Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad Nacional de Agronomía Medellín
Print version ISSN 0304-2847
Rev. Fac. Nac. Agron. Medellín vol.67 no.1 Medellín Jan./June 2014
https://doi.org/10.15446/rfnam.v67n1.42649
http://dx.doi.org/10.15446/rfnam.v67n1.42649
Evaluation of the Antioxidant Properties and Aromatic Profile During Maturation of The Blackberry (Rubus glaucus Benth) and The Bilberry (Vaccinium meridionale Swartz)
Evaluación de las Propiedades Antioxidantes y el Perfil Aromático Durante la Maduración de Mora (Rubus glaucus Benth) y Agraz (Vaccinium meridionale Swartz)
Luisa Juana Bernal1; Laura Angélica Melo2 and Consuelo Díaz Moreno3
1 Master Student. Universidad Nacional de Colombia - Sede Medellín - Facultad de Ciencias Agrarias - Departamento Ciencias Agronómicas. A.A. 1779, Medellín, Colombia. <ljbernalr@unal.edu.co>
2 Chemical Engineering Student. Universidad Nacional de Colombia - Sede Bogotá - Facultad de Ingeniería - Departamento Ingeniería Química. Carrera 30 No. 45-03, Bogotá, Colombia. <lamelot@unal.edu.co>
3 Associate Professor. Universidad Nacional de Colombia - Sede Bogotá - Instituto de Ciencia y Tecnología de Alimentos ICTA. Carrera 30 No. 45-03, Bogotá, Colombia. <acdiazmo@unal.edu.co>
Received: October 25, 2012; accepted: August 29, 2013.
Abstract. The blackberry (Rubus glaucus Benth) and the bilberry (Vaccinium meridionale Swartz) are natural sources of antioxidants; they are known for their preventive role against degenerative diseases. In this study, the aromatic profile was evaluated using an electronic nose, including the antioxidant properties and the vitamin C, phenolic and anthocyanin contents during three stages of blackberry and bilberry ripening. A completely random statistical design was followed and the results presented differences in the aromatic profile: a higher anthocyanin content (1.59 mg of cyn-3-glu g-1 in the bilberry and 0.26 mg of cyn-3-glu g-1 in the blackberry) and total phenols (5.57 mg of caffeic acid g-1 bilberry and 2.68 mg caffeic acid g-1 blackberry). The behavior of the evaluated properties was independent in each of the fruits.
Keywords: Antioxidant activity, electronic nose, Folin-Ciocalteu, FRAP, TEAC.
Resumen. Los frutos como la mora (Rubus glaucus Benth) y el agraz (Vaccinium meridionale Swartz) son fuentes naturales de sustancias antioxidantes reconocidas por su papel preventivo en el desarrollo de enfermedades degenerativas. En este estudio se evaluó el perfil aromático por medio de nariz electrónica, las propiedades antioxidantes y el contenido de vitamina C, fenoles y antocianinas totales, durante tres estados de maduración de mora y agraz. El diseño estadístico que se siguió fue completamente aleatorio y los resultados muestran que las frutas en el último estado de madurez evaluado se diferencian por su perfil aromático, un contenido mayor de antocianinas (1,59 y 0,26 mg cyn-3-glu g-1 en agraz y mora, respectivamente) y fenoles totales (5,57 y 2,68 mg ácido caféico g-1 en agraz y mora, respectivamente). El comportamiento de las propiedades evaluadas es independiente en cada una de las frutas.
Palabras clave: Actividad antioxidante, nariz electrónica, Folin- Ciocalteu, FRAP, TEAC.
The blackberry (Rubus glaucus Benth) is a fruit composed of drupes and is characterized by its red-blue color and its aroma. It is a source of vitamins, minerals and phytochemicals. In Colombia, the most cultivated species is known as "mora de Castilla" (Rubus glaucus Benth); harvest is constant during the whole year and is only influenced by the rainy season. For cultivation, altitudes between 1,800 and 2,000 masl are preferred. The climacteric character of this fruit is unclear and, additionally, it is a product that is difficult to handle due to its high perishability and sensitivity to mechanical damage (Perkins-Veazie et al., 2000).
The bilberry (Vaccinium meridionale Swartz), also known as "mortiño", is a small shrub that has two harvests per year. The fruit is fleshy with numerous seeds, has a sour taste, and is characterized by a dark purple color when maturity is reached. It is found between 2,200 and 3,400 masl, with the Colombian regions of Boyacá and Antioquia being the areas where domestic production is focused (Ligarreto, 2011). It belongs to the same genus as blueberries and the climacteric fruits are harvested in the state of sensory maturity, which ensures the aroma and flavor characteristics that are desirable by consumers (Mitcham et al., 1998).
These red fruits are a natural source of antioxidants, with a predominant group of phenolic acids and flavonoids, the latter being responsible for the color (Pietta et al., 2003; Castrejón et al., 2008). Antioxidants retard oxidation reactions, key deterioration reactions that result from the relocation of the aromatic rings that compose them (Balasundram et al., 2006). In addition, recent studies have shown the importance of preventing degenerative diseases such as Alzheimer's and Parkinson (Wang and Stoner, 2008). It is a fact that the content of phenolic compounds in berries is affected by genetic differences, pre- and post-harvest conditions, and, also, the degree of maturity.
In fruits, ripening control is important at the time of establishing the point of harvest, storage conditions and the evolution of the sensory characteristics. During this process, the biochemical changes undergone by the fruits give way to the generation of volatiles and changes in color, texture and flavor. A nondestructive method that provides results in real time is the electronic nose, an instrument that emulates the human olfactory process and that is comprised of a system that is sensitive to volatiles and another that is in charge of converting responses into electrical signals. Applications in this area have been numerous: peaches (Benedetti et al., 2008), pears (Brezmes et al., 2000), and apples (Pathange et al., 2006) have been analyzed and distinguished in different stages of ripening, postharvest operations (Torri et al., 2010) and fermentations (Bhattacharyya et al., 2007). In fruits such as berries, the available information is limited (Peris and Escuder-Gilabert, 2009); however, there is a report from Li et al., (2010), who employed bilberries for decease detection.
In Colombia, the blackberry and bilberry have gained commercial positioning among consumers as a food and a source of functional substances. So far, no studies have been conducted to evaluate changes in antioxidant properties and the aromatic profile during the ripening stage. This study aimed to evaluate changes in the antioxidant capacity by using spectrophotometric techniques and in the aromatic profile by using an instrumental measure of aroma in three ripening stages (green, intermediate and harvesting).
MATERIALS AND METHODS
Samples. The blackberries were collected from a crop located in the village of El Triunfo, Icononzo-Tolima and the bilberries were collected from wild bushes in the village of Arrayanes, Tinjacá - Boyacá. Three stages of ripening were selected that were associated with a subjective color indicator: green (G), where the fruits had developed their size and the surfaces had a green color with small yellow and pink areas; intermediate (I), characterized by a pink color with small yellow drupes on the surface of the blackberries and a red surface in the bilberries; and harvesting (H), the latter corresponded to the state of commercial maturity where the blackberries had developed a deep red color and the bilberries a purple tone. For every physicochemical analysis, a representative sample was taken for use in the corresponding procedure.
Physical and chemical analyses. For analyses of fruit characterization, official methods were employed:
Moisture. 10 g of sample were dried at 105 ºC (ThermoHeraus™ Instruments Function Line Oven) for 4 hours. A.O.A.C. Official Method 934.06; A.O.A.C. Official Method 934.01. This measurement was performed in triplicate.
Total soluble solids (TSS). The content of ºBrix was determined by using a refractometer according to Colombian Technical Standard NTC 4624. This measurement was performed in triplicate.
pH and acidity. A potentiometric titration was performed (SCHOTT Handy labpH11) according to the method described in the Colombian Technical Standard NTC 440 and A.O.A.C. Official Method 942.15.
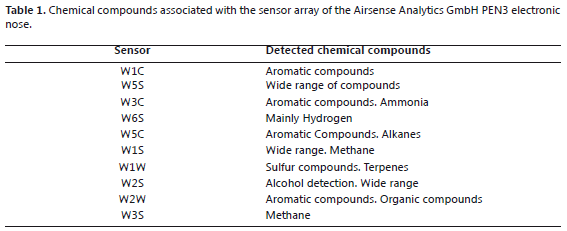
Aromatic profile determination. An Airsense Analytics™ GmbH PEN3 Electronic Nose was employed with 10 semi-conductor metal oxide sensors - MOS (Table 1). 25 g of sample were weighed and kept in a plastic chamber with a hermetic seal that measured 11.0 x 10.6 x 10.6 cm and a cover that measured 3.8 x 11.2 x 11.2 cm at room temperature (15 ºC approx.) for 5 min to stabilize the content of volatile compounds. The aromatic profile was evaluated in cycles of 150 s with an air chamber flow of 70 mL min-1 and a flow injection of 60 mL min-1. The sample analysis was performed randomly, leaving a time of 450 s for cleaning the sensors between each cycle of analysis in order to avoid the interference and undesirable effects on the responses resulting from the dragging of volatile residues from the previous sample (Zuluaga et al., 2011).
Vitamin C. The extraction was carried out with distilled water; before injection, the solution was purified with a C18 cleaning cartridge. For the detection and quantification, HPLC chromatography was employed (Jasco™ Pump PU980, Detector UV/VIS975) with a Phenomenex Rezex™ ROA-Organic Acid H+ 8% ion exchange column, at a flow of 0.5 mL/min and with a mobile phase of H2SO4 4 mM at room temperature. The employed wavelength was 254 nm. The data were reported as mmol of vitamin C g-1 of fresh fruit (Shui and Leong, 2002; Castañeda et al., 2009). 20 g of fruit were prepared and homogenized (Ultra-Turrax™ IKA T18 Basic), 20 mL of extraction solution were added and, then, vortex agitation and heating in a magnetic stirring plate were carried out. The mixture was centrifuged for 10 min (Tecnovetro™ 4235); this procedure was repeated twice with the solid. The extracts were brought to a volume of 100 mL by using the extraction solution. Each sample was performed in triplicate and stored at -18 °C until analysis.
Total anthocyanin content. The differential pH method was employed. A.O.A.C. Official Method 2005.02. Results were reported as mg of cyaniding-3-glycoside (cyn-3-glu) g-1 of fresh fruit.
Total phenolic content. The Folin-Ciocalteau reagent was used (Panreac, Spain). This is a method described by Vasco et al. (2008) and Oliveira et al. (2011). The wavelength at which the reading was taken was 765 nm and the quantification was done by reference to a calibration curve of caffeic acid, Sigma Aldrich, (R2 = 0.995). The data were reported as mg of caffeic acid g-1 of fresh fruit.
Antioxidant capacity. In TEAC, radical discoloration was read at 734 nm after a 6min reaction. Each extract was read in triplicate and the results were reported as mmol trolox g-1 of fresh fruit with respect to a trolox 97% calibration curve, Sigma Aldrich™, (R2 = 0.989) (Murcia et al., 2009).
In FRAP, 330 mL of extract were mixed with 10 mL of a solution made from buffer acetate pH 3.6 (300 nM), TPTZ diluted in HCl (40 mM) and FeCl3 (20 mM) at a 10:1:1 ratio, respectively. The reaction was held for an hour and the reading was taken at 593 nm. Each extract was read in duplicate and the results were reported according to the Trolox calibration curve (R2 = 0.996) in mmol trolox g-1 of fresh fruit (Gorinstein et al., 2009; Müller et al., 2010).
Statistical analysis. The data were analyzed using one-way analysis of variance ANOVA, in conjunction with a Tukey-Kramer's multiple comparison test, using an alpha of 0.05. In addition, the principal component analysis-PCA was performed as a multivariate technique for exploration of the data obtained with the electronic nose. The statistical analyses were carried out with the MATLAB software, v. 7.9 (MathWorks™).
RESULTS AND DISCUSSION
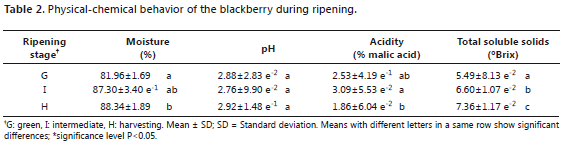
The "mora de Castilla" is a fruit whose maturity is determined by changes in color, size, percentage of acidity and soluble solids content, according to NTC 4106. For this fruit, seven stages of maturation can be differentiated. The ° Brix of the samples evaluated correspond to the zero stage, a state corresponding to green; stage three: an intermediate state; and stage five: the harvest state. It could be observed (Table 2) that, as the fruits matured, humidity increased and the percentage of acidity decreased. No pH changes were noticeable in these states.
In the bilberry, in addition to the obvious changes of color, parameters such as pH and concentration of soluble solids were indicators of fruit maturation (Table 3). The study of the bilberry is gaining strength given its wild characteristic. Ávila et al. (2007) reported, for the harvesting stage, acidity values close to 1.44%. The behavior of this fruit is similar to that of the cranberry, which belongs to the same genus (Castrejón et al., 2008).
Aromatic profile. The Release of aromatic compounds, together with the loss of the green color and acid flavor, is one of the changes that occur during the ripening of any fruit.
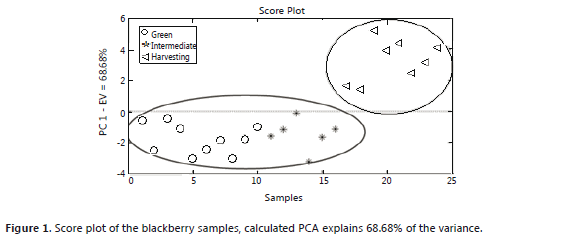
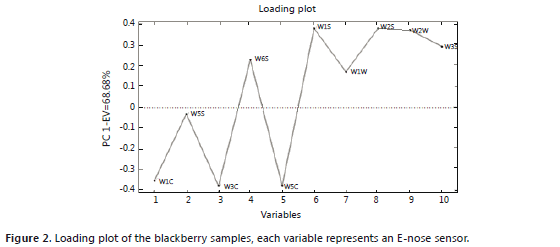
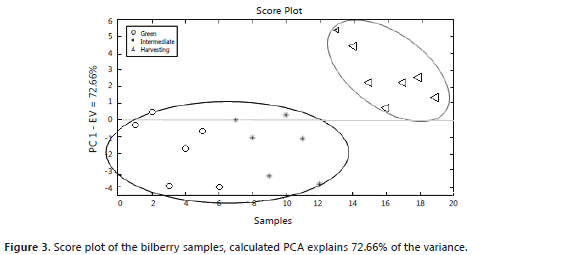
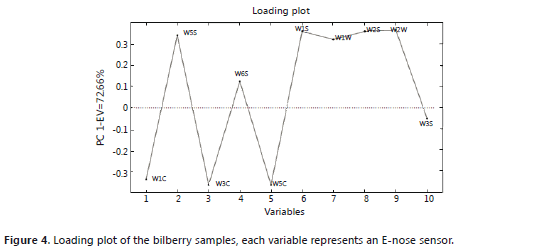
The principal component analysis for the blackberry (Figures 1 and 2) showed that the green and intermediate maturation stages are distinguished from the harvesting stage by the response of a group of sensors that perceive the presence of aromatic functional groups of low polarity: W1C, W5S, W3C and W5C (Table 1). In the harvesting stage, the presence of terpenes, alcohols, and aromatic compounds such as methanol can be considered because the group of sensors that shows responses includes: W6S, W1S, W2S, W3S, W1W and W2W. These results agree with the findings of a study conducted with volatiles of ready-to-eat blackberries, where the presence of benzoic acid, 2-heptanol, terpene-4-ol, ethyl and methyl benzoate was reported (Meret et al., 2011); also, in the Rubus sp. genus, during maturation, the production of alcohols, aldehydes, esters and ketones is favored (Perkins-Vezzie et al., 2000). The bilberry (Figures 3 and 4) exhibited the same behavior as that of the blackberry; fruits in the harvesting stage can be differentiated from the other two stages by sensors that respond to volatile aromatic functional groups, alcohols and terpenes. Studies on the Vaccinium sp. genus report the presence of 1-hexanol, 2-hexen-1-ol, 2-hexenal, 2-butyl-1-octanol, hexadecanol, etc. (Diban, 2008).
Both score plots explain more than 50% of the variance of the groups in each case. In the loading plots, a difference between the aromatic profile of the blackberry and the bilberry is seen due to different responses from the group of sensors that actuates during ripening.
Vitamin C content. Vitamin C has biological activity as an antioxidant because the double bond and substitutions have two hydroxyl groups within the chemical structure, is synthesized in plants in response to stress conditions, and, additionally, its concentration is determined by genetic factors (Asard et al., 2004).
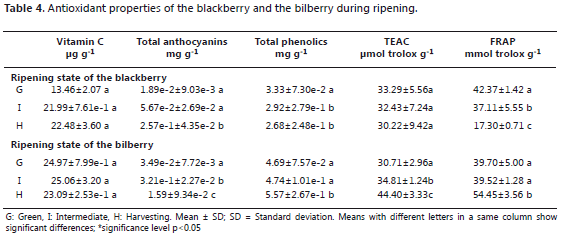
The evaluated samples contained between 13.46 and 22.48 mg of vitamin C g-1 of blackberries and 23.09 and 25.06 mg of vitamin C g-1 of bilberries (Table 4); there were no significant differences in either case, but the p value of the blackberries was close to the alpha value (P=0.057), suggesting that, for one of the stages of maturity, the content is different, statistical analysis according to the value found corresponds to the first stage of maturation, where it presented high variability. Contrary to expectations, the vitamin C content of the evaluated blackberry sample increased from state zero to state three. This could be explained by the non-climacteric behavior and the variability in agricultural practices and environmental conditions.
It has been reported that there is an accumulation of vitamin C and phenolic compounds (Cocetta et al., 2012) in the Vaccinium sp genus. during ripening, a behavior corresponding to that found in the bilberry. Rodriguez et al. (2007) reported a higher content of vitamin C for this fruit in the harvesting stage, about 80 mg of vitamin C g-1 of fresh fruit.
The vitamin C content of the blackberry and the bilberry was considerably lower than that reported in the composition table of the USDA (2010); for the blackberry: 209.72 mg of vitamin C g-1 of fresh fruit and for the blueberry: 97.24 mg of vitamin C g-1 of fresh fruit. On the other hand, some Colombian fruits have a vitamin C content between 0.53 and 257 mg 100 g-1 of fresh fruit (Contreras et al., 2011); the present data are outside this range, so it is important to note the differences that exist between families and the ecophysiological conditions of fruits, which determine composition and affect behavior patterns.
Anthocyanins content. The blackberry and the blueberry are sources of anthocyanins, the most important flavonoids responsible for attracting insects to plants and protecting them from various diseases and predators (Brown et al., 2006; Chemler et al., 2009). In general, the anthocyanin structure is linked to a sugar and can even be copolymerized, which affects the availability and quantification (AOAC, 2005) Different authors report that predominate anthocyanin in blackberry and blueberry is the cyn-3-glu (Dai et al., 2009; Garzón et al., 2010).
During maturation, the increased anthocyanin content, in this way is synthesized from shikimic acid and malonic acid. (Chemler et al., 2009). In the case of the blackberry, there are no significant differences between the green and intermediate stages but an increase of nearly 14 times more than the initial value has been observed in the harvesting stage. This is comparable to that described by Chen et al. (2012) for the early stages of ripening, reporting a value of 0.106 mg cyn-3-glu g-1 of fresh fruit and, for the subsequent maximum peak in the maturing state, 1.46 mg cyn-3-glu g-1 of fresh fruit. The bilberry presents significantly different contents in the three stages, with the harvesting stage having 45 times more anthocyanins than the green stage.
The contents of anthocyanins found in the blackberry and the bilberry allow for the comparison of the blackberry stage of harvesting with the intermediate stage of the bilberry, leading to the conclusion that, among mature fruits, the bilberry is an important source of these bioactive compounds.
Total phenolic content. The concentrations of phenolic compounds and nutrients, vitamins, and minerals, among others, respond to multiple environmental and genetic factors. The composition of a food is never constant and it is not uncommon to find differences between fruits, even in the same crop. For example, it has been found that the leaves and fruits that are in direct contact with solar radiation have increased contents of phenolic compounds, vitamin C and carotenoids, in comparison to those found in the shade (Brown et al., 2006; Vicente et al., 2009). The total phenolic content in the case of the bilberry, at any stage of maturity, was greater than that of the blackberry and, while polyphenols increased in the bilberry, in the blackberry, they decreased. The variety of compounds present in each fruit may influence the final result, especially considering that the Folin-Ciocalteu method may not necessarily respond to polyphenolic substances such as aromatic amines or even ascorbic acid (Magalhães et al., 2008).
Antioxidant capacity. The antioxidant power of a fruit can be measured by different in vitro methods, which give an idea of the ability of the sample to scavenge free radicals (Niki, 2010). Non-competitive methods employing ABTS•+ and the complex [Fe (II) (TPTZ)2]+2 introduce a radical in the sample and quantify how much was inhibited by the substances present. During ripening, the bilberry increased its antioxidant capacity (Table 4), whereas the blackberry presented no evidence of significant changes (such as TEAC), similar to the findings of Livani et al. (2013), who studied two ripening stages of Rubus anatolicus with the DPPH radical. However, the FRAP data of the blackberry showed that the stage of harvesting had a lower antioxidant capacity than the green stage. Acosta-Montoya et al. (2011) evaluated samples of Rubus adenotrichus Schltdl with H-ORAC during ripening, finding that the antioxidant capacity increased while the total phenol content remained unchanged; these results show the differences that can be found among the same family and that might be associated with genetic characteristics or crop and postharvest conditions.
CONCLUSIONS
The behavior of the antioxidant properties during ripening in the blackberry and the bilberry are independent. In the blackberry, there was a decrease but, in the bilberry, an increase was evidenced, a better characteristics that makes the latter fruit an attractive food. The electronic nose allowed for the formation of the aromatic profile in different stages of maturity, revealing differences in the harvesting stage for both fruits.
ACKNOWLEDGEMENTS
The authors thank the Food Science and Technology Institute - ICTA of Universidad Nacional de Colombia and the Corporación Colombiana de Investigación Agropecuaria - CORPOICA.
BIBLIOGRAPHY
Acosta-Montoya, Ó., F. Vaillant, S. Cozzano, C. Mertz, A. Pérez and M. Castro. Phenolic content and antioxidant capacity of tropical highland blackberry (Rubus adenotrichus Schltdl.) during three edible maturity stages. Food Chemistry 119(4): 1497-1501. [ Links ]
Asard, H., J.M. May and N. Smirnoff. 2004. Vitamin C, function and biochemistry in animals and plants. Garland Science, London, UK. 374 p. [ Links ]
Association of Analytical Communities A.O.A.C. 2005. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. Journal of AOAC International 88(5):1269-1278. [ Links ]
Ávila, H., J. Cuspoca, G. Fischer, G. Ligarreto and M. Quicazan. 2007. Caracterización fisicoquímica y organoléptica del fruto de agraz (Vaccinium meridionale Swartz) Almacenado 1 a 2 ºC. Revista Facultad Nacional de Agronomía, Medellín 60(2): 4179-4193. [ Links ]
Balasundram, N., K. Sundramand and S. Samman. 2006. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chemistry 99(1): 191-203. [ Links ]
Benedetti, S., S. Buratti, A. Spinardi, S. Mannino and I. Mignani. 2008. Electronic nose as a non-destructive tool to characterise peach cultivars and to monitor their ripening stage during shelf-life. Postharvest Biology and Technology 47(2): 181-188. [ Links ]
Bhattacharyya, N., S. Seth, B. Tudu, P. Tamuly, A. Jana, D. Ghosh, R. Bandyopadhyay, M. Bhuyan and S. Sabhapandit. 2007. Detection of optimum fermentation time for black tea manufacturing using electronic nose. Sensors and Actuators B: Chemical 122(2): 627-634. [ Links ]
Brezmes, J., E. Llobet, X. Vilanova, G. Saizand and X. Correig. 2000. Fruit ripeness monitoring using an electronic nose. Sensors and Actuators B: Chemical 69(3): 223-229. [ Links ]
Brown, J.E., V. Cheynier, M. Clifford, O. Dangles, K.M. Davies, C. Dufour, G.G. Duthie, D. Ferreira, T. Fossen, K.S. Gould, R.J. Grayer, K. Hostettmann, M. Jay, M. Jordheim, J.A. Kyle, C. Lister, J.P. Marais, A. Marston, K.E. Schwinn, D. Slade, K.M. Valant-Vetschera, N.C. Veitch, C.A. Williams, H. Wiseman and E. Wollenweber. 2006. Flavonoids: chemistry, biochemistry, and applications. Taylor and Francis Group, Boca Raton, USA. 1.212 p. [ Links ]
Castañeda, A., M.D. Pacheco, M.E. Páez, J.A. Rodríguez and C.A. Galán. 2009. Chemical studies of anthocyanins: A review. Food Chemistry 113(4): 859-871. [ Links ]
Castrejón, A.D., I. Eichholz, S. Rohn, L.W. Kroh and S. Huyskens-Keil. 2008. Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chemistry 109(3): 564-572. [ Links ]
Chemler, J.A, K.M. Davies, V. de Freitas, S. Deroles, K.S. Gould, J.H. Hatier, M.A. Koffas, E. Leonard, S. Lev-Yadun, M.A. Lila, N. Mateus, T. Nakayama, S. Rasmussen, K. Saito, W.J. Steyn, M. Yamazaki and K. Yonekura-Sakakibara. 2009. Anthocyanins biosynthesis, functions, and applications. Springer, New York, USA. 345 p. [ Links ]
Chen, Q., H. Yu, H. Tang and X. Wang. 2012. Identification and expression analysis of genes involved in anthocyanin and proanthocyanidin biosynthesis in the fruit of blackberry. Scientia Horticulturae 141(0): 61-68. [ Links ]
Cocetta, G., K. Karppinen, M. Suokas, A. Hohtola, H. Häggman, A. Spinardi, I. Mignani and L. Jaakola.2012. Ascorbic acid metabolism during bilberry (Vaccinium myrtillus L.) fruit development. Journal of Plant Physiology 169(11): 1059-1065. [ Links ]
Contreras, J., L. Calderón, E. Guerra and B. García. Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from Colombia. Food Research International 44(7): 2047-2053. [ Links ]
Dai, J., A. Gupte, L. Gates and R.J. Mumper. 2009. A comprehensive study of anthocyanin-containing extracts from selected blackberry cultivars: Extraction methods, stability, anticancer properties and mechanisms. Food and Chemical Toxicology 47(4): 837-847. [ Links ]
Diban G.N. 2008. Separación de aromas en etapas del procesado de zumos de frutas y bebidas. Tesis de doctorado en Ingeniería Química y Química Inorgánica: Facultad de Ingeniería. Universidad de Cantabria. 34 p. [ Links ]
Garzón, G.A., C.E. Narváez, K.M. Riedl and S.J. Schwartz. 2010. Chemical composition, anthocyanins, non-anthocyanin phenolics and antioxidant activity of wild bilberry (Vaccinium meridionale Swartz) from Colombia. Food Chemistry 122(4): 980-986. [ Links ]
Gorinstein, S., Z. Jastrzebski, H. Leontowicz, M. Leontowicz, J. Namiesnik, K. Najman, Y. S. Park, B. G. Heo, J. Y. Cho andJ. H. Bae. 2009. Comparative control of the bioactivity of some frequently consumed vegetables subjected to different processing conditions. Food Control 20(4): 407-413. [ Links ]
Instituto Colombiano de Normas Técnicas y Certificación (ICONTEC). 1999. NTC 4624. Jugos de frutas y hortalizas. Determinación del contenido de sólidos solubles. método refractométrico. Icontec, Bogotá [ Links ].
Instituto Colombiano de Normas Técnicas y Certificación (ICONTEC). 1999. NTC 440. Productos alimenticios. Métodos de ensayo. Icontec, Bogotá [ Links ].
Instituto Colombiano de Normas Técnicas y Certificación (ICONTEC). 1999. NTC 4106. Frutas frescas. Mora de castilla. Especificaciones. Icontec, Bogotá [ Links ].
Li, C., G.W. Krewer, P. Ji, H. Scherm and S.J. Kays. 2010. Gas sensor array for blueberry fruit disease detection and classification. Postharvest Biology and Technology 55(3): 144-149. [ Links ]
Ligarreto, G.A. 2011. Agraz (Vaccinium meridionale Swartz), algunas prácticas de cultivo y poscosecha. Bogotá, Colombia. Universidad Nacional de Colombia. Facultad de Agronomía. Ministerio de Agricultura y Desarrollo Rural, Bogotá D.C. 23 p. [ Links ]
Livani, F., M. Ghorbanli and A. Sateeyi. Changes in antioxidant activity and content of phenolic compounds during the ripening process of elm-leaved blackberry fruit. International Journal of Agronomy and Plant Production 4(1): 88-93. [ Links ]
Magalhães, L. M., M.A. Segundo, S. Reis and J.L. Lima. 2008. Methodological aspects about in vitro evaluation of antioxidant properties. Analytica Chimica Acta 613(1): 1-19. [ Links ]
Meret, M., P. Brat, C. Mertz, M. Lebrun and Z. Günata, 2011. Contribution to aroma potential of Andean blackberry (Rubus glaucus Benth). Food Research International 44(1): 54-60. [ Links ]
Mitcham, E., C. Crisosto and A. Kader. 1998. Bushberries: blackberry, blueberry, cranberry, raspberry: recommendations for maintaining postharvest quality. In: University of California, http://postharvest.ucdavis.edu/PFfruits/Bushberries/; accessed: May 2012. [ Links ]
Müller, L., S. Gnoyke, A.M. Popken and V. Böhm. 2010. Antioxidant capacity and related parameters of different fruit formulations. LWT - Food Science and Technology 43(6): 992-999. [ Links ]
Murcia, A.M., A.M. Jiménez and M. Martínez-Tomé. 2009. Vegetables antioxidant losses during industrial processing and refrigerated storage. Food Research International 42(8): 1046-1052. [ Links ]
Niki, E. 2010. Assessment of Antioxidant Capacity in vitro and in vivo. Free Radical Biology and Medicine 49(4): 503-515. [ Links ]
Oliveira, I., P. Baptista, R. Malheiro, S. Casal, A. Bento and J.A. Pereira. 2011. Influence of strawberry tree (Arbutus unedo L.) fruit ripening stage on chemical composition and antioxidant activity. Food Research International 44(5): 1401-1407. [ Links ]
Pathange, L.P., P. Mallikarjunan, R.P. Marini, S. O'Keefeand and D. Vaughan. 2006. Non-destructive evaluation of apple maturity using an electronic nose system. Journal of Food Engineering 77(4): 1018-1023. [ Links ]
Peris, M. and L. Escuder-Gilabert. 2009. A 21st century technique for food control: Electronic noses. Analytica Chimica Acta 638(1): 1-15. [ Links ]
Perkins-Veazie, P., J.R. Clark, D.J. Huber and E.A. Baldwin. 2000. Ripening physiology in 'Navaho' Thornless blackberries: color, respiration, ethylene production, softening, and compositional changes. Journal of the American Society for Horticultural Science 125(3): 357-363. [ Links ]
Pietta, P., M. Minoggio and L. Bramati. 2003. Plant polyphenols: structure, occurrence and bioactivity. pp: 257-312. In: Atta-Ur, R. (ed.). Studies in natural products chemistry. Elsevier, Amsterdam, The Netherlands. 491 p. [ Links ]
Shui, G. and L.P. Leong. 2002. Separation and determination of organic acids and phenolic compounds in fruit juices and drinks by high-performance liquid chromatography. Journal of Chromatography A 977(1): 89-96. [ Links ]
Torri, L., N. Sinelliand and S. Limbo. 2010. Shelf life evaluation of fresh-cut pineapple by using an electronic nose. Postharvest Biology and Technology 56(3): 239-245. [ Links ]
United States Department of Agriculture USDA. 2010. USDA National Nutrient Database for Standard Reference, Release 22 Nutrient Lists, http://www.ars.usda.gov/Services/docs.htm?docid=18877; accessed: September 2010. [ Links ]
Vasco, C., J. Rualesand and A. Kamal-Eldin. 2008. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chemistry 111(4): 816-823. [ Links ]
Vicente, A., G.A. Manganaris, G. Sozzi and C. Cristoso. 2009. Nutritional quality of fruits and vegetables. pp: 57-106. In: Florkowski W., R. Shewfelt, B. Brueckner and S. Prussia. (eds.). Postharvest handling: a systems approach. Second edition. Academic Press, Massachusetts, USA. 640 p. [ Links ]
Wang, L.S. and G.D. Stoner. 2008. Anthocyanins and their role in cancer prevention. Cancer Letters 269(2): 281-290. [ Links ]
Zuluaga, C.M., A.C. Diaz and M.C. Quicazán. 2011. Estandarización y validación del método de análisis del perfil aromático por nariz electrónica. Ingeniería e Investigación 31(2): 65-73. [ Links ]