Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad Nacional de Agronomía Medellín
Print version ISSN 0304-2847
Rev. Fac. Nac. Agron. Medellín vol.66 no.1 Medellín Jan./June 2013
Dormancy and Germination of Castilla Blackberry Seeds (Rubus glaucus Benth)
Latencia y Germinación de Semillas de Mora de Castilla (Rubus glaucus Benth)
Cipriano Arturo Díaz Diez1; Mario Lobo Arias2; José Régulo Cartagena Valenzuela3 and Clara Inés Medina Cano4
1 Researcher. Corpoica C.I. La Selva. A.A. 100, Rionegro, Antioquia, Colombia. <cdiaz@corpoica.org.co>
2 Senior Researcher. Corpoica C.I. La Selva. A.A. 100, Rionegro, Antioquia, Colombia. Associate Professor, Universidad Nacional de Colombia - Sede Medellin - Facultad de Ciencias Agrarias - Departamento de Ciencias Agronómicas. A.A. 1779, Medellín, Colombia. <mloboa@unal.edu.co>
3 Associate Professor. Universidad Nacional de Colombia - Sede Medellín - Facultad de Ciencias Agrarias - Departamento de Ciencias Agronómicas. A.A. 1779, Medellin, Colombia. <jrcartag@unal.edu.co>.
4 Principal Researcher, Corpoica C.I. La Selva. A.A. 100, Rionegro, Antioquia, Colombia. <cmedina@corpoica.org.co>
Received: January 21, 2013; accepted: March 07, 2013.
Abstract
Abstract. We categorized the dormancy and germination of blackberry (Rubus glaucus Benth) seeds from the Germplasm Bank System for Food and Agriculture of the Colombian Nation. A tetrazolium test showed normal seedling production viability, but seed coat impermeability prevented imbibition, which is considered an expression of exogenous dormancy; however, this was released by immersing the seeds in 5.25% sodium hypochlorite for 16 to 21 hours. The treatment was applied to 10 accessions of Castilla blackberry, harvested during the dry and rainy seasons. The seeds were germinated under light and dark conditions. The highest germination counts were obtained with the dry-season-collected seeds incubated in darkness and with the rainy-season seeds incubated under light conditions. Differential germination responses were also independently determined by genotype, incubation conditions (light or darkness) and collecting season.
Key words: Plant genetic resources, sexual propagation, Andean fruits, seed physiology.
Resumen
Se categorizaron la latencia y la germinación en semillas de mora de Castilla (Rubus glaucus Benth), provenientes del Sistema de Bancos de Germoplasma para la Alimentación y la Agricultura de la Nación Colombiana. La prueba del tetrazolio demostró que las semillas tenían la capacidad para dar origen a una plántula normal; sin embargo, al contacto con el agua la imbibición fue restringida por la impermeabilidad de la testa, lo que se considera como una expresión de latencia exógena. Ésta pudo ser removida con la inmersión de las semillas durante 16 a 21 horas en hipoclorito de sodio al 5,25%. El tratamiento fue aplicado a 10 accesiones de mora de Castila cosechadas en épocas seca y lluviosa; las semillas se germinaron bajo condiciones de luz y oscuridad, con una mayor germinación al incubar las provenientes de tiempo seco en condiciones de oscuridad y en aquellas de época lluviosa al ser germinadas con luz. También al aplicar el procedimiento de remoción de latencia exógena se observaron respuestas germinativas diferenciales determinadas por el genotipo, las condiciones de incubación (luz y oscuridad) y la época de recolección.
Palabras clave: Recursos genéticos vegetales, propagación sexual, frutales andinos, fisiología de semillas.
Blackberry (Rubus glaucus Benth) agricultural propagation is usually done with vegetative material. The development of this and other Andean fruit crops has been carried out by farmers without a technical approach capable of a comprehensive vision of the productive function components: genotype, environment, and their interaction (Lobo, 2000; 2004). Nevertheless, the blackberry holds an important place among the fruit crops of the Colombian Andes, with a planted area of 11,651 ha in 2011, distributed in 17 departments, and contributing a production of 94,151 t (MADR, 2010). The planted area is projected to reach 26,000 ha by 2026 (Tafur et al., 2006).
In this context, it is important to conduct a breeding program for the productive system of this crop, through individual selection and hybridization between the genetic resources of the species, which must be adequately identified, preserved and characterized. In fact, these materials constitute the basis of breeding research (Rodríguez et al., 2009) and make it possible to obtain the cultivars required by the different agents of a crop's productive chain at all stages. Among these genetic resources, local materials are especially valuable, inasmuch as they are spontaneously adapted to different environmental conditions. Thus, it is important to undertake the collection and characterization of the wild and cultivated species of Rubus, in order to enrich the currently existing genetic bank of this crop. This implies collecting seeds (not to damage the plants) in order to obtain recombinants.
Blackberry seed germination is usually slow and irregular, as has also been observed in other Rubus species such as the raspberry (R. idaeus L.) and blackberry (R. chloocladus W. C. R. Watson). Among other factors, this is attributable to dormancy, (Ellis et al., 1985), i.e., non-germination of mature, intact and viable seeds, despite having the necessary conditions (Finch-Savage and Leubner-Mettzger, 2006). Seed dormancy, indeed, is a common characteristic of incipiently domesticated species.
Dormancy can be of different types, with some seeds exhibiting more than one type. Exogenous dormancy consists of embryo growth restriction due to seed coat impermeability or excessive hardness, or to the presence of chemical inhibitors. Endogenous dormancy results from deficiencies in the embryo axis or from metabolic limitations in the cotyledons (Desai, 2004). Several methods are employed to release dormancy, namely physical and chemical ones; including the application of gibberellic acid (AG3) (Doria, 2010). In Rubus, chemical and mechanical seed scarification are the most common ones, but they usually do not produce uniform results within and among species (Suzuki, 1993; Zasada and Tappeiner III, 2003).
For these reasons, it is necessary to characterize seed dormancy in the blackberry R. glaucus and related taxa, and to develop release protocols, in order to facilitate collection and preservation, as well as the consequent development, of improved cultivars.
Materials and methods
Location. This research was conducted at the Seed Laboratory of the Germplasm Bank System for Food and Agriculture of the Colombian Nation (Sistema de Bancos de Germoplasma de la Nación Colombiana para la Alimentación y la Agricultura -SBGNCAA), at "La Selva" Research Center (Rionegro, Antioquia, Colombia), which belongs to Corpoica.
Biological material and its extraction. The seeds were obtained from fruits of R. glaucus Benth at ripening stage 6, according to the Colombian standard for the Castilla blackberry (NTC 4106) (ICONTEC et al., 1997). These fruits were gathered from the blackberry field collection established at "La Selva" Research Center. The seeds were extracted by squeezing them in water and, after a 72 h resting period, application of pressurized water so that the seeds were retained in a sieve. They were dried at 17 °C and 78% relative humidity for 48 h, after which the moisture content was 40%. Next, the seeds were kept in a Fisher Scientific® drying chamber with silica gel at a 1:10 proportion (in order to prevent moisture absorption) for a period of five days.
Seed viability determination protocol. For such purpose, we used the tetrazolium staining technique (Ribeiro et al., 2010), which, based on mitochondrial function catalyzing dehydrogenase activity, indicates an oxidation-reduction reaction resulting in tissue color change (França-Neto et al., 1999). Under this technique, normal tissues show standard staining while abnormal ones show incomplete staining or no staining (ISTA, 2005; Marcos-Filho, 2005; ). In the case of the genus Rubus, sub-genus Rubus, which includes R. glaucus, viable seeds were identified by their (total or partial) red-stained embryos; while non-viable seeds showed red-stained coats and unstained embryos (Wada and Reed, 2011).
Determination of imbibition. This procedure intends to assess whether the seed coat is more or less permeable to water (Baskin and Baskin, 2001), and thus more or less dormant, which is indicated by weight gain (Bansal et al., 1980). Since blackberry seeds exhibit coarse surfaces, to which water is likely to adhere, the latter was measured by immersing seeds of known weight in water for 5 s, drying them with absorbent napkins and weighing them again. The difference, which corresponded to the superficially adhered water, was used to correct the seed imbibition data, which were obtained with Mettler AE200® scales by weighing the seeds every 60 min for a period of 24 h. The trial was carried out under a completely randomized experimental design with five replications. The experimental unit was defined as 1 g of seeds. The data were subjected to Analysis of Variance with the program SAS 9.1.
Germination assessment. The germination assessment was carried out under dark conditions at 29 °C in a Seedburo 1022 W® germinator, and under continuous 16 A white light in a Conviron CMP 3244 phytotron, Model E-16®. According to the methodology of Baskin and Baskin (2001), the seeds were placed on filter paper in Petri dishes that were periodically wetted over a period of 30 days, after which they were quantified, discriminating between germinated, viable non-germinated and dead seeds. The latter two categories were recognized through tetrazolium staining, according to Wada and Reed's criterion for Rubus spp. (2011).
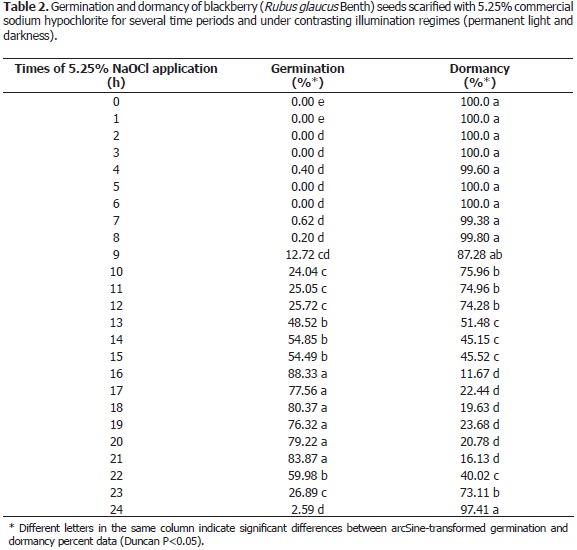
Evaluation of dormancy release with commercial sodium hypochlorite. A 5.25% solution of sodium hypochlorite was evaluated every hour for a period of 24 h, along with an absolute control without hypochlorite. The experiment made use of fresh seeds at a degree of maturity of 6, following NTC 4106 (ICONTEC et al., 1997). The trial was carried out under a completely randomized design with four replications, with a 25 x 2 factorial arrangement. The factors corresponded to chemical agent application time and germination test conditions (light and darkness) as recommended, when the data showed ample dispersion (Gómez, 1997). The current results were subjected to arcSine transformation and then processed in SAS 9.1. The germination and dormancy calculations were based on the total numbers of germinable and viable dormant seeds as assessed through the tetrazolium test. The data were taken on day 30 after the beginning of the embryo emergency test. To assess the significance of the obtained results, a Duncan test for mean values was applied (P<0.05) through SAS 9.1 procedure.
Validation of exogenous dormancy release in materials of the SBGNCAA blackberry germplasm bank through 5.25% hypochlorite application. Based on the results of the above mentioned 5.25% sodium hypochlorite test, this agent's effectiveness was evaluated in ten ecotypes from the blackberry field collection of the SBGNCAA, which is managed by Corpoica at "La Selva" Research Center, in Rionegro, Antioquia. The seeds, obtained both in the rainy and dry seasons, were scarified with 5.25% sodium hypochlorite for a period of 16 h, and then evaluated through a germination test, following the above mentioned protocol. The trial was conducted under a completely randomized design with a 10 x 2 x 2 factorial arrangement with five replications of 100 seeds. The factors in question corresponded to 10 blackberry populations, seeds obtained in two climatic (rainy and dry) seasons, and two illumination regimes: light and darkness. Statistical analysis was conducted in SAS 9.1, including arcSine data transformation by a model that estimated the mentioned factors and their interactions. The differences between the arcSinetransformed mean values were estimated through Duncan's test.
Results and discussion
Seed viability. A tetrazolium test interpreted with the criteria of Nurse and Di Tomaso (2005) and Borza et al. (2007) (who found that, in Rubus spp., light pink or non-stained embryos are not viable) indicated that the seed batch studied in the present research was 97% viable.
Imbibition assessment. The analysis of variance applied to the arcSine of the percentage of change in seed weight with regards to time zero found no significant differences attributable to water absorption (Table 1). This indicates seed coat impermeability and, hence, physical dormancy, because the embryo remains dry until the coat is broken (Fenner and Thompson, 2005). Lack of imbibition has been observed in several Rubus spp. (Ourecky 1975; Warr et al., 1979; Nybom 1980; Jennings, 1988). For that reason, Ourecky (1975), as well as Peacock and Hummer (1996) remarked on the importance of scarifying these seeds.
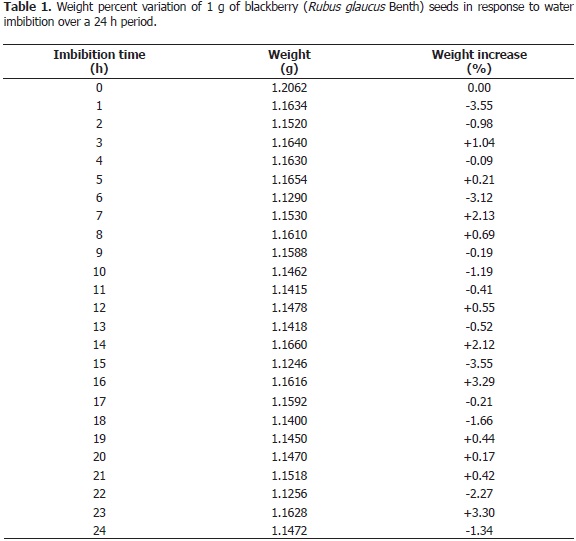
The restrained imbibition, coupled with the 97% viability observed with the tetrazolium test, indicated dormancy mechanisms. Suzuki (1997) pointed out that dormancy release in Rubus spp. is associated with the permanence in the soil, and that Rubus spp. life histories, reproductive phenology and growth are diverse. These attributes reveal fine-tuned environmental adaptation, a fundamental part of which corresponds to seed dormancy variation. Yokoyama (1982) reported that immediate germination after seed dispersion is scarce, even under ideal moisture and temperature conditions. Harrington (1972) observed that, after dormancy release, seed viability may last for long periods of time. This is explained by the fact that germination in natural ecosystems is associated with canopy opening (Washitani and Tanaka, 1987; Suzuki, 1993), which allows light penetration, thus favoring seedling survival and germination.
Germination assessment. No germination was observed after 30 days, which led to the extension of the experiment for 500 days, with periodical checks, but still with negative results. This coupled to the mentioned result of no imbibition, points to deep dormancy. In this respect, Ourecky (1975); Nybom (1980); Jennings (1988) reported dormancy in Rubus spp. resulting from seed coat impermeability, which constitutes a mechanical impediment for embryo germination. In turn, Heit and Slate (1950); Scott and Ink (1957); Feder and Spangelo (1971); Moore et al. (1974); Rantala (1976); Nybom (1980) have recorded hindered and scattered germination in different Rubus taxa resulting from exogenous dormancy. This condition has been attributed to seed coat (endocarp) impermeability, mechanical resistance to growth, presence of chemical inhibitors, and dormancy of the embryo itself (Zasada and Tappeiner III, 2003).
In this context, several researchers (Krepting and Roe, 1949; Nybom, 1980; Dale and Jarvis, 1983; Barnes, 1985; Maxwell, 1990; Zasada et al., 1994) have determined that germination of Rubus spp. seeds under field conditions might take from two to three years, and that some of them are likely to remain dormant for decades, depending on the taxa and on the conditions to which the seeds are exposed.
Evaluation of dormancy release with 5.25% commercial sodium hypochlorite. The analysis of variance of the arcSine-transformed seed germination and dormancy data (which were corrected according to the results of the tetrazolium test as applied to viable seeds) revealed significant differences between 5.25% commercial hypochlorite application times, and no significant differences among germination light regimes or in their interaction with treatment duration (Table 2). The highest germination counts were recorded between 16 and 21 h, when the scarification effect seems to be at an optimum point. Longer times were apparently harmful for the embryo. In this respect, sodium hypochlorite has been reported to have scarifying effects on Cattleya mendellii (Salazar-Mercado, 2012), also overcoming dormancy, whose lowest scores were observed during the same time period reported in the current research. These results may correspond to one of the multiple germination episodes postulated by Cain and Shelton (2003), for Rubus blackberries, which are related to the germination potential of the soil seed bank as it changes over time, and associated, in turn, with delayed germination and population survival. In this sense, Dübbern de Souza and Marcos-Filho (2001) highlighted the fact that seed coat softening determines permeability to water and oxygen, which facilitates imbibition and further activation of metabolic processes such as repair mechanisms (membranes, proteins and DNA), nutritious reserve oxidation and conversion to carbohydrates as the energetic source of radicle growth (Dubreucq et al., 2000; Doria, 2010).
Validation of a seed exogenous dormancy release protocol (consisting of 5.25% sodium hypochlorite application) tested on seeds of ten blackberry materials from the Colombian Rubus collection. The arcSine-transformed germination data in Table 3 revealed significant differences between seed collection times (dry and rainy seasons), illumination conditions (light and darkness) and blackberry ecotypes. Furthermore, significant differences were seen due to double and triple interactions between these factors. The dry season germination percentage (52.83%) was considerably higher than that of the rainy season (38.61%). For the interactions, those seeds harvested during the dry season and germinated under dark conditions rendered the highest result (71.31%, averaging all materials), while those harvested in the rainy season and germinated in light scored the second highest average result (59.67%), thus showing contrasting germination behaviors.
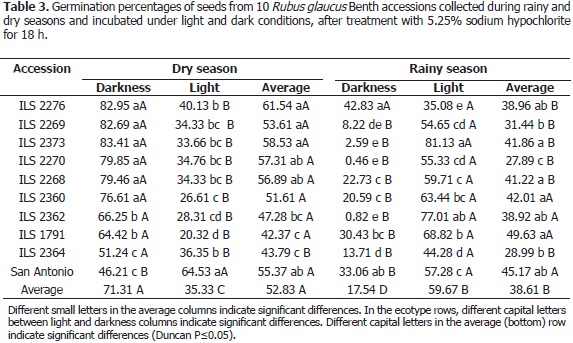
Among the dry season seed ecotypes, cv. San Antonio exhibited significantly higher germination counts in the light than in the darkness, while the other ecotypes showed better performances under dark conditions (Table 3). Regarding the rainy season seeds, accession ILS 2276 was the only one that showed significantly similar scores under light and dark conditions, while the other ecotypes performed better in the light. Seeds from accession ILS 2373 collected during the dry season reached larger significant counts in the darkness, while those collected in the rainy season germinated more abundantly in the light. These results show how not all materials germinate the same when harvested in different seasons or treated with contrasting light regimes.
These results are consistent with the fact that the blackberry (R. glaucus) is an incipiently domesticated crop whose dormancy has only been scarcely released, because, among other reasons, its propagation is mostly asexual, which limits intense selection of desirable characteristics. Finch-Savage and Leubner-Metzger (2006) indicated that seed dormancy is an innate characteristic associated with the environmental conditions to which the species germination is adapted. At this point, it is worth noting that the studied accessions kept in the Colombian Rubus collection have been obtained from different localities and therefore, may have contrasting dormancy depths, probably coupled to varied germination signal requirements, as observed by Fenner and Thompson (2005). Along these lines, Finch-Savage and Leubner-Metzger (2006) have pointed out that, as an adaptation of higher plants to different climatic regions, dormancy not only has a genetic component, but is also under an important environmental influence. The analysis of this characteristic in natural populations indicates that it prevents germination under inadequate conditions, thus acquiring an important adaptive value in terms of survival (Baskin and Baskin, 1998).
Wada (2010) reported that diverse Rubus species, including R. glaucus, tend to acquire deep dormancy after the drying process they undergo when stored in the cold rooms of germplasm banks. The release of this particular mode of dormancy may take place under cold storage conditions over months or years, probably setting a significant contrast with initial seed viability. As a consequence, the effect of storage conditions (namely temperature and moisture) on seeds should be thoroughly studied.
In studying the germination pattern of 47 native Rubus species, Naruhashi et al. (1999) found embryo emergency rates with inter- and intraspecific variation. This is in agreement with the intraspecific variation observed in the current study, which is probably due to the fact that the studied materials had been collected in different localities.
Conclusions
Under natural conditions, Castilla blackberry seeds show high viability, as assessed with the tetrazolium test, which allows for recognizing living embryos. Nevertheless, this viability is not reflected in immediate germination.
The Castilla blackberry seeds presented exogenous dormancy due to their impermeable coat, as shown by their negative germination behavior when incubated under adequate moisture, temperature and aeration conditions for embryo development, and by the negative results of the water imbibition test.
The dormancy of Castilla blackberry seeds can be released by reducing coat impermeability through scarification with 5.25% sodium hypochlorite applied for 16 to 21 h.
After the hypochlorite treatment, the germination of the studied materials (with different geographical origins, but cultivated in the same place) showed contrasting germination behaviors. Similarly, the seed-collection season and incubation light regime determined corresponding germination variations.
As a first step in the collection, preservation and study of the currently existing variability of this crop, the results obtained in this study are expected to support future efforts aimed at the development of improved blackberry varieties, both for small farmers and large producers.
As a means of obtaining and preserving plant variability for the sustainable development of the species and a better use of the available Rubus glaucus germplasm, seed behavior should be studied in-depth, especially in terms of its reaction to long term storage. Furthermore, it is advisable to extend this study to the other Rubus species available in the Colombian Rubus collection currently managed by Corpoica.
Acknowledgements
The authors want to thank Corpoica and its employees, who are in charge of the Germplasm Bank System for Food and Agriculture of the Colombian Nation - SBGNCAA, for support provided within the framework of the different experiments conducted in this study.
Bibliography
Bansal, R.P., P.R. Bathi and D.N. Sen. 1980. Differential specificity in water imbibition of Indian arid zone seeds. Biologia Plantarum 22(5): 327-331. [ Links ]
Barnes, H.W. 1985. Production of Rubus deliciosus by seed. Plant Propagation 31(3): 6-7. [ Links ]
Baskin, C.C and J.M. Baskin, 1998. Seeds: ecology, biogeography and evolution of dormancy and germination. Academic Press, London. 666 p. [ Links ]
Baskin, C.C. and J.M. Baskin. 2001. Seeds. Academic Press. San Diego, CA. 627 p. [ Links ]
Borza, J.K., P.R. Westerman and M. Liebman. 2007. Comparing estimates of seed viability in three foxtail (Setaria) species using the imbibed seed crush test with and without additional tetrazolium testing. Weed Technology 21(2): 518-522. [ Links ]
Cain, M.D. and M.G. Shelton. 2003. Fire effects on germination of seeds from Rhus and Rubus: competitors to pine during natural regeneration. New Forests 26: 51-64. [ Links ]
Dale. A. and B.C. Jarvis. 1983. Studies on germination in raspberry. Crop Research 23: 73-81. [ Links ]
Desai, B.B. 2004. Seeds handbook. Biology, production, processing, and storage. Second edition. Marcel Dekker Inc., New York. 627 p. [ Links ]
Doria, J. 2010. Generalidades sobre las semillas: su producción, conservación y almacenamiento. Cultivos Tropicales 31(1): 74-85. [ Links ]
Dübbern de Souza, F. and J. Marcos-Filho. 2001. The seed coat as a modulator of seed-environment relationships in Fabaceae. Brazilian Journal of Botany 24(4): 365-375. [ Links ]
Dubreucq B., N. Berger, E. Vincent, M. Boisson, M. Caboche and L. Lepiniec. 2000. The Arabidopsis AtEPR1 extensin-like gene is specifically expressed in endosperm during seed germination. The Plant Journal: for Cell and Molecular Biology 23(5): 643-652. [ Links ]
Ellis, R.H., T.D. Hong and E.H. Roberts. 1985. Chapter 62. Rosaceae. pp. 343-346. In: Handbooks for Genebanks: No. 3. Department of Agriculture and Horticulture. University of Reading, UK. International Board for Plant Genetic Resources. Rome. [ Links ]
Feder, S.O. and L.P.S. Spangelo. 1971. Seed germination in the red raspberry. Fruit Varieties and Horticultural Digest 25(4): 75-76. [ Links ]
Fenner, M. and K. Thompson. 2005. The ecology of seeds. Cambridge University. Cambridge, UK. 250 p. [ Links ]
Finch-Savage, W.E. and G. Leubner-Metzger. 2006. Seed dormancy and the control of germination. New Phytologist 171: 501-523. [ Links ]
França-Neto, J.B., F.C. Krzyzanowski e N.P. Costa. 1999. Capitulo 8: metodología do teste de tetrazólio em sementes de soja. pp. 1-28. In: Krzyzanowski, F.C., R.D. Vieira e J.B. França-Neto (eds.). Vigor de sementes: conceitos e testes. Londrina, Brasil. 218 p. [ Links ]
Gómez, H. 1997. Estadística experimental con aplicaciones a las ciencias agrícolas. Facultad de Ciencias Agropecuarias. Universidad Nacional de Colombia, Medellin. 571 p. [ Links ]
Harrington, J.F. 1972. Seed storage and longevity. pp. 145-245. In: T.T. Kozlowski (ed.). Seed biology (III). Academic Press, New York. 422 p. [ Links ]
Heit, C.E. and G.L. Slate. 1950. Treatment of blackberry seed to secure first year germination. Proceedings of the American Society for Horticultural Science 55: 297-301. [ Links ]
Hummer, K.E. and D.N. Peacock. 1994. Seed dimension and weight of selected Rubus species. HortScience 29(9):1034-1036. [ Links ]
ICONTEC, MADR, Federación Nacional de Cafeteros de Colombia, Cenicafé. 1997. Norma Técnica Colombiana NTC 4106. Frutas frescas. Mora de castilla. Especificaciones. Bogotá. 15 p. [ Links ]
ISTA, 2005. International rules for seed testing. International Seed Testing Association. Bassersdorf, Suiza. In: http://www.seedtest.org; accessed: April, 2011. [ Links ]
Jennings, D.L. 1988. Raspberries and blackberries: their breeding diseases and growth. Academic Press, New York. USA. 20 p. [ Links ]
Krepting, L.W and E.I. Roe, 1949. The role of some birds and mammals in seed germination. Ecological Monographs 19(3): 269-286. [ Links ]
Lobo, M. 2000. Papel de la variabilidad genética en el desarrollo de los frutales andinos como alternativa productiva. pp. 27-36. En: Memorias III Seminario de Frutales de Clima Frío Moderado. Centro de Desarrollo Tecnológico de Frutales, Manizales. [ Links ]
Lobo, A.M. 2004. Posibilidades y perspectivas del desarrollo de programas de mejoramiento en frutales andinos. Visión conceptual. pp. 27-36. In: Memorias V Seminario de Nacional e Internacional de Frutales de Clima Frío. CDTF. Manizales. [ Links ]
Marcos-Filho, J. 2005. Fisiologia de sementes de plantas cultivadas. First edition. FEALQ, Piracicaba. Brasil. 49 p. [ Links ]
Maxwell, B.D. 1990. The population dynamics and growth of salmonberry (Rubus spectabilis) and thimbleberry (Rubus parviflorus.). Ph.D. Dissertation. Oregon State University. Corvallis, Oregon. 206 p. [ Links ]
Ministerio de Agricultura y Desarrollo Rural (MADR). 2010. Estadísticas por producto. La mora. En: http://www.agronet.gov.co; consulta: marzo 2011. [ Links ]
Moore, J.N., G.R. Brown and C. Lundergan. 1974. Effect of duration of acid scarification on endocarp thickness and seedling emergence of blackberries. HortScience 9: 124-126. [ Links ]
Naruhashi, N., W. Nakata, M. Shibata, and H. Takeda. 1999. Germination patterns of Japanese Rubus under immediate sowing after harvest. Acta Horticulturae 505: 379-383. [ Links ]
Nurse, R.E. and A. Di Tommaso. 2005. Corn competition alters germinability of velvetleaf (Abutilon theophrasti) seeds. Weed Sciences 53(4): 479-488. [ Links ]
Nybom, H. 1980. Germination in Swedish blackberries (Rubus L. subgen. Rubus). Botaniska Notiser 133(4): 619-631. [ Links ]
Ourecky, D.K. 1975. Brambles, pp. 98-129. In: J. Janick and J.N. Moore (eds.). Advances in fruit breeding. Purdue University Press. West Lafayette, Indiana. 640 p. [ Links ]
Peacock, D.N. and K.E. Hummer. 1996. Pregermination studies with liquid nitrogen and sulfuric acid on several Rubus species. HortScience 31(2): 238-239. [ Links ]
Rantala, E.M. 1976. Sexual reproduction in the cloudberry. Annales Agriculturae Fenniae 15: 295-303. [ Links ]
Ribeiro, C., O. De Castro, M. Ingredi and M. Panobianco. 2010. Tetrazolium test for evaluating triticale seed viability. Revista Brasileira de Sementes 32(3): 163-169. [ Links ]
Rodríguez, R., S. Montes, J.A. Rangel, M. Mendoza y L. Latournerie. 2009. Caracterización morfológica de la calabaza pipiana (Cucurbita argyrosperma Huber). Agricultura Técnica en México 35(4): 378-388. [ Links ]
Salazar-Mercado, S. 2012. Germinación asimbiótica de semillas y desarrollo in vitro de plántulas de Cattleya mendelii Dombrain (Orchidaceae). Acta Agronómica 61(1): 69-78. [ Links ]
Scott, D.H and P.D. Ink. 1957. Treatment of Rubus seeds prior to after-ripening to improve germination. Journal American for Society Horticultural Science 69: 261-267. [ Links ]
Suzuki, W. 1993. Germination of Rubus palmatus var. coptophyllus and R. microphyllus seeds buried in soil for 7.5 years. Ecological Research 8: 107-110. [ Links ]
Suzuki, W. 1997. Germination responses of Rubus palmatus var. coptophyllus and R. parvifolius seeds with different burial durations to a variable light and temperature regime. Ecological Research 12:167-174. [ Links ]
Tafur, R., J. Toro, J. Perfetti, D. Ruiz y J. Morales. 2006. Plan Frutícola Nacional (PFN). Ministerio de Agricultura y Desarrollo Rural, Fondo Nacional de Fomento Hortifrutícola, Asohofrucol, SAG. Valle del Cauca. Colombia. 43 p. [ Links ]
Tomlik, W.A., J. Zielinsky and M. Guzicka. 2010. Morphology and anatomy of blackberry pyrenes (Rubus L., Rosaceae) elementary studies of the European representatives of the genus Rubus L. Flora-Morphology Distribution Functional Ecology of Plants 205(6): 370-375. [ Links ]
Wada, S. 2010. Evaluation of Rubus seed characteristics: seed coat morphology, anatomy, germination requirements and dormancy breaking. Ph.D dissertation. Oregon State University. Corvallis, Oregon. 207 p. [ Links ]
Wada, S. and B. Reed. 2011, Standardizing germination protocols for diverse raspberry and blackberry species. Scientia Horticulturae 132: 42-49. [ Links ]
Warr, H.J., D.R. Savory and A.K. Bal. 1979. Germination studies of bakeapple (cloudberry) seeds. Canadian Journal of Plant Science 59: 69-74. [ Links ]
Washitani, I. and A. Tanaka. 1987. Gap-detecting mechanism in the seed germination of Mallotus japonica (Thunb.) Muell. Arg., a common pioneer tree of secondary succession in temperate Japan. Ecological Research 2:191-201. [ Links ]
Yokoyama, T. 1982. Germination of Rubus palmatus var. captophylus seed extracted from droppings of birds (Zoasterops palpebrosa japonica). pp. 287-288. In: Transactions of the 93rd Meeting of the Japanese Forestry Society. [ Links ]
Zasada, J.C., J.C. Tappeiner III, B.D. Maxwell and M.A. Radwan. 1994. Seasonal changes in shoot and root production and in carbohydrate content of salmonberry (Rubus spectabilis) rhizome segments from the central Oregon Coast Ranges. Canadian Journal of Forest Research 24(2): 272-277. [ Links ]
Zasada, J.C. and J.C. Tappeiner III. 2003. Rubus L. blackberry, raspberry. Rosaceae Rose family. The Woody Plant Seed Manual U.S.D.A. Forest Service, pp. 1629-1638. [ Links ]













