Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad Nacional de Agronomía Medellín
Print version ISSN 0304-2847
Rev. Fac. Nac. Agron. Medellín vol.65 no.2 Medellín June/Dec. 2012
EFFECT OF TWO PROTOCOLS OF CRYOPRESERVATION ON FERTILIZING CAPACITY OF STALLION (Equus caballus) SEMEN
EFECTO DE DOS PROTOCOLOS DE CRIOPRESERVACIÓN SOBRE LA CAPACIDAD FECUNDANTE DE SEMEN EQUINO (Equus caballus)
Giovanni Restrepo Betancur1; Juan Esteban Duque Cortés2 y Juan David Montoya Páez3
1 Professor. Politécnico Colombiano Jaime Isaza Cadavid. Carrera 48 No. 7 - 151. Medellín, Colombia. <grestrepo@elpoli.edu.co>
2 Professor Researcher. Politécnico Colombiano Jaime Isaza Cadavid. Animal Biotechnology Research Group Carrera 48 No. 7 - 151. Medellín, Colombia. <duque8778@hotmail.com>
3 Professor Researcher. Politécnico Colombiano Jaime Isaza Cadavid. Animal Biotechnology Research Group. Carrera 48 No. 7 - 151. Medellín, Colombia. <juanchon29@hotmail.com>
Received: August 21, 2012; accepted: October 04, 2012.
Abstract. Semen cryopreservation is a fundamental process for the development of biotechnologies for assisted reproduction in horses. The use of cryopreservation techniques with changes in concentrations and the nature of the cryoprotectant, as well as, the different types of vials for storage of semen, have become an alternative to improve the protocols used. The objective of this work was to evaluate the effect of two protocols of cryopreservation (freezing and vitrification) on the fertilizing capacity of stallion semen. The study was conducted with horses of the Criollo Colombiano breed. For freezing was used a extender supplemented with egg yolk (4%) and dimethyl formamide (5%), and 0.5 mL straws as vials, whereas for vitrification, the extender was supplemented with egg yolk (8%) and dimethyl formamide (8%), and cryovials were used as carriers. As post thaw parameters were evaluated: progressive motility, vitality, normal morphology and integrity of the plasma membrane through the hypoosmotic swelling test (HOS). For statistical evaluation was fitted a generalized linear model (GLM) and means were compared by the Tukey test. Were found average percentages of progressive motility, vitality, normal morphology and HOS of 41.6 ± 11.8 and 37 ± 8.5, 54.3 ± 10.2 and 52.3 ± 7.8, 83.1 ± 5.4 and 83.6 ± 5.8, 41.7 ± 9.8 and 38.9 ± 3.6, for cryopreserved semen by freezing and vitrification, respectively. There were no statistically significant differences (P ≤ 0.05) between treatments for any of the parameters evaluated. The fertilizing capacity of equine semen cryopreserved by vitrification is comparable to that obtained by conventional freezing.
Key words: Artificial insemination, seminal quality, freezing, vitrification.
Resumen. La criopreservación de semen es un proceso fundamental en el desarrollo de biotecnologías para la reproducción asistida en equinos. El uso de diferentes técnicas de criopreservación con cambios en las concentraciones y la naturaleza de los crioprotectores, así como en los diferentes tipos de soportes para el almacenamiento del semen, se ha constituido en una alternativa para mejorar los protocolos empleados. El objetivo de este trabajo fue evaluar el efecto de dos protocolos de criopreservación (congelación y vitrificación), sobre la capacidad fecundante del semen equino. El estudio se realizó con equinos de la raza Criollo Colombiano. Para la congelación se empleó un diluyente suplementado con de yema de huevo (4%) y dimetilformamida (5%), y pajillas de 0,5 mL como soportes; mientras que para la vitrificación, el diluyente fue suplementado con yema de huevo (8%) y dimetilformamida (8%) y se usaron crioviales como soportes. Post-descongelación, se evaluaron los parámetros: movilidad progresiva, vitalidad, morfología normal e integridad de la membrana plasmática (HOS). Para la evaluación estadística se ajustó un modelo lineal generalizado (GLM) y las medias se compararon por la prueba de Tukey. Se encontraron porcentajes promedio de movilidad progresiva, vitalidad, morfología normal y HOS de 41,6±11,8 y 37,0±8,5, 54,3±10,2 y 52,3±7,8, 83,1±5,4 y 83,6±5,8, 41,7±9,8 y 38,9±3,6, para el semen criopreservado por congelación y vitrificación, respectivamente. No se encontraron diferencias estadísticamente significativas (P ≤ 0,05) entre los tratamientos para ninguno de los parámetros evaluados. La capacidad fecundante del semen equino criopreservado por vitrificación es equiparable a la obtenida por congelación convencional.
Palabras clave: Inseminación artificial, calidad seminal, congelación, vitrificación.
There are several methods for cryopreservation of semen, which mainly modify the temperature drop rates, the concentrations of cryoprotectants, and the storage vials used. Many cryoprotectants have been evaluated in the cryopreservation process, such as glycerol, dimethyl sulfoxide, ethylene glycol and dimethyl formamide, among others (Chenier et al. 1998; Mantovani et al., 2002; Medeiros et al., 2002). The methodology used mainly for conservation of stallion semen, is possibly the refrigeration at 4 °C. This technique results in a reduction in the metabolic rate of sperm, allowing extending their survival. However, the sperm can only be stored for a few hours due to rapid reduction of fertility (Ponglowhapan et al., 2004), which depends primarily on the resistance of the plasma membrane damage caused by temperature changes and thermal shock (Sánchez et al., 2006).
Freezing and storage in liquid nitrogen allow the preservation of semen for long periods of time, reaching post-thaw fertility rates between 60% and 70%, in species as bovine (Liu et al., 1998) and canine (Alamo et al., 2005). For the case of stallion semen is still limited the effective use of frozen semen, mainly by its association with the low fertility and the inconsistency in the results. As is known, the equine sperm are extremely sensitive to alterations generated by the freezing, by osmotic stress resulting from exposure to hypertonic media, and by the osmotic changes induced during the process (Ball, 2008). The osmotic stress has been associated with adverse effects on the motility, viability and mitochondrial membrane potential of equine sperm cells, which could be associated with oxidative stress (Ball and Vo, 2001; Pommer et al., 2002). The osmotic stress in both hypotonic and hypertonic conditions can increase the production of superoxide anion in the equine sperm (Burnaugh et al., 2007).
Equine sperm cryopreservation causes cellular changes associated with apoptosis, such as alterations in the percentage of active caspases, in the mitochondrial activity, in the plasma membrane permeability and in the total and progressive motility (Brum et al., 2008). Hence it is considered that different markers of apoptosis can be used to predict the resistance for freezing of the equine sperm cells (Ortega et al., 2009). Mitochondria suffer significant damage by cryopreservation, since in the post-thaw morphological evaluation has been observed moderate swelling of the mid piece, associated with distention of these organelles (Ball, 2008). Have also been reported in the cryopreserved equine spermatozoa, changes similar to those that occur in sperm capacitation, in a process called "cryo-capacitation" (Thomas et al., 2006).
Rapid and ultra-rapid freezing can prevent the intracellular ice formation due to the cell dehydration and the cell exposure to high concentrations of permeable cryoprotectants (4 to 6 mol L-1) and sugars. The rapid freezing involves the cell exposure to liquid nitrogen vapors (Albarracin, 2005). Vitrification corresponds to a cryopreservation technique which triggers the formation of a vitreous state similar to glass, without the presence of intracellular and extracellular ice crystal, thereby decreasing cell damage (Bailey et al., 2000; Orief et al., 2005).
The advantages of vitrification are large compared to the traditional methods of freezing, because of lower equipment costs, simplicity and reduced exposure time at low temperatures. However, it has adverse effects of osmotic shock by the use of hypertonic solutions, toxic effects caused by the chemical nature and high concentrations of cryoprotectants, and cellular changes related to exposure to low temperatures (Lopera et al., 2007). New techniques of vitrification of human spermatozoa without cryoprotectants, made by direct exposure of these cells to liquid nitrogen in special vials, have managed to preserve their ability to fertilize reaching motility rates 2.87 times higher than the vitrification processes with cryoprotectant (Isachenko et al., 2004; Saki et al., 2009). The attainment of the application of vitrification processes in equine semen can mean a breakthrough in the fertility rates of cryopreserved semen. The objective of this research was to evaluate the effect of two protocols of cryopreservation (freezing and vitrification) on equine sperm fertilizing capacity.
MATERIALS AND METHODS
Stallion semen collection. The study was conducted with three horses (Equus caballus) of the Criollo Colombiano breed, located at the municipality of Girardota (Antioquia, Colombia). Animals had standard conditions of feeding, body condition, environment and reproductive management. Were collected between 3 and 5 ejaculates per stallion, with a maximum rest period of 7 days. The procedure for collection of semen was performed using the method of artificial vagina, with a Missouri model vagina (Minitube®, Tiefenbach, Germany) lubricated with non-spermicidal gel. The gel fraction of the ejaculate was removed by filtration. A mare was used to enhance sexual stimulation of the stallion. The raw semen was kept at 37 °C and later was diluted in a 1:1 ratio in EquiPro® extender (Minitube®, Tiefenbach, Germany). Subsequently, the semen was transported in refrigeration at 4 °C to the Laboratory of Animal Biotechnology of the Politécnico Colombiano Jaime Isaza Cadavid, within eight hours of collection.
Evaluation of semen quality. In each ejaculate were performed classical evaluations to determine the seminal quality. Sperm motility was determined using a phase contrast microscope Eclipse E200 (Nikon® Inc., Mellville, USA) by averaging the progressive motility of five different fields of view (400X). The concentration of the fresh semen was established through a SpermaCue® photometer (Minitube®, Tiefenbach, Germany). The assessment of sperm viability and morphology was made using a eosin-nigrosine technique modified by Barth and Oko (1989). On a glass slide at 37 °C a drop of semen was mixed with a drop of eosin-nigrosin (Sigma-Aldrich® 861006 and N4763) for 30 seconds; moreover, was made a semen smear and then fixed with heat. Through a phase contrast microscope (1000X) the 200 spermatozoa morphology was assessed, being classified as abnormal those that presented primary or secondary anomalies. Stained spermatozoa were classified as dead, while those who did not incorporate the dye were classified as living. Finally, were established general rates of morphologically normal spermatozoa and live spermatozoa.
For the assessment for the integrity of cell membranes of cryopreserved spermatozoa the hypoosmotic swelling test (HOS) was used. 0.5 mL for thawed semen was diluted in 1 mL for sodium citrate (2.94%) (Aldrich W302600) and was centrifuged at 1500 rpm for 8 min. The pellet was resuspended in 500 mL of sodium citrate (2.94%). Then, 50 mL of this solution were added to a tube containing 500 mL of hypoosmotic solution (100 mOsmol L-1) composed by 5.4% sucrose (Sigma S0389) and 2.94% sodium citrate in reagent grade water. This mixture was incubated at 38.5 °C for 30 min and then evaluated the spermatic swelling at least in 100 cells in five different fields (400X) using phase contrast microscopy. Finally, the total proportion of reacted sperm was determined. As selection criteria were processed only the ejaculates with minimum parameters of 60% of progressive motility, 100 million of sperm mL-1 and 70% of normal spermatozoa.
Cryopreservation of stallion semen. Semen cryopreservation was performed using two protocols (rapid freezing and vitrification). For rapid freezing each ejaculate was centrifuged at 1500 rpm for 15 min, then the pellet was resuspended in 2 mL of EquiPro® (Minitube, Tiefenbach, Germany) supplemented with dimethyl formamide (DMF; 5%) and egg yolk (4%). Then, the semen was resuspended in the same medium in sufficient quantity for a final concentration of 100 x 106 spermatozoa mL-1. The extended semen was maintained in refrigeration at 4 °C for 30 min and was packaged in 0.5 mL straws. The straws were cooled again at 4 °C for 15 min and then were subjected to rapid cryopreservation in liquid nitrogen vapors, being placed horizontally at a distance of 4 cm from the surface of liquid nitrogen for 15 min. Finally, straws were immersed in a storage tank of liquid nitrogen at -196 °C. After one week the straws was thawed at 37 °C for one minute in a water bath.
Vitrification of semen was performed according to the procedure reported by Peirouvi et al. (2007), which was modified for use in equine semen. The semen was diluted with EquiPro® supplemented with egg yolk (8%) and DMF (8%) in sufficient volume to a sperm concentration of 100 x 106 spermatozoa mL-1. For packaging 1.8 mL cryovials were used, which was added in a volume of 500 mL. The cryovials were exposed to 4 cm nitrogen vapor for 10 min and then were stored in a liquid nitrogen tank. A week later, each cryovial was removed from the tank and the semen was devitrified in a water bath at 37 °C for 5 min.
Statistical design. For statistical evaluation was performed a completely randomized design and the data were analyzed using a generalized linear model (GLM) for each of the dependent variables (progressive motility, vitality, normal morphology and HOS). The means for each treatment were compared by the Tukey test.
The adjusted model was:
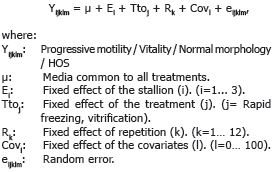
According to the dependent variable for each fitted model were included as covariates the progressive motility, vitality, normal morphology and HOS. To determine the association between variables was developed a Pearson correlation analysis according to the expression: r (X, Y) = cov (X, Y) / sxsy. All analyzes were developed using SAS 9.2 software.
Results. Sixteen ejaculates were collected and evaluated, of which 10 were processed and cryopreserved by freezing and vitrification. A total of 32 straws and 35 cryovials were post-thaw assessed (Table 1), for an average of 3.35 repetitions per processed ejaculate and treatment. For the progressive motility was found an average of 43.7% with a variation coefficient of 18.6%. The determination coefficient (r2) of the fitted model was 0.84, and the effects of stallion, HOS and vitality were statistically significant (P≤0.05).
For the vitality was found an average of 56.47% with a variation coefficient of 12.6%. The determination coefficient (r2) for the fitted model was 0.80, with statistically significant effects of stallion, treatment and motility. For the normal morphology was found an average of 82.95% with a variation coefficient of 31.57%. The main abnormalities observed were proximal and distal cytoplasmic droplets, micro heads, reflected mid piece and double head. The determination coefficient (r2) of the fitted model was 0.29, being the stallion the only statistically significant effect. And for the HOS was found an average of 44.71% of reacted spermatozoa with a variation coefficient of 15%, the determination coefficient (r2) of the fitted model was 0.84, and were statistically significant the effects of stallion, treatment and progressive motility.
The results of the treatments for the variables of progressive motility, vitality, normal morphology and HOS are described in the Table 1.
Correlation coefficients indicated a high and positive association between the variables progressive motility, vitality and HOS. While between progressive motility and vitality with the normal morphology were found low levels of association. No significant association was found between normal morphology and HOS. The correlation coefficients between the variables are presented in the Table 2.
DISCUSSION
Cryopreservation of stallion semen is known for reducing different parameters of fertilizing capacity of sperm, such as total motility, progressive mobility, permeability of the plasma membrane, morphology and even mitochondrial activity (Parks and Graham, 1992; Brum et al., 2008). With the results of this study was observed a detrimental effect of equine semen cryopreservation by the procedures of freezing and vitrification, on the parameters of progressive motility, vitality and membrane integrity, compared to the fresh stallion semen. Salazar et al. (2011), observed a reduction in the parameters of total motility, progressive motility, and the curvilinear and linear velocities, in semen cryopreserved with rapid and slow rates of cooling. In this study there was no effect of cryopreservation on the normal morphology of stallion semen, while Blottner et al. (2001) reported several alterations in the morphology of cryopreserved equine sperm, and Vlasiu et al. (2008) describe changes in the head dimensions of the cryopreserved sperm.
The values for the parameters of fresh semen quality found in this work are comparable and, in some cases, higher than those reported in the literature, mainly in relation to normal morphology and plasma membrane integrity. Results are presented for progressive motility: 41.5% ± 5.4% (Kankofer et al., 2005); 68.3% ± 3.7% (Pérez et al., 2008) and 73% ± 6.3% (Cocchia et al., 2011). Results of vitality of 81% ± 5.2% (Cocchia et al., 2011); 55.1% ± 14.4% (Henry et al., 2002) and 86.6% ± 4.3% (Sieme et al., 2003). And normal morphology values: 43.4% ± 19.9% (Neild et al., 2000), and also results are presenten for: 56.1% ± 14.2% (Neild et al., 2000) and 56.2% ± 8.6% (Perez et al., 2008). Being clear the variability among the different reports, which could be attributed to factors such as race, the environment and the evaluation method.
For the cryopreserved stallion semen were found progressive motility values of 56.1% ± 20.9% (Hoffmann et al., 2011); 23% (Squires et al., 2004) and 55.3% ± 14.3% (Henry et al., 2002). Results of vitality of 32.6% ± 6.52% (Hoffmann et al., 2011); 44.1% (Medeiros et al., 2002) and 12% ± 9.9% (Henry et al., 2002); and a HOS value of 52.8% (Mantovani et al., 2002). These results were lower in some cases, and in other cases similar to those obtained in this study, being important to consider the differences in the cryopreservation protocols, the origin of the animals, as well as, in the quality cryopreserved semen.
Between treatments for equine semen cryopreservation (freezing vs. vitrification), no statistically significant difference was found for any of the parameters evaluated as indicators of the fertilizing capacity: progressive motility, vitality, normal morphology and HOS. Thus can be considered that vitrification is comparable to the rapid freezing regarding equine sperm fertilizing ability after cryopreservation.
As mentioned previously, in different investigations has been evident decline in post-thaw quality of cryopreserved stallion semen compared to fresh semen, being slow freezing procedures (Padilla and Foote, 1991) and rapid freezing in nitrogen vapor (Clulow et al., 2008), the predominantly used. Vitrification of semen has been reported primarily in humans, which has demonstrated great efficiency in maintaining the integrity and fertility of spermatozoa (Isachenko et al., 2004a, 2004b; Peirouvi et al., 2007), for that reason was not available for this analysis reports regarding the vitrification of stallion semen. However, equivalence found between vitrified semen quality compared to the frozen semen quality, may be attributed to a combination of factors such as: the increasing of concentration of the cryoprotective and the use of cryovials for vitrification. Thus could expect a good protection of integrity of the sperm through proper heat transfer during freezing and thawing, as well as, a significant reduction in the appearance of extracellular and intracellular ice crystals.
It is known that the storage system affects the outcome of the semen cryopreserved (Samper and Morris, 1998). Oliveira et al. (2006) in a study with donkey semen from the Brazilian breed Pêga, found no statistical difference between the use of French straw of 0.5 mL and the macrotube of 2.5 mL. Kozink et al. (2006) found a 39.3% for the motility of equine semen packaged in 0.5 mL straws, and of 31.7% for the motility of semen packaged in 3.6 mL cryovials. While in an investigation from Gomez et al. (2011), under conditions of cryopreservation in cryovials similar to those used in this work, found a post-thaw motility rates between 30% and 55.6%. These results show mobility rates fairly close to those reported in this study for both packaging systems.
According to the above, vitrification can be considered as a viable alternative for cryopreservation of stallion semen, for artificial insemination. Given that addition to providing a fertilizing capacity comparable to that achieved with rapid freezing, could allow the storage of higher doses of semen in the cryovials, facilitating logistics for conducting artificial insemination (Gómez et al., 2011), and reducing the risk of failure when is necessary thaw and join multiple doses of semen (Samper and Morris, 1998). Clulow et al. (2008) reported that different fertilizing capacity parameters are not affected by changing the dose of semen packaged.
The high and positive association between the parameters of progressive motility, vitality and HOS, suggests the importance of the interrelationship between them, possibly explained by its nature as indicators of integrity and functionality of the plasma membrane, as well as, fertilizing capacity of sperm (Nie and Wenzel, 2001). The low association found between normal sperm morphology and the other parameters of semen quality would indicate that the morphology could behave independently in semen quality analysis, being to highlight their low impact on the motility of the stallion semen.
CONCLUSION
The fertilizing capacity of stallion semen cryopreserved by vitrification is comparable to that obtained in semen cryopreserved by rapid freezing, so that could be considered the use of vitrification as a viable alternative for the cryopreservation of equine semen intended for the artificial insemination.
BIBLIOGRAPHY
Álamo, D., M. Batista, F. González, N. Rodríguez, G. Cruz, F. Cabrera and A. Gracia. 2005. Cryopreservation of semen in the dog: use of ultra-freezers of - 152 ºC as a viable alternative to liquid nitrogen. Theriogenology 63(1): 72-82. [ Links ]
Albarracín, M. 2005. Vitrificación de ovocitos bovinos mediante la técnica open pulled straw: estudio estructural de cromosomas microtúbulos y microfilamentos y posterior desarrollo embrionario in vitro. Tesis de doctorado. España: Facultad de Veterinaria, Universidad Autónoma de Barcelona. 133 p. [ Links ]
Bailey, J., J. Bilodeau and N. Cormier. 2000. Semen cryopreservation in minireview domestic animals: a damaging and capacitating phenomenon. Journal of Andrology 21(1): 1-7. [ Links ]
Ball, B. 2008. Oxidative stress, osmotic stress and apoptosis: Impacts on sperm function and preservation in the horse. Animal Reproduction Science 107(3-4): 257-267. [ Links ]
Ball, B. and A. Vo. 2001. Osmotic tolerance of equine spermatozoa and the effects of soluble cryoprotectants on equine sperm motility, viability and mitochondrial membrane potential. Journal of Andrology 22(6): 1061-1069. [ Links ]
Barth, A. and R. Oko. 1989. Anormal morfology of bovine spermatozoa. First edition. lowa State University Press, Ames, Iowa. 20 p. [ Links ]
Blottner, S., C. Warnke, A. Tuchscherer, V. Heinen and H. Torner. 2001. Morphological and functional changes of stallion spermatozoa after cryopreservation during breeding and non-breeding season. Animal Reproduction Sciences 65(1-2): 75-88. [ Links ]
Brum, A., K. Sabeur and B. Ball. 2008. Apoptotic-like changes in equine spermatozoa separated by density-gradient centrifugation or after cryopreservation. Theriogenology 69(9): 1041-1055. [ Links ]
Burnaugh, l., K. Sabeur and B. Ball. 2007. Generation of superoxide anion by equine spermatozoa as detected by dihydroethidium. Theriogenology 67(3): 580-589. [ Links ]
Chenier, T., K. Merkies, C. Plante and W. Johnson. 1998. Evaluation of cryoprotective agents for use in the cryopreservation of equine spermatozoa. AAEP Proceedings 44: 5-6. [ Links ]
Clulow, J.R., L.J. Mansfield, L.H. Morris, G. Evans and W.M. Maxwell. 2008. A comparison between freezing methods for the cryopreservation of stallion spermatozoa. Animal Reproduction Science 108(3-4): 298-308. [ Links ]
Cocchia, N., M. Pasolinia, R. Mancinib, O. Petrazzuoloc, I. Cristofarod and I. Rosapane. 2011. Effect of SOD (superoxide dismutase) protein supplementation in semen extenders on motility, viability, acrosome status and ERK (extracellular signal-regulated kinase) protein phosphorylation of chilled stallion spermatozoa. Theriogenology 75(1): 1201-1210. [ Links ]
Gómez, S.L., N.S. Arruda and J.L. Rodrigues. 2011. Cryopreservation of equine semen loaded in cryovials. Original article 962. Acta Scientiae Veterinariae 39(2): 1-7. [ Links ]
Henry, M., Snoeck, P. and Cottorello, A. 2002. Post-thaw spermatozoa plasma membrane integrity and motility of stallion semen frozen with different cryoprotectants. Theriogenology 58(2): 245-248. [ Links ]
Hoffmann, N., H. Oldenhof, C. Morandini, K. Rohn and H. Sieme. 2011. Optimal concentrations of cryoprotective agents for semen from stallions that are classified 'good' or 'poor' for freezing. Animal Reproduction Science 125(1-4): 112- 118. [ Links ]
Isachenko, E., V. Isachenko, I. Katkov, G. Rahimi, T. Schondorf, P. Mallmann, S. Dessole and F. Nawroth. 2004. DNA integrity and motility of human spermatozoa after standard slow freezing versus cryoprotectant-free vitrification. Human Reproduction 19(4): 932-939. [ Links ]
Isachenko, V., E. Isachenko, I. Katkov, M. Montag, S. Dessole, F. Nawroth and H. Van der ven. 2004. Cryoprotectant-free cryopreservation of human spermatozoa by vitrification and freezing in vapor: effect on motility, DNA integrity, and fertilization ability. Biology of Reproduction 71(4): 1167-1173. [ Links ]
Kankofer, M., G. Kolm, J. Aurich and C. Aurich. 2005. Activity of glutathione peroxidase, superoxide dismutase and catalase and lipid peroxidation intensity in stallion semen during storage at 5 °C. Theriogenology 63(5): 1354-1365. [ Links ]
Kozink, D.M., C. Rubio and C.R. Pinto. 2006. Cryopreservation of stallion spermatozoa in polypropilene cryovial vials. Animal Reproduction Science 94(1-4): 96-98. [ Links ]
Liu, Z., R. Foote and C. Brockett. 1998. Survival of bull spem frozen at different rates in media varying in osmolarity. Cryobiology 37(3): 219-230. [ Links ]
Lopera, V., I. Méndez, M. Peña y A. Góngora. 2007. Vitrificación de oocitos bovinos inmaduros por el método de la pajilla abierta y estirada (Open pulled Straw - OPS). Revista Colombiana de Ciencias Pecuarias 20: 532-540. [ Links ]
Medeiros, A., G. Gomes, M. Carmo, F. Papa and M. Alvarenga. 2002 Cryopreservation of stallion sperm using different amides. Theriogenology 58(2): 273-276. [ Links ]
Mantovani, R., A. Rota, M. Falomo, I. Bailoni, and I. Vincenti. 2002. Comparison between glycerol and ethylene glycol for the cryopreservation of equine spermatozoa: semen quality assessment with standard analyses and with the hypoosmotic swelling test. Reproduction, Nutrition and Development 42(3): 217-226. [ Links ]
Nie, G. and J. Wenzel. 2001. Adaptation of the hypoosmotic swelling test to assess functional integrity of stallion spermatozoal plasma membranes. Theriogenology 55(4): 1005-1018. [ Links ]
Oliveira, J.V., M.A. Alvarenga, C.M. Melo, L.M. Macedo, J.A. Delláquajr and F.O. Papa. 2006. Effect of cryoprotectant on donkey semen freezability and fertility. Animal Reproduction Science 94(1-4): 82-84. [ Links ]
Ortega, C., B. Macías, J.M. Gallardo, I. González, H. Rodríguez, J.A. Tapia and F.J. Peña. 2009. Apoptotic markers can be used to forecast the freezeability of stallion spermatozoa. Animal Reproduction Science 114(4): 393-403. [ Links ]
Parks, J. and J. Graham. 1992. Effects of cryopreservation procedures on sperm membranes. Theriogenology 38(2): 209-222. [ Links ]
Peirouvi, T., G. Farjah, J. Rad and M. Novin. 2007. Vitrification induced apoptosis in spermatozoa from fertile and subfertile men. Iranian Journal of Reproductive Medicine 5(3): 117-120. [ Links ]
Pérez, J., F. Mello, G. Juliani, M. Lagares, L. Lago and M. Henry. 2008. Effect on post-thaw viability of equine sperm using stepwise addition of dimethyl formamide and varying cooling and freezing procedures. Animal Reproduction Science 5(3-4): 103-109. [ Links ]
Pommer, A., J. Rutllant and S. Meyers. 2002. The role of osmotic resistance on equine spermatozoal function. Theriogenology 58(7): 1373-1384. [ Links ]
Saki, G., F. Rahim and M. Zergani. 2009. Vitrification of small volume of normal human sperms: use of open pulled straw carrier. Journal of Medical Sciences 9(1): 30-35. [ Links ]
Salazar, J., S. Teague, C. Love, S. Brinsko, T. Blanchard and D. Varner. 2011. Effect of crypreservation protocol on postthaw characteristics of stallion sperm. Theriogenology 76(3): 409-418. [ Links ]
Samper, J. and C. Morris. 1998. Current methods for stallion semen cryopreservation: a survey. Theriogenology 49(5): 895-903. [ Links ]
Sánchez, R., P. Cartagena y O. Berland. 2006 Comparación del efecto de dos diluyentes sobre la fertilidad potencial de semen canino refrigerado. Revista de Investigaciones Veterinarias del Perú 17(1): 1-7. [ Links ]
Squires, E., S. Keith and L. Graham. 2004. Evaluation of alternative cryoprotectants for preserving stallion spermatozoa. Theriogenology 62(6): 1056-1065. [ Links ]
Sieme, H., G. Martinsson, H. Rauterberg, K. Walter, C. Aurich, R. Petzoldt and E. Klug. 2003. Application of techniques for sperm selection in fresh and frozen-thawed stallion semen. Reproduction of Domestic Animals 38(2): 134-140. [ Links ]
Thomas, A., S. Meyers and B. Ball. 2006. Capacitation-like changes in equine spermatozoa following cryopreservation. Theriogenology 65(8): 1531-1550. [ Links ]
Vlasiu, T., I. Groza, I. Morar and R. Catana. 2008. The effect of different freezing procedures on sperm head morphometry in stallions. Bulletin UASVM, Veterinary Medicine 65(2): 146-151. [ Links ]