Costa Rica. 288 p. [ Links ]
Guzmán, O. y J. Castaño. 1997. Reconocimiento de nematodos fitopatogenos en plátano Dominico-Hartón (Musa AAB Simmonds) África, FHIA 20 y FHIA 21, en la granja Montelindo, Municipio de palestina (Caldas). Revista de la Academia Colombiana de Ciencias Exactas Físicas y Naturales 28 (107): 295-301. [ Links ]
Hayward, A. 1991. Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annual review of Phytopathology 29: 65-87. [ Links ]
Hanlin, R.T. 1990. Illustrated genera of Ascomycetes. Second edition. Vol. I. The American Phytopathological Society. APS. 247 p. [ Links ]
Hanlin, R.T. 1998. Illustrated genera of Ascomycetes. Vol. II. The American Phytopathological Society. APS. 244 p. [ Links ]
Lins, S. y R. Coelho. 2004. Ocorrência de doenças em plantas ornamentais tropicais no Estado de Pernambuco. Fitopatologia Brasileira 29(3): 332-335. [ Links ]
López, N. 2009. Diagnóstico mediante técnica ELISA de los virus que afectan los cultivos de plátano y banano (Musa sp.) en el eje cafetero. Trabajo de grado de Agronomía. Facultad de Ciencias Agropecuarias. Universidad de Caldas. 75 p. [ Links ]
Madriz, R., G. Smits y R. Noguera. 1991. Principales hongos patógenos que afectan algunas especies ornamentales del género Heliconia. Agronomía Tropical 41(5-6): 265-274. [ Links ]
Mai, W., H. Mullin, H. Lyon y K. Loeffler. 1996. Plant parasitic nematodes. A pictorial key to genera. Fifth edition. Comstock Publishing Associates, Cornell University Press. 277 p. [ Links ]
Mata, F., C. Contreras y H. López. (no date). Alternativas geoespaciales para el monitoreo y vigilancia epidemiológica del marchitamiento vascular (Fusarium oxysporum f. sp cubense raza 4) en México. En: http://www.selper-mexico.org.mx/XT%20PDF/EPIDEMIOLOGIA/EPIDEM-01.pdf. 6 p.; consulta: abril 2012. [ Links ]
Nakati, L. (no date). Aspectos de fungos fitopatogênicos em plantas ornamentais e seu controle. En: http://www.biologico.sp.gov.br/rifib/XIVRifib/coutinho.PDF. 8 p.; consulta: abril 2012. [ Links ]
Qiangqiang, Z. W Jiajun and L. LI. 1998. Storage of fungi using sterile distilled water or lyophilization: comparison after 12 years. Mycoses 41(5-6): 255-257. [ Links ]
Rabelo, C. 2007. Fungos asociados ás plantas ormanetais tropicais no Distrito Federal. Universidade de Brasília. 114 p. En: http://repositorio.bce.unb.br/bitstream/10482/3141/1/2007_CarolineRabeloCosta.PDF. consulta: abril 2012. [ Links ]
Reis, N. 2010. Murcha de Fusarium em Heliconia spp: ocorrência, variabilidade e resistência genética em espécies ornamentais cultivadas em Pernambuco, Alagoas e Sergipe. The Biblioteca Digital de Teses e Dissertaçñes (BDTD) of the Instituto Brasileiro de Informação em Ciência e Tecnologia (IBICT). En: http://biblioteca.universia.net/html_bura/ficha/params/id/49065032.html. consulta: abril 2012. [ Links ]
Sewake, K. and J. Uchida. (no date). Pest management guidelines: diseases of heliconia in Hawaii. College of Tropical Agriculture and Human Resources, and Hawaii Cooperative Extension Service. University of Hawaii In: http://www.extento.hawaii.edu/kbase/reports/heliconia_pest.htm. consulta: marzo 2012. [ Links ]
Schaad, R. 1988. Laboratory guide for identification of plant pathogenic bacteria. The American Phytopathological Society (APS), Minnesota. 253 p. [ Links ]
Thorne, G. 1961. Principles of nematology. Mc Graw- Book Company, USA. 547 p. [ Links ]
Villegas, N., J. Alarcón y R. Galindo. 2005. Enfermedades limitantes en la producción de heliconias en los departamentos de Caldas, Risaralda y Quindío. Fitopatología Colombiana 29(2): 53-58. [ Links ]