Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad Nacional de Agronomía Medellín
Print version ISSN 0304-2847
Rev. Fac. Nac. Agron. Medellín vol.66 no.2 Medellín July/Dec. 2013
Determination of Soursop (Annona muricata L. cv. Elita) Fruit Volatiles during Ripening by Electronic Nose and Gas Chromatography Coupled to Mass Spectroscopy
Determinación de Compuestos Volátiles en Frutos de Guanábana (Annona muricata L. cv. Elita), durante la Maduración, por Nariz Electrónica y Cromatografía de Gases Acoplada a Espectroscopía de Masas
Carlos Julio Márquez Cardozo1; José Régulo Cartagena Valenzuela2 and Guillermo Antonio Correa Londoño2
1 Associate Professor. Universidad Nacional de Colombia - Sede Medellín - Facultad de Ciencias Agrarias - Departamento de Ingeniería Agrícola y Alimentos. A.A. 1779, Medellín, Colombia. <cjmarque@unal.edu.co>
2 Associate Professor. Universidad Nacional de Colombia - Sede-Medellín - Facultad de Ciencias Agrarias - Departamento de Ingeniería Agronómica. A.A. 1779, Medellín, Colombia. <jrcartag@unal.edu.co>
3 Associate Professor. Universidad Nacional de Colombia - Sede Medellín - Facultad de Ciencias Agrarias - Departamento de Ingeniería Agronómica. A.A. 1779, Medellín, Colombia. <gcorrea@unal.edu.co>
Recibido: Mayo 02 de 2013; aceptado: septiembre 11 de 2013.
Abstract. As an exotic highly perishable tropical fruit commercially grown in Colombia, soursop (Annona muricata L.) is currently in need of postharvest handling studies. Thus, the present research was conducted to characterize the volatile compounds of soursop cv. Elita during postharvest. For this purpose, fruit ripeness was evaluated, for one thing, by a volatile compound measuring system known as electronic nose (EN), and for another thing, by headspace solid phase microextraction and gas chromatography mass spectrometry (HS-SPME/GC-MS). The profile of volatile substances in fruits is one of the main indicators of the sensory attributes that typify the organoleptic quality of these products. The EN constitutes an economical, relatively simple, fast and innovative alternative to determine groups of volatile compounds in whole or fractionated samples from fruits of commercial interest. In contrast, and despite its being a highly selective technique, the use of SPME/CG-MS might be limited by its elevated cost. Based on EN assessment, fruit ripening stages were classified as unripe, half ripe, ripe and overripe. The most active EN sensors were numbers 2 sensitive to nitrogen oxides), 6 (sensitive to methane) and 8 (sensitive to alcohols and partially aromatic compounds). HS-SPME/GC-MS analysis allowed establishing that during postharvest, the major proportion of volatile compounds corresponded to esters, predominantly Methyl hexanoate. Particularly in overripe fruits, the presence of alcoholic compounds coincides with the EN assessment, which, for its part, detected mainly alcohols and a wide range of aromatic substances. The study contributes to the characterization of postharvest volatiles, which are one of the major sensory attributes of tropical fruits.
Key words: Aroma, sensorial contribution, postharvest, fruit quality.
Resumen. La guanábana (Annona muricata L.) es una fruta tropical exótica que se cultiva comercialmente en Colombia. Su condición altamente perecedera justifica los estudios de manejo en poscosecha. Por esta razón, la evaluación de la maduración se hizo en primer lugar, por un sistema de medición de compuestos volátiles conocido como nariz electrónica (NE) y por otro lado mediante cromatografía de gases acoplada a espectrometría de masas mediante microextracción en fase sólida del espacio de cabeza (CG-EM/MEFS). El estudio del perfil de sustancias volátiles en las frutas es uno de los principales indicadores de los atributos sensoriales que tipifica la calidad organoléptica de los vegetales. La NE se constituye en una alternativa rápida, novedosa, económica y relativamente simple para determinar grupos de sustancias volátiles en frutos de interés comercial, bien sea enteros o en fracciones. En contraste, el uso de la CG-EM/MEFS puede verse limitado por su alto costo, no obstante ser una técnica altamente selectiva. Con base en la evaluación de la pulpa realizada con NE fue posible clasificar el estado de madurez de las frutas así: inmaduro, madurez intermedia, maduro y sobremaduro, siendo los sensores de mayor impacto el 2 (reactivo con óxidos de nitrógeno), el 6 (sensible al metano) y el 8 (sensible a alcoholes y compuestos parcialmente aromáticos). Por CG-EM/MEFS, se logró establecer que durante la etapa de poscosecha, la mayor proporción de compuestos volátiles pertenece al grupo de los ésteres, predominando el Hexanoato de metilo. De manera particular en frutas sobremaduras, la presencia de compuestos alcohólicos, coincide con la evaluación hecha con la NE, la cual mostró sensibilidad a alcoholes y compuestos aromáticos de amplio rango para las frutas evaluadas. El estudio realizado aporta a la caracterización en poscosecha de los volátiles, uno de los principales atributos sensoriales en las frutas tropicales.
Palabras clave: Aroma, contribución sensorial, poscosecha, calidad de la fruta.
Fruits and vegetables are important components of a healthy diet. Their sufficient daily consumption can help prevent major disorders such as cardiovascular diseases and certain cancers. While the World Health Organization recommends a minimum intake of 120 kg / person / year (OMS, 2008), some European countries like France, Germany and Belgium meet this recommendation adequately, whereas the U.S. consumption is about 90 kg / person / year and in Colombia this figure is only about 40 kg / person / year (Langreo, 2002; Espinal et al., 2005; Jacaboy and Keller, 2006). Annonaceae fruits are among the most desirable ones in the world due to their sweetness, creamy flesh and excellent flavor when ripe. Of the nearly 100 species, five are the most commercially important ones, including soursop (Annona muricata L.) (Pareek et al., 2011). This species is grown in the Caribbean, mainly in Bermuda, Bahamas, Cuba, the Dominican Republic, St. Vincent and the Grenadines, and Puerto Rico; in Central America (southern Mexico and Costa Rica); in South America (Colombia, Brazil, Ecuador and Venezuela); and elsewhere in southeastern China, Vietnam, Australia, New Zealand, West Africa, the Pacific Islands and, in general, in the equatorial belt (Rami et al., 1995; Orellana and Martínez, 2002; Cuadros, 2008).
Fruit ripening is a complex, genetically controlled process of synthesis and degradation of plant compounds (Giovannoni, 2004; Vrebalov et al., 2009, Klee and Giovannoni, 2011). Many of the changes that occur during ripening and postharvest directly affect the savor, shelf life, nutritional quality and sensorial attributes of fruits (Wu et al., 2005; Yashoda et al., 2005; Ornelas et al., 2008). Among the latter, aroma is a determining feature of fruit taste, resulting from the synthesis of a series of low molecular weight compounds of varying concentration and quantitative importance that are volatile at room temperature (Kader, 2008; Dávila-Aviña et al., 2011). Recent evidence on different types of fruits indicates that more than 600 compounds make up an aroma (Pereira et al., 2005), but only a very limited number of them (commonly known as impact compounds) is responsible for the characteristic odor of fruits and other foods (Gutiérrez et al., 2010).
A fruit's aroma is the result of a particularly varied and complex mixture of volatile compounds. The composition of this mixture is species specific, sometimes indicating the variety, cultivar and site of origin or ripening stage of the fruit (Cantillo et al., 2011; El Hadi et al., 2013). Different proportions of volatiles, as well as the presence or absence of other biomolecules, determine the aromatic properties of a fruit (Ayala-Zavalla et al., 2004). For its part, flavor arises from the interaction between organic acids, sugars and volatiles (Baldwin et al., 2008). Playing an important role in consumer satisfaction, this attribute certainly exerts further influence on fruit consumption (Pelayo et al., 2003).
The climacteric ripening of the Annonaceae was discovered by Biale and Barcus (1970). It is characterized by an increase in respiratory activity promoted by high ethylene production (Taiz and Zeiger, 2006), a process that is, in turn, highly dependent on ATP availability and, therefore, associated to fruit respiration (El Hadi et al., 2013). Many of the parameters of interest in fruit ripening such as pulp softening, color changes and volatile production (some of them responsible for aroma), and in general, the cascade of events featuring the postharvest period, are subject to the production and action of ethylene (Génard and Gouble, 2005; Song et al., 2008; Mattheis, 2013).
The determination of volatile plant compounds can be done by chromatographic techniques or by the "electronic nose" (EN) technique, an instrument that enables the qualitative and / or quantitative assessment of volatile substances. This is a quick measurement equipment which can be used in a non-destructive way with the whole fruit. However, to facilitate and speed up the process, this device can be used with fractions of fruit pulp, seeds or skin tissues, among others. The EN allows differentiating and recognizing volatile compounds with the aid of sensors, thus operating as an artificial smelling instrument (Rodríguez et al., 2009).
The EN technique has been used in combination with gas chromatography coupled to mass spectrometry to determine the evolution of volatile compounds in several fruits like "Jonagold" apple (Malus sylvestris subsp. mitis Wallr) stored for 15 days under different conditions (Saevels et al., 2004); pear (Pyrus communis L.), in a study conducted to classify four cultivars through their volatile content during postharvest, using principal component analysis and linear discriminant analysis (Benedetti et al., 2008); mango (Mangifera indica L.), where volatile compounds were analyzed during postharvest (Lebrun, 2008); and tangerine cv. China (Citrus reticulata Blanco), assessed for volatiles in fruits harvested at different times (Hernández, 2006). Also, this instrument has been used in apple and pear as nondestructive analysis technique to evaluate postharvest quality and classify the fruits according to their degree of maturity in unripe, ripe and overripe (Brezmes, 2000).
The objective of this research was to determine the volatile groups present in the fruit pulp of soursop cv. Elita during ripening (unripe, half ripe, ripe and overripe stages), using volatiles measurement instrumental techniques, namely portable EN (E-nose AIRSENSE PEN3®) and gas chromatography coupled to mass spectroscopy, applying headspace solid phase microextraction (HS-SPME/GC-MS).
MATERIALS AND METHODS
Plant material. Elita soursop fruits obtained from orchards established in the agribusiness area of the Department of Valle del Cauca (Colombia) were employed for the trial. The experimental site is located in the rural area of the municipality of Pradera, Agrícola Varahonda (1,070 m.a.s.l.; average temperature of 23 °C; 1,225 mm of annual precipitation; average solar radiation of 4.8 Wm-2day-1; and average relative humidity of 83%). The fruits were harvested during the main production peak, which corresponds to the month of March. The fruits were all collected at the same ripening stage, 16 weeks after floral bud formation. Then, they were transported in styrofoam containers to Universidad Nacional de Colombia, Bogota campus, where they were stored at 20 °C and 65% RH in the Food Quality Control Laboratory of the Food Science and Technology Institute (Instituto de Ciencia y Tecnología de Alimentos - ICTA).
Determination of volatile compounds by EN. Volatiles were analyzed at the following ripening stages: unripe, half ripe, ripe and overripe, corresponding to postharvest days 1, 3, 6 and 9, respectively. For all evaluations, the experimental unit (EU) was one fruit, with six replications per ripening stage.
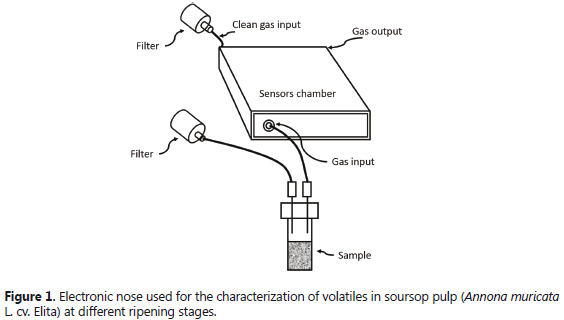
The EN equipment was a portable AIRSENSE PEN3® (Win Muster AIRSENSE Analytics Inc.; Schwerin, Germany). It possesses a small (1.8 cm3 volume) inner chamber containing 10 metal oxide semiconductor sensors that recognize gas molecular patterns and emit corresponding signals, which are received by a computer system and processed by a Win Muster 1.6.2 software package. Figure 1 presents an overview of this equipment.
Table 1 shows the 10 sensors used in the EN, their names and position numbers within the chamber and the general description of the gases they detect (Benedetti et al., 2008).
Sensor response is expressed in ohms as a resistivity resulting from changes in conductivity occurred by absorption of the volatile molecules in their gas phase. Having a detection limit of 1 ppm, the sensors are heated to 400 °C by a resistor that burns and transforms the volatiles into CO2 and water, which are evacuated.
Four grams of fruit pulp were extracted from each EU, then homogenized and placed in a 40 mL vial. Headspace conditioning time was 20 min; gas flow was 200 mL min-1; running time for each sample determination was 100 s; and sensor cleaning air flow time was 10 min. A septum inserted with two 20 gauge needles was hermetically installed on top of the vial. One of these needles led to an activated carbon filter air cleaning system. The other one took the gas with the volatiles to a Teflon duct (3 mm internal diameter) which ran from the headspace to the sensors chamber of the EN. The gas with the volatiles produced by the fruit pulp was sucked by a pump and conducted from the headspace to the sensors chamber. The dilution of the gases was carried out by a second pump which introduced clean air into the chamber. This clean air was also used to purge the system and take it back to baseline conditions for a new sample determination.
At each measurement, the signals from all the sensors were stored, plotted and statistically analyzed by principal component (PCA), linear discriminant (LDA) and Biplot analyses. PCA is a descriptive dimensionality reduction technique that facilitates assessing interdependence between groups of variables by representing information on a smaller dimensional space (Vivanco, 1999; Rencher, 2002; Brezmes, 2006; Bern, 2010). LDA allows building linear discriminant functions based on which the maximum possible separation between maturity stages is obtained (Bern, 2010). Biplot analysis approximates the distribution of a multivariate sample in a reduced dimensional space (usually a plane) on which it overlaps representations of the variables (Santos et al., 1991; Gower and Hand, 1996). Two software packages, namely Win Muster 1.6.2 (AIRSENSE Analytics GmbH 2006) and Matlab 7.0 were used for data analysis.
Determination of volatile compounds by HS-SPME/GC-MS. Volatile compounds were analyzed in three fruits per each of the target ripening stages (unripe, half ripe, ripe and overripe). Thirty grams of seedless fruit pulp were extracted from each fruit and homogenized for 5 min, to be deposited into a 110 mL vial which was sealed and allowed an equilibrium time of 20 min at 20 °C. Then, for the solid phase microextraction, a 30 mm fiber of the DVB/CAR/PDMS type was introduced into the headspace and left immersed in the vial for 30 min at 20 °C without agitation, so as to extract the volatiles. This fiber was then removed and immediately placed in the injection port of a GC-MS-QP5050 Shimadzu® gas chromatograph with a desorption time of 5 min. The experiment was performed thrice for each ripening stage, using a different fruit in each case, for a total of 12 EU. The operating conditions of the chromatograph were 250 °C for the injection port and the interface; initial oven temperature of 50 °C; heating rate of 4 °C min-1 up to 300 °C; RTX-5 chromatographic column (30 m x 0.32 mm x 0.25 mm); helium at 1.5 mL min-1 flow as carrier gas; and 30 min run time. HS-SPME and GC-MS conditions had been previously standardized by Vela et al. (2005).
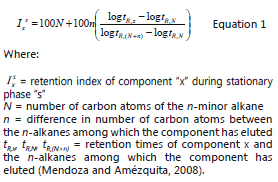
The identification of volatiles was done by comparing the mass spectrum data of the sampled compounds with the information of the CLASS-5000 library and by the Kovats Index (KI) as calculated through equation 1.
RESULTS AND DISCUSSION
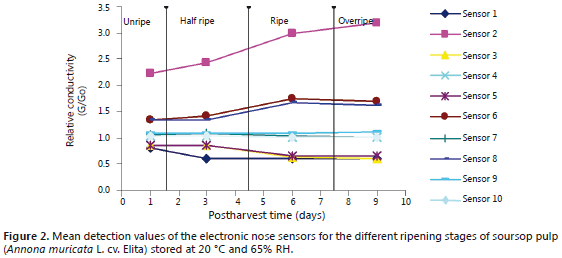
Determination of volatile compounds by EN. The EN response to the volatile compounds in the pulp of unripe soursop fruit was characterized by its resemblance to that obtained with the reference substances nitrogen oxide (NO2 - sensor 2-W5S), methane (CH4) and a wide range of organic substances (sensor 6-W1S), and alcohols and partially aromatic compounds (sensor 8-W2S).
The response of the sensors to the half ripe fruit volatiles showed slightly increased levels with respect to those found for the immature fruit pulp. The response to CH4 and the wide range organic substances of Sensor 6-W1S was very similar to that observed in immature fruit pulp.
In the case of the ripe fruits, there was an increase in the detection of NO2 by sensor 2-W5S; CH4 by sensor 6-W1S; and alcohols and partly aromatic compounds, by sensor 8-W2S. The other sensors showed no change in behavior. The observed increase may be related to the respiration shift that is proper of the climacteric nature of soursop (Pareek et al., 2011).
In turn, the overripe fruits (as compared to the ripe ones) were featured by even higher detected levels of NO2, CH4, and alcohols and partially aromatic compounds. These results are certainly useful to classify the ripening stages of this fruit (Gómez et al., 2007).
Figure 2 presents the responses generated by the ten electronic nose sensors.
Figure 2 shows how the impact of fruit ripeness on pulp olfactory trace is detected by some of the EN sensors (those with the most pronounced slope). The chemical groups associated to such sensors were also detected by Cheong et al. (2010), who identified 35 volatile compounds represented by esters, alcohols, terpenes, acids, aromatic ketones and an aldehyde. Although the reports on soursop pulp volatiles reveal chemical group similarities, this does not necessarily cover the specific volatile compounds themselves. In this regard, some authors suggest that the lack of agreement in the results on the specific volatile composition of these groups may be due to evaluation methodology contrasts and to differences among cultivars and regions of origin (Franco and Janzantti, 2005). However, according to Gómez et al. (2007) and Hernández et al. (2007), EN data can be used to establish a plant material's origin, facilitate taxonomic identification and determine postharvest (ripening) stages. Furthermore, it should be noted that volatile component contributions to a plant's aroma are not necessarily related to the quantitative expression of these products (Mahattanatawee et al., 2005).
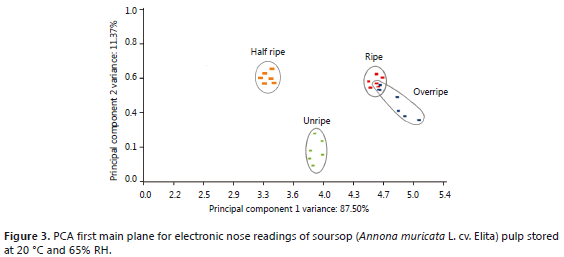
Principal component analysis (PCA). After subjecting the results of the 10 EN sensors (as obtained across ripening stages) to PCA, it can be observed how the first two principal components collect 98.87% of total variability. In Figure 3, the average results of the sampling dates are displayed on the main plane, where colors identify the ripening stage.
Figure 3 shows a good level of separation between almost all ripening stages, except for ripe and overripe fruits, indicating that in terms of volatile compounds, these two would be the least differentiated groups.
In addition to the main separation between groups given on the first dimension, the unripe stage is separated from the other groups on the second dimension, suggesting that this fruit condition has a clearly distinct volatile composition.
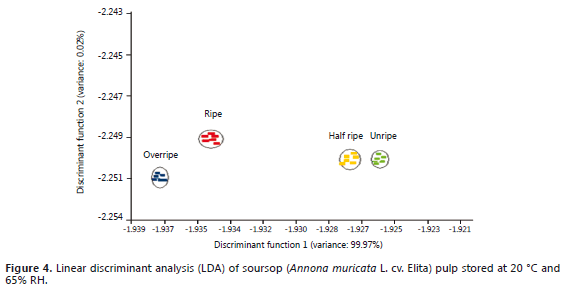
Linear discriminant analysis (LDA). Figure 4 illustrates the positioning of the ripening stage sample data on the plotting of the two first discriminant functions.
Figure 4 shows that the first linear discriminant function is enough to separate the samples according to the four predefined ripening stages. The second function could be used to discriminate between ripe and overripe stages, thus confirming the statement by Cantillo et al. (2011) in the sense that the formation of volatile compounds during ripening is characterized by a dynamic process wherein the concentration of the constituents changes both qualitatively and quantitatively. Moreover, the usefulness of the EN appears as a rapid instrumental alternative to evaluate quality attributes such as volatiles, some of them responsible for aroma in whole or processed fruits, which can be advantageous to define storage conditions (Quicazán et al., 2011).
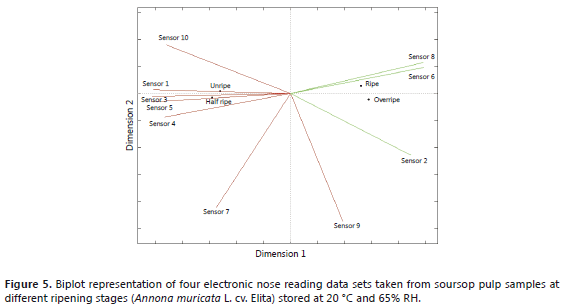
Biplot Analysis. Figure 5 shows the first principal plane with the different soursop ripening stages represented by their corresponding EN sensor readings.
Figure 5 shows that the ripening stages are distributed along the first dimension, with the early stages (half ripe and unripe) on the left, and the final states (ripe and overripe) on the right. The sensors that contribute the most to that dimension are 2-W5S, 8-W2S, 1-W1C, 3-W3C, 5-W5C and 4-W6S.
Among the active sensors we can count those sensitive to NO2 (sensor 2 - W5S), methane and a wide range of organic substances (sensor 6 - W1S), and partially aromatic substances (sensor 8 - W2S), all of which exhibit a strong negative correlation with the group of aromatic compound detecting sensors (sensor 1-W1C and sensor 3-W3C), together with those sensitive to hydrogenated compounds (sensor 4 - W6S) and alkanes and lower polarity aromatic substances (sensor 5 - W5C).
The major contributions on the second dimension are due to the sensors detecting sulfur compounds, terpenes and some organic sulfur substances (sensor 7-W1W); and aromatic and organic sulfur compounds (sensor 9-W2W); none of which has been observed to have a considerable impact on the analysis of volatiles.
From the above, it can be deduced that the early ripening stages (unripe and half ripe) are characterized by elevated aromatic, hydrogenated and lower polarity aromatic compound contents. As aging progresses, these substances decrease, to be replaced by methane and a range of organic and partially aromatic compounds.
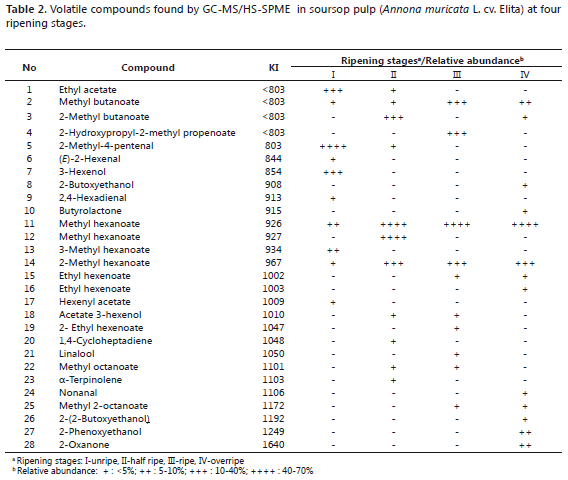
Determination of volatiles by HS-SPME/GC-MS. The HS-SPME/GC-MS results of the volatile compound separation analysis applied to soursop pulp samples at four different ripening stages are shown in Table 2. The Kovats Index (KI) was calculated from the application of equation 1, and the relative abundance was found as a function of gas chromatography percentage areas.
The compounds detected through the different studied ripeness stages (pooled data) correspond to esters, aldehydes, alcohols, terpenes and ketones, esters being the most abundant ones and showing an increasing trend along postharvest time, except for the overripe stage. The unripe fruit pulp contained 60% esters, predominantly Ethyl acetate; 30% aldehydes, mostly 2-Methyl-4-pentenal; and 10% alcohols, mainly 3-Hexenol.
The half ripe fruit pulp contained 70% esters (predominantly Ethyl hexenoate, Methyl hexanoate and 2-Methyl butanoate) and 10% aldehydes, alkenes and terpenes (mostly 2-Methyl-4-pentenal, 1,4-Cycloheptadiene and a-Terpinolene).
The highest amount of esters was found in the ripe fruit pulp (90%), mainly corresponding to Methyl hexanoate and 10% Linalool. The overripe fruit pulp showed a general decrease in ester content, which went down to 62%. However, within this group there was an increment in the relative abundance of Methyl hexanoate. In turn, alcohol levels rose to 23%, dominated by 2-phenoxyethanol; whereas aldehyde content (majorly Nonanal) was 8%. Belonging to the group of ketones, a new compound (2-oxanone) appeared at this ripening stage.
The ripe fruit pulp composition coincided with a previous report on other soursop cultivars by MacLeod and Pieris (1981), who found that 80% of the volatiles corresponded to esters, out of which 31% was Methyl hexanoate and 27% was 2-Methyl hexanoate. In turn, Vela et al. (2005) reported 58.6% of esters in the volatile composition of fresh soursop fruit, the most representative compounds being Methyl butanoate, (E) 2-Methyl butanoate and Methyl hexanoate.
Besides the mentioned esters, this same fruit has been found to contain Ethyl acetate, 3-methyl hexanoate, 2-methyl octanoate and Linalool (Chong et al., 2011; Lemos et al., 2011). In connection with the volatile compounds which make up the aroma of exotic fruits, Lasekan and Abbas (2012) state that esters and terpenes are the most abundant components in crops like durian (Durio zibethinus L.), mangosteen (Garcinia mangostana L.), rambutan (Nephelium lappaceum L.), langsat (Lansium domesticum Corr.) and sapodilla (Manilkara zapota van Royer) (Laohakunjit et al., 2007).
Ripening determines the emergence of new compounds and changes in the concentrations of the existing ones. This situation can be used as a physiological indicator of the ripening of soursop and other fruits of commercial interest (Sampaio and Nogueira, 2006; Nunes et al., 2008).
At ripeness, the pulp presents alcoholic compounds, mainly butoxyethanol, 2 - (2-butoxyethanol) and 2-phenoxyethanol, probably indicating the beginning of the fermentative stage. This coincides with the EN results, which detected alcohols and a range of aromatic compounds (sensor 8-W2S) in the overripe fruit pulp, facilitating in this way plant material identification through its olfactory trace during postharvest. In general, some esters can serve as precursors, thus participating in the formation of alcohols, which explains the decrease of these compounds at this stage (Villatoro et al., 2008).
For the other ripening stages no relation was found between the compounds detected by the EN and the evaluation of volatile compounds by HS-SPME/GC-MS, which corroborates the statement of Correa et al. (2004), who studied the aroma of apple, emphasizing the difficulty to find any correlation between EN and HS-SPME/GC-MS techniques. This sets a contrast with previous research by Lebrun et al. (2008), who conducted a study on the volatiles produced during mango fruit ripening and observed high coincidence between the results obtained with both methods.
CONCLUSIONS
The analysis of volatiles by EN allowed classifying the studied soursop fruit groups according to their ripening stages (unripe, half ripe, ripe and overripe), the most active sensors being 2-W5S (sensitive to nitrogen oxides), 6 - W1S (sensitive to methane) and 8 - W2S (sensitive to alcohols and partially aromatic compounds).
The EN data were processed through principal component analysis (PCA) and linear discriminant analysis (LDA), which allowed separating the different stages of maturity for the postharvest period of soursop cv. Elita.
The Biplot representation analysis allowed the visualization of the main changes in volatile components taking place during the ripening process. The unripe and half ripe stages are characterized by elevated levels of aromatic, hydrogenated and lower polarity aromatic compounds. As aging progresses, these substances decrease, to be replaced by methane, partially aromatic compounds and a range of organic products.
Out of the observed volatiles, the major group corresponded to esters, predominantly Methyl hexanoate. Likewise, changes in the relative abundance of volatiles were accompanied by the disappearance of certain compounds and the appearance of some new ones. The current EN assessment detected alcohols and a wide range of aromatic compounds in overripe fruits.
ACKNOWLEDGEMENTS
We thanks the Direction of Research in the Universidad Nacional de Colombia, Medellín campus (DIME) for their finantial assistance under the QUIPU project 20201006039, and the Laboratories of Food Quality Control and Chemistry of Aromas in the Universidad Nacional de Colombia, Bogotá campus by the EN and GC equipments. Also the autors wish to thank Engineer Fernando Arenas Gil for his valuable assistance in the lab.
BIBLIOGRAPHY
Ayala-Zavala, J.F., S.Y. Wang, C.Y. Wang and G.A. Gonzalez-Aguilar. 2004. Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit. LWT 37(7): 687-695. [ Links ]
Baldwin, E.A., K. Goodner and A. Plotto. 2008. Interaction of volatiles, sugars and acids on perception of tomato aroma and flavor descriptors. Journal of Food Science 73(6): S294-S307. [ Links ]
Benedetti, S., S. Buratti, A. Spinardi, S. Mannino and I. Mignani. 2008. Electronic nose as a non-destructive tool to characterize peach cultivars and to monitor their ripening stage during shelf-life. Postharvest Biology and Technology 47(2): 181-188. [ Links ]
Berna, A. 2010. Metal oxide sensors for electronic noses and their application to food analysis. Sensors 10(4): 3882-3910. [ Links ]
Biale, J.B. and D.E. Barcus. 1970. Respiratory patterns in tropical fruits of Amazon Basin. Tropical Science 12: 93-104. [ Links ]
Brezmes, J. 2006. Diseño de una nariz electrónica para la determinación no destructiva del grado de maduración de las frutas. Dissertation (Science Ph.D.). Universidad Politécnica de Catalunya. Departament de Teoria del Señyal i Comunicacions. 195 p. [ Links ]
Brezmes, J. 2000. Fruit ripeness monitoring using an electronic nose. Sensors and Actuators 69(3): 223-229. [ Links ]
Cantillo, J., D. Sinuco, M. Solarte y L. Melgarejo. 2011. Estudio comparativo de los compuestos volátiles de tres variedades de guayaba (Psidium guajava L.) durante la maduración. Revista Colombiana de Química 40(1): 79-90. [ Links ]
Cheong, K.W., C.P. Tan, H. Mirhosseini, N.S.A. Hamid, A. Osman and M. Basri. 2010. Equilibrium headspace analysis of volatile flavor compounds extracted from soursop (Annona muricata) using solid-phase microextarction. Food Research International 43(5): 1267-1276. [ Links ]
Cheong, K.W., C.P. Tan, H. Mirhosseini, C.T. Chin, Y.B. Che Man, N S.A. Hamid, A. Osman and M. Basri. 2011. Optimization of equilibrium headspace analysis of volatile flavor compounds of Malaysian soursop (Annona muricata): comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry (CGxGC-TOFMS). Food Chemistry 125(4): 1481-1489. [ Links ]
Correa, E.C., P. Barreiro, M. Ruiz, M.L. López y G. Echevarria. 2004. Aplicación de una nariz electrónica para la medida de aromas emitidos por manzanas cv. Fuji conservadas con diferentes tecnologías. Fruticultura Profesional 29(141): 5-12. [ Links ]
Cuadros, V. 2008. Guanábana: Manejo del Cultivo y Poscosecha. Proexant, Quito, Ecuador. 25 p. [ Links ]
Dávila-Aviña, J.E., G.A. González, J. Ayala-Zavala, D.R. Sepúlveda y G. Olivas. 2011. Compuestos volátiles responsables del sabor del tomate. Revista Fitotecnia Mexicana 34(2): 133-143. [ Links ]
Espinal, C.F., J. Martínez y Y. Peña. 2005. La cadena de los frutales de exportación en Colombia. Ministerio de Agricultura y Desarrollo Rural, Observatorio Agrocadenas, Bogotá. 68 p. [ Links ]
El Hadi, A.M., F. Zang, F. Wu, C.H. Zhou and J. Tao. 2013. Advances in fruit aroma volatile research. Molecules. 18(7): 8200-8229. [ Links ]
Franco, M.R. and N.S. Janzantti. 2005. Aroma of minor tropical fruits. Flavour and Fragance Journal 20(4): 358-371. [ Links ]
Génard, M. and B. Gouble. 2005. ETHY. A theory of fruit ethylene emission. Plant Physiology 139(1): 531-545. [ Links ]
Giovannoni, J.J. 2004. Genetic regulation of fruit development and ripening. Plant Cell 16 Suppl. 1: S170-S180. [ Links ]
Gómez, H.A., G.A. Pereira and J. Wang. 2007. Using electronic nose technique to monitoring tomatoes maturity states during shelf live. Revista Ciencias Técnicas Agropecuarias 16(1): 24-30. [ Links ]
Gower, J.C. and D.J. Hand. 1996. Biplots. Chapman and Hall, London. 277 p. [ Links ]
Gutiérrez, D., C. Sinuco y C. Osorio. 2010. Caracterización de los compuestos volátiles activos olfativamente en uchuva (Physalis peruviana L.). Revista Colombiana de Química 39(3): 389-399. [ Links ]
Hernández, G.A. 2006. Electronic nose technique potential monitoring mandarin maturity. Sensors and Actuators 113(1): 347-353. [ Links ]
Hernández, A., J. Wang, G. Hu and A. García. 2007. Discrimination of storage shelf-life for mandarin by electronic nose technique. LWT - Food Science and Technology 40(4): 681-689. [ Links ]
Jacoby, E. y I. Keller. 2006. La promoción del consumo de frutas y verduras en América Latina: Buena oportunidad de acción intersectorial por una alimentación saludable. Revista Chilena de Nutrición 33 Suppl. 1: 226:231. [ Links ]
Kader, A.A. 2008. Flavor quality of fruits and vegetables. Journal of the Science of Food and Agriculture 88(11): 1863-1868. [ Links ]
Klee, H.J. and J. Giovannoni. 2011. Genetics and control of tomato fruit ripening and quality attributes. Annual Review of Genetics 45: 41-59. [ Links ]
Langreo, A. 2002. Consumo de frutas y hortalizas en la Unión Europea: estrategias agroalimentarias. Distribución y Consumo 12(63): 24-35. [ Links ]
Laohakunjit, N., O. Kerdchoechuen, F.B. Matta, J.L. Silva and W.E. Holmes. 2007. Postharvest survey of volatile compounds in five tropical fruits using headspace-solid phase microextraction (HS SPME). HortScience 42(2): 309-314. [ Links ]
Lasekan, O. and K.A. Abbas. 2012. Distinctive exotic flavor and aroma compounds of some exotic tropical fruits and berries: a review. Critical Reviews in Food Science and Nutrition 52(8): 726-735. [ Links ]
Lebrun, M. 2008. Discrimination of mango fruit maturity by volatiles using the electronic nose and gas chromatography. Postharvest Biology and Technology 48(1): 122-131. [ Links ]
Lemos, J., G. Molina, A.P. Dionísio, F.F. Cavalcante, R. Wagner, M.R. Maróstica Jr. and G.M. Pastore. 2011. Volatile constituents of exotic fruits from Brazil. Food Research International 44(7): 1843-1855. [ Links ]
Macleod, J.A. and M.N. Pieris. 1981. Volatile flavor components of soursop (Annona muricata). Journal of Agricultural Food Chemistry 29(3): 488-490. [ Links ]
Mahattanatawee, K., K.L. Goodner and E.A. Baldwin. 2005. Volatile constituents and character impact compounds of selected Florida's tropical fruit. Proceedings of the Florida State Horticultural Society 118: 414-418. [ Links ]
Mattheis, J. 2013. Apple fruit volatiles: Impact of fruit development, ethylene, and storage environment. Horticulture Seminars. Cornell University. [ Links ]
Mendoza, D. and F. Amézquita. 2008. Taller básico de cromatografía de gases. Facultad de Química. Universidad de Guanajuato, Guanjuato, México. 50 p. [ Links ]
Nunes, C., C. Santos, G. Pinto, J.A. Lopes-da-Silva, J.A. Saraiva, and M.A. Coimbra. 2008. Effect of candying on microstructure and texture of plums (Prunus domestica L.). LWT - Food Science and Technology 41 (10): 1776-1783. [ Links ]
Orellana, A.D. y E. Martínez. 2002. Distribución geográfica de anonáceas en Guatemala. ICTA- FAUSAC, Guatemala. 22 p. [ Links ]
OMS. 2008. Organización Mundial de la Salud. Alimentos saludables. Serie de Informes Técnicos. OMS/FAO, Ginebra. 20 p. [ Links ]
Ornelas-Paz, J.J., E.M. Yahia and A.A. Gardea, 2008. Changes in external and internal color during postharvest ripening of "Manila" and "Ataulfo" mango fruit and relationship with carotenoid content determined by liquid chromatography and mass spectrometry. Postharvest Biology and Technology 50(2-3): 145-152. [ Links ]
Pareek, S., E.M. Yahia, O.P. Pareek and R.A. Kaushik. 2011. Postharvest physiology and technology of Annona fruits. Food Research International 44(7): 1741-1751. [ Links ]
Pelayo, C., S.E. Ebeler and A.A. Kader. 2003. Postharvest life and flavor quality of three strawberry cultivars kept at 5°C in air or air + 20kPa CO2. Postharvest Biology and Technology 27: 171-183. [ Links ]
Pereira, C.C., J.R. Rufino, A.C. Habert, R. Nobrega, L.M. Cabral and C.P. Borges. 2005. Aroma compound recovery of tropical fruit juice by pervaporation: membrane material selection and process evaluation. Journal of Food Engineering 66(1): 77-78. [ Links ]
Quicazán, M.C., A.C. Díaz y C. Zuluaga. 2011. La nariz electrónica, una novedosa herramienta para el control de procesos y calidad de la industria agroalimentaria. Revisión. Vitae 18(2): 209-217. [ Links ]
Rasai, S., A.P. George and A.S. Kantharajah 1995. Tissue culture of Annona spp. (cherimoya, atemoya, sugar apple and soursop). A review. Scientia Horticulturae 62 (1-2): 1-14. [ Links ]
Rencher, A. 2002. Methods of Multivariate Analysis. Second edition. John Wiley and Sons Inc., New York. 715 p. [ Links ]
Rodríguez, D., A. Lamagna y A. Boselli. 2009. Método para la determinación de intensidad de olores y nariz electrónica. Comisión Nacional de Energía Atómica, Buenos Aires. 35 p. [ Links ]
Santos, C., S.S. Muñoz, Y. Gutiérrez, E. Hebrero, J.L. Vicente, P. Galindo and J.C. Rivas. 1991. Characterization of young red wines by application of HJ biplot analysis to anthocyanin profiles. Journal of Agricultural and Food Chemistry 39(6): 1086-1090. [ Links ]
Saevels, S. 2004. An electronic nose and a mass spectrometry-based electronic nose for assessing apple quality during shelf life. Postharvest Biology and Technology 31(1): 9-19. [ Links ]
Sampaio, S.T. and L.P. Nogueira. 2006. Volatile components of mangaba fruit (Hancornia speciosa G.) at three stages of maturity. Food Chemistry 95(4): 606-610. [ Links ]
Song, J. and C.F. Forney. 2008. Flavour volatile production and regulation in fruit. Canadian Journal of Plant Science 88: 537-550. [ Links ]
Taiz, L. and E. Zeiger. 2006. Plant physiology. Fourth edition. Sinauer Assocites. Sunderland, MA, USA. 764 p. [ Links ]
Vela, E.A., A.L. Morales y C. Duque. 2005. Aporte al estudio del aroma libre y creación de un aroma artificial de guanábana (Annona muricata). Undergraduate thesis (Chemical engineer). School of Sciences. Department of Chemistry. Universidad Nacional de Colombia, Bogota, D.C. 82 p. [ Links ]
Villatoro, R.C., G. Altisent, J. Echeverría, M.L. Graell and I.L. López. 2008. Changes in biosynthesis of aroma volatile compounds during on tree maturation of "Pink Lady"® apples. Postharvest Biology and Technology 47(3): 286-295. [ Links ]
Vivanco, M. 1999. Análisis Estadístico Multivariable. Colección Textos Universitarios. Editorial Universitaria. Santiago de Chile. 234 p. [ Links ]
Vrebalov, J., I.L. Pan, A.J. Matas, R. McQuinn, M.Y. Chung, M. Poole, J. Rose, G. Seymour, S. Grandillo, J. Giovannoni and V.F. Irish. 2009. Fleshy fruit expansion and ripening are regulated by the tomato Shatterproof gene TAGL1. The Plant Cell 21(10): 3041-3062. [ Links ]
Wu, B.H., B. Quilot, M. Génard, J. Kervella and S.H. Li. 2005. Changes in sugar and organic acid concentrations during fruit maturation in peaches, (Prunus davidiana) and hybrids as analyzed by principal component analysis. Scientia Horticulturae 103(4): 429-439. [ Links ]
Yashoda, H.M., T.N. Prabha and R.N. Rudrapatnam. 2005. Mango ripening chemical and structural characterization of pectic and hemicellulosic polysaccharides. Carbohydrate Research 340(87): 1335-1342. [ Links ]