Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Facultad Nacional de Agronomía Medellín
versão impressa ISSN 0304-2847
Rev. Fac. Nac. Agron. Medellín vol.68 no.2 Medellín jul./dez. 2015
https://doi.org/10.15446/rfnam.v68n2.50950
Effectiveness of a rock phosphate solubilizing fungus to increase soil solution phosphate impaired by the soil phosphate sorption capacity
La capacidad de adsorción de fosfato del suelo limita la efectividad de microorganismos solubilizadores de roca fosfórica para incrementar la concentración de fosfato en solución
Nelson Walter Osorio Vega1; Mitiku Habte2 and Juan Diego León Peláez3
1 Associated Professor. Universidad Nacional de Colombia - Sede Medellín - Facultad de Ciencias - Escuela de Biociencias. A.A. 3840, Medellín, Colombia.<nwosorio@unal.edu.co>
2 Professor University of Hawaii - Department of Tropical Plant and Soil Sciences. St. John 102, 3190 Maile Way, 96822, Honolulu, HI, USA. <mitiku@hawaii.edu>
3 Full Professor. Universidad Nacional de Colombia - Sede Medellín - Facultad de Ciencias Agrarias - Departamento de Ciencias Forestales. A.A. 1779, Medellín, Colombia.<jdleon@unal.edu.co>
Received: September 12, 2014; Accepted: May 4, 2015
DOI: http://dx.doi.org/10.15446/rfnam.v68n2.50950
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Abstract. Available phosphate (P) deficiency in tropical soils has been recognized as a major factor that limits soil quality and plant performance. To overcome this, it is necessary to add high amounts of soluble P-fertilizers; however, this is inefficient and costly. Alternatively, rock phosphates (RP) can be used, but their low reactivity limits their use. Phosphate solubilizing microorganisms (PSM) can enhance RP dissolution and, thus, improve the RP agronomic effectiveness as fertilizer. Nonetheless, their effectiveness may be impaired by the soil P fixation capacity. An experiment was carried out to assess the in vitro effectiveness of the fungus Mortierella sp. to dissolve RP in an axenic culture medium and, thus, enhance the solution P concentration in the presence of aliquots of soils with contrasting P fixation capacity. The results showed that the fungus was capable of lowering the medium pH from 7.7 to 3.0 and, thus, dissolving the RP. The presence of soil aliquots in the medium controlled the effectiveness of the fungus to increase the concentration of the soluble P. In the presence of soils with a low or medium P sorption capacity, the concentration of the soluble P was high (63.8-146.6 mg L-1) in comparison with the inoculated (soilless) treatment (50.0 mg L-1) and the uninoculated control (0.7 mg L-1). By contrast, with very-high P fixing soil aliquots, the concentration of the soluble P was very low (3.6-33.1 mg L-1); in addition, in these soils, the fungus immobilized more P into its mycelia than in soils with a low or medium P fixation capacity. The capacity of a soil to fix P seems to be a good predictor for the effectiveness of this fungus to increase the soluble P concentration via RP dissolution.
Key words: Mortierella, apatite, phosphorus, Mollisol, Oxisol, Ultisol, Andisols.
Resumen. Se realizó un experimento de laboratorio para evaluar la efectividad del hongo Mortierella sp. para disolver in vitro roca fosfórica (RP) en un medio de cultivo axénico y así aumentar la concentración de P soluble, en presencia de alícuotas de siete suelos con capacidad contrastante para adsorber P. Los resultados mostraron que el hongo fue capaz de disminuir el pH del medio de 7.7 a 3.0 y de esta manera disolver la RP. La presencia de alícuotas de suelo en el medio controló la efectividad del hongo para incrementar el nivel de P soluble. En presencia de suelos con baja y media capacidad de fijación de P el hongo fue efectivo para aumentar la concentración de P soluble del medio (63.8-146.6 mg L-1) en comparación al tratamiento inoculado sin suelo (50.0 mg L-1) y el control no-inoculado (0.7 mg L-1). En contraste, con los suelos de alta capacidad para fijar P la concentración de P soluble fue muy baja (3.6-33.1 mg L-1); adicionalmente, en estos suelos el hongo inmovilizó en su micelio mayor cantidad de P en suelos que en los suelos con baja y media fijación de P. La capacidad de fijación de P por el suelo parece ser un buen predictor de la efectividad del hongo para aumentar la concentración de P soluble vía disolución de RP.
Palabras claves: Mortierella, apatita, fósforo, Mollisol, Oxisol, Ultisol, Andisol.
Available soil phosphate (P) deficiency has been widely recognized as a factor that severely impairs soil quality and plant performance in agriculture and forestry ecosystems in the tropics (Turner et al., 2006; León and Osorio, 2014). To overcome this problem, it is necessary to apply high amounts of P fertilizers; however, this is inefficient and costly. Although rock phosphates (RP) can be used effectively in high P fixing soils (Gyaneshwar et al., 2002; Osorio and Habte, 2013), the low reactivity of most RP restricts their widespread use (Shrivastava et al., 2007; Ojo et al., 2007). The use of phosphate solubilizing microorganisms (PSM) to enhance RP dissolution has been considered a viable approach to improve the efficacy of RP as a source of P for agricultural purposes (Osorno and Osorio, 2014; Singh and Reddy, 2011; Vyas et al., 2007; Delvasto et al., 2006; Welch et al., 2002).
Many authors have reported positive effects on the P uptake and yield response of several plant species from inoculation of the soil with PSM (Osorio and Habte, 2013; Barea et al., 2002; Whitelaw, 2000). Most of these positive responses have been obtained in low P sorbing soils, such as Mollisols and sandy soils (Peiz et al., 2001); however, in the high P sorbing soils of the tropics and volcanic ash soils (Loaiza-Usuga et al., 2013), where cost-effective approaches are needed to overcome the effects of high P fixation by soils, PSM have been little studied. The effectiveness of these microorganisms in enhancing plant P uptake may be limited because the solubilized P would be rapidly re-fixed by the soil constituents (Osorno and Osorio, 2014). However, there are no published data to refute or confirm this argument. The objective of the current investigation was to determine the effectiveness of the fungus Mortierella sp. at increasing the soluble P by dissolving RP in the presence of soil samples that differed in their P sorbing capacity.
MATERIALS AND METHODS
The in vitro experiments were conducted in the Soil Microbiology Laboratory of the Universidad Nacional de Colombia at Medellín (6º15' N, 75º35' W). 250 mL Erlenmeyer flasks had 75 mL of a liquid medium added that contained, per liter: NH4NO3 1.0 g, NaCl 1.0 g, CaCl2.2H2O 0.2 g, MgSO4.7H2O 0.4 g, glucose 10.0 g, and Huila RP 3.5 g as the only source of P. The empirical formula of the Huila RP was Ca9.69 Na0.22Mg0.09(PO4)5.14(CO3)0.86F2.34 (Osorio, 2008). which was passed through a 0.5 mm aperture sieve and had a P content of 130 g kg-1. The liquid medium was also amended with soil samples (0.5 to 2.0 mm diameter) at the rate of 0.6 g/flask. The soil samples were collected from different places of Colombia (Table 1) and were selected due to their widely differing P sorption capacities. The medium was then autoclaved (120°C, 0.1 MPa, 30 min).
The medium was either not inoculated or inoculated with two mL of a three-day-old suspension of Mortierella sp. containing 3x105 colony forming units (CFU=spores and mycelial fragments) per mL, which was determined in a Petri dish culture with a selective medium (PDA+ 100 µg mL-1 cycloheximide + 100 µg mL-1 benomyl) (Osorio and Habte, 2013). The fungus was originally isolated from an Andisol of Hawaii (Osorio and Habte, 2001) and multiplied and stored on yeast mannitol agar (YMA) slants (KH2PO4 0.5 g, MgSO4.7H2O 0.2 g, NaCl 0.1 g, mannitol 10.0 g, yeast extract 1.0 g, agar 15.0 g L-1) at 4°C. Then, it was multiplied in Petri dishes on a YMA medium for three days at 28°C; the mycelium was removed from the surface of the agar with a sterile loop and suspended in sterile, deionized water and shaken by hand until the mycelial clumps and spores were dispersed. The uninoculated flasks received two mL of sterile, deionized water. The flasks were continuously shaken with an orbital shaker (model Innova 4400, New Brunswzc Scientific Co., Inc., Edison, NJ) at 100 rpm and 25 °C for seven days.
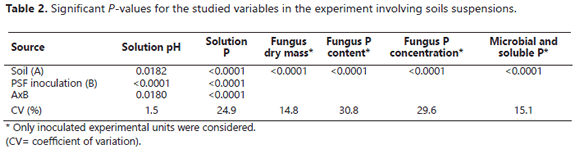
After the incubation period, the medium was filtered through a Whatman No. 42 filter paper fitted to a Buchner funnel; a vacuum was exerted at 350 mm Hg. The fresh fungal matter was collected on the filter paper and then dried in an oven at 60°C for 30 h for dry mass determination after correcting for the weight of the remaining RP and soil particles. The filtrate was centrifuged at 5000xg for 15 minutes and passed through a Millipore membrane filter (0.45 µm) for the solution pH determination by means of a pH-meter and for the solution P concentration by the molybdate blue method (Murphy and Riley, 1962). The net solution gain for each soil sample was calculated by subtracting the P released in the absence of the PSF from that released in the presence of the PSF. In order to determine the fungus P content and concentration, the fungal samples (5 mg) were oven-dried and then ashed in a muffle furnace at 500°C for 3 hours. The ash was dissolved in one mL of 0.1 M HCl and then brought up to 10 mL with deionized water. The fungal P content was determined by the molybdate blue method (Murphy and Riley, 1962).
The experiment design was completely randomized; the treatments were arranged in an 8x2 factorial with three replicates per treatment. The data were subjected to analysis of variance (F-test) and mean separation was achieved by employing the Duncan's multiple range test at a P-value of 0.05. Regression models were estimated to fit the relationship between the soil P sorption capacity and the net P solubility by the fungus; the analyses of data were achieved by employing the statistical package Statgraphics Centurion XV (StatPoint Technologies, Inc.).
RESULTS AND DISCUSSION
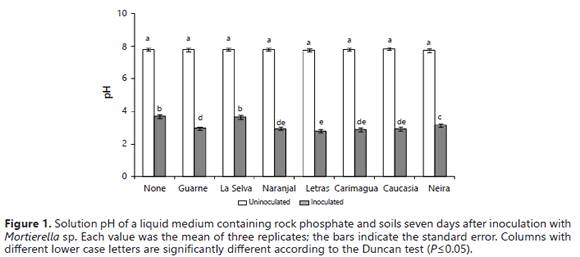
The fungus exhibited a strong capacity to reduce pH even in the presence of soils with a high pH buffer capacity (Table 2). The decline in the pH produced by the activity of the fungus was significantly higher in the presence of some soils (Letras, Naranjal, Carimagua, Caucasia, and Guarne, pH values ≤ 3.0) than in their absence (no soil: pH 3.7) (Figure 1). It is very well documented that a major mechanism for the microbial dissolution of RP is acid production (Osorio and Habte, 2013; Vyas et al., 2007; Chen et al., 2006; Radersma and Grierson, 2004; Welch et al., 2002; Whitelaw et al., 1999; Illmer and Schinner, 1995). The presence of Mortierella sp. was associated with decreases in the pH of the growth medium despite the presence of soil samples that were expected to buffer the medium pH (Bolan et al., 1999; Prabhakaran, 1996).
The lowest pH observed in the presence of these soil samples was 2.75 (Figure 1), which was lower than what is commonly reported in the literature (pH 3.5-4.5) (Pandey et al., 2006; Cerezine et al., 1988). This reduction in the pH of the growth medium indicates that the RP dissolution was related to a microbial induced acidification of the growth medium. An inverse relationship between pH and solution P concentration was previously obtained by Osorio and Habte (2001, 2013) under similar experimental conditions without soil samples.
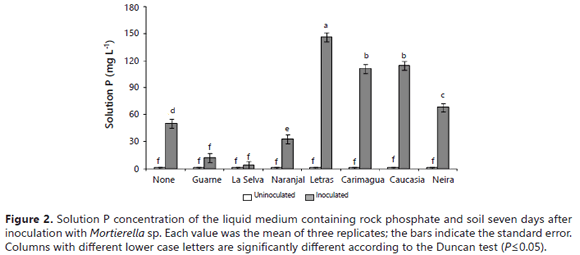
The quantity of P observed in the solution was significantly affected by the presence of soil samples in the liquid medium. In this way, the soluble P detected in the growth medium was significantly lower in the presence of soils than in their absence (Table 2).
This effect was much more pronounced in the presence of soils with a very high P sorbing capacity (Guarne, La Selva, and Naranjal) than with soils with a lower P sorbing capacity (Figure 2). In fact, the P released into the solution in the presence of the rest of the soils was significantly higher than that observed in the absence of the soils. The extent of this stimulatory effect of the soils was had the following order: Letras > Carimagua, Caucasia > Neira.
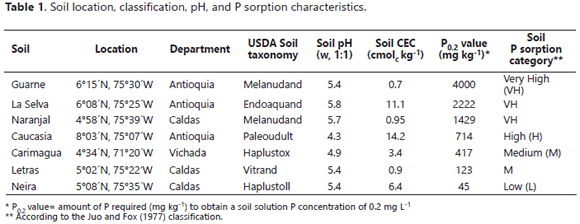
The mechanism of Mortierella sp. for the RP dissolution was likely by means of oxalic acid production, as mentioned by Osorio (2008). However, the presence of soil samples controlled, to different degrees, the amount of P that remained in solution. For instance, in the presence of soil samples with a very high P sorption capacity (P0.2 >1000 mg kg-1), less P was left in solution; conversely, more P remained in solution in the presence of soil samples that exhibited a lower P sorption capacity. Thus, although the RP dissolution occurred in the presence of all of the soil samples, the effectiveness of Mortierella sp. in increasing the solution P varied widely. The active surfaces of the soil samples controlled the extent to which the P released by the fungus stayed in solution. The presence of samples of Andisols with a very high P sorbing capacity (Guarne, La Selva, and Naranjal) was particularly effective in lowering the concentration of P measured in the liquid medium. These results are understandable in light of the tendency of allophanes to strongly sorb large quantities of P in volcanic ash soils (Bolan et al., 1994; Shoji et al., 1993). These types of soils are very well known for their very high P sorbing capacity (Jackman et al., 1997; Shoji et al., 1993) due to the large active surface area they have coupled with the high binding strength they exert on P (Osorio and León, 2013). These properties appear to be responsible for the low level of P detected in the solution when Mortierella sp. was incubated in the presence of soils compared to the level of P observed in their absence. Among the very high P sorbing soils studied, Guarne and La Selva were more effective at adsorbing the P released from RP due to the solubilization activity of Mortierella sp. To obtain a soil solution concentration of 0.2 mg L-1 in the presence of these two soils, it was necessary to add 4126 and 2222 mg of P kg-1 to the growth medium (i.e., P0.2 of 4126 and 2222, respectively). Naranjal was the Andisol with lowest P sorbing capacity (P0.2= 1429 mg kg-1); apparently, this lower capacity (among Andisols) was reflected in a lower reduction in the concentration of P detected in the medium in the presence of Mortierella sp. (Figure 2). The results obtained with these three Andisols support the hypothesis of Tinker (1980) and Osorio (2008), regarding the low or nil effectiveness of PSM in the presence of high P sorbing soils. However, the presence of Mortierella sp. was associated with higher levels of P in solution if soils with high to moderate P sorbing capacities were present than if they were absent. For instance, in the presence of the Caucasia soil, an Ultisol with a high P sorbing capacity (P0.2=714 mg kg-1), the P concentration in the liquid medium was more than twice that noted in the absence of the soil. The effect of the Carimagua soil (an Oxisol with a medium P fixing capacity (P0.2= 417 mg kg-1) was similar to that of the Caucasia soil; kaolinite was the dominant soil mineral in these two soils, a soil mineral that did not reduce the P concentration measured in solution after the growth of Mortierella in the medium in the presence of RP.
The positive effects on the amount of P released after dissolution of RP by Mortierella sp. activity observed in the presence of Neira (Montmorillonite as the dominant soil mineral) and the Letras soils were not unexpected because of their low P-sorbing capacities (Letras P0.2= 123, Neira P0.2= 45). However, the magnitude of the positive effect they had is surprising, particularly regarding the Letras soil (Vitrand). This soil had a sandy texture, 94% sand and only 2% clay, which explained, at least in part, why it did not impair the effectiveness of Mortierella sp. in increasing the soluble P in the liquid medium.
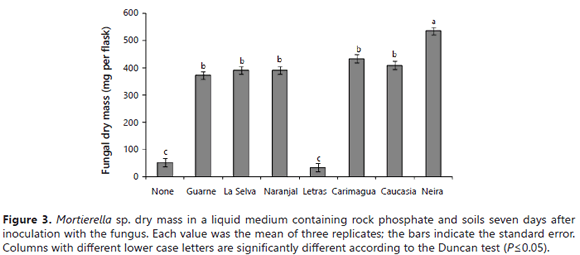
On the other hand, the Mortierella sp. dry matter was significantly higher (P= 0.05) in the presence of soils than in their absence, except in the presence of the Letras soil (Figure 3). The highest fungus dry matter yield was observed in the presence of the Neira soil (the soil with the lowest P sorbing capacity and highest value of cation exchange capacity) (Table 1).
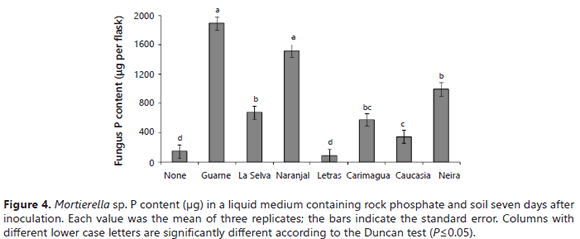
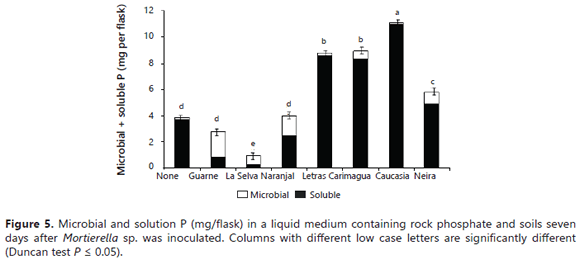
The fungus P uptake was significantly stimulated by the presence of some soils (Guarne, Naranjal > La Selva, Neira, Carimagua), but not by others (Letras and Caucasia) (Figure 4). Surprisingly, the fungus took up significantly more P in the presence of high P-fixing soils (Guarne and Naranjal) than in the presence of lower P-fixing soils. The lowest fungus P content was observed in flasks not amended with soil and in those amended with the Letras soil. Similar results were obtained for the tissue P concentration of the fungus (data not shown). The relative proportions of microbial-P and soluble-P in the growth medium varied depending on the type of soil present in the medium (Figure 5). In the absence of soil samples in the medium, the fungus mobilized only 3.9 mg of the P, leaving 96% of the P in solution (Figure 5). In the two soils with the higher P-sorbing capacity (Guarne and La Selva), the proportion of orthophosphate was only 27-31% of the total P, the balance being immobilized in the fungus mycelium. In the Naranjal soil, also a soil with a very high P sorption capacity, but lower than the other two Andisols, the distribution of the P was significantly different: orthophosphate constituting 62% of the total P and microbial-P constituting the balance.
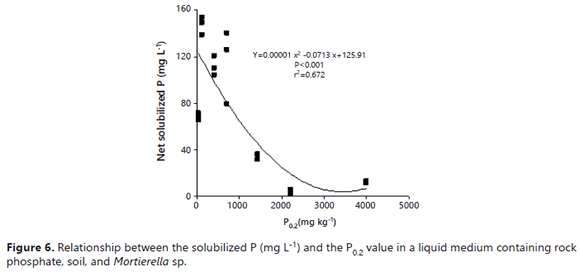
The values observed in the presence of soils that stimulated the effectiveness of the fungus at increasing the P in solution were comparable to those observed in the soil-free medium. In the absence of soil, the solution P ranged 84-99%, while the microbial-P amounted to only1-16% of the total P (Figure 5). It is hard to explain the positive effect that soil minerals and the presence of soils had on the fungal dry mass, P content, and P concentration of Mortierella sp. However, some explanations may lie in the growth characteristics exhibited by the fungus. First, Mortierella sp. has a tendency to grow on solid surfaces, its mycelium tending to adhere to internal flask walls, RP particles, minerals, soil particles, and also on root surfaces, forming a biofilm-like pattern (Atlas and Bartha, 1997). This ability to grow on soil mineral surfaces may be advantageous for the fungus because these surfaces contain large quantities of sorbed P. The capacity of this fungus to produce oxalic acid could help this fungus to desorb P for its own uptake, as reported by Osorio and Habte (2013, 2014) using the same soils. The fact that some PSM can utilize sorbed P was also demonstrated by Hoberg et al. (2005) and He and Zhu (1997). The fact that Mortierella sp. can grow on these P-adsorbent surfaces is an advantage since it can remove P from the immediate vicinity of these surfaces. It is probable that some essential nutrients were also concentrated on these surfaces. The fungus could displace and utilize these anions with the oxalic acid that it excretes. The fungus could make them available to meet its own demands without the need to leave behind much soluble P in solution (Figure 6).
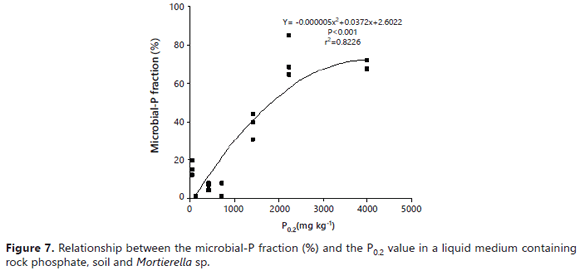
The auto-immobilization of Mortierella sp. cells on surfaces might explain the better performance of the fungus in the presence of some soil samples. Vassilev et al. (2001) observed that immobilized cells of PSF were more effective in dissolving RP under in vitro conditions than if freely suspended. The higher ability of Mortierella sp. to absorb P in the higher P fixing soils suggests that P ions were present on the soil surfaces (Figure 7). This alternative pool of P for uptake by the fungus explains how the fungus was able to grow despite the very low P concentration in the growth medium. The results of the regression analysis revealed that the net amount of P measured in solution was inversely related to the P0.2 value (r2= 0.67) (Figure 6). On the other hand, the amount of microbial-P (immobilized) was directly related to it (r2= 0.82) (Figure 7).
The data on the relative distribution of P between the fungal cells and the growth medium (Figures 5 and 6) clearly showed that, under limited P availability (as when the very high P fixing soils were present), the tendency of Mortierella sp. was to accumulate P in its cells instead of releasing it into the growth medium. Notice that the relative amount of microbial-P (69-73%) was higher than that of P in solution (27-31%) in the presence of the Guarne and La Selva soils (the higher P fixing soils). In the soil that was ranked third for its P fixing capacity (Naranjal soil), the proportion of the total P immobilized by the fungus was only 38%. Likewise, the presence of soils with a much lower P sorbing capacity was associated with a proportionally lower immobilization of P into the microbial cells (0.8-16%) and, consequently, a lower fungal dry mass. The soil in which Mortierella released the highest quantity of P in solution (11 mg/flask, 99.2% of which was in solution) was the Letras soil, but it was the worst regarding P uptake by Mortierella sp. (0.8% in fungal cells). As mentioned before, the Letras soil was very sandy (94% sand and only 2% clay) and, hence, had a very low capacity to sorb P on its limited active surfaces, making it unfavorable for the growth of Mortierella sp., but highly favorable for the accumulation of soluble P in the growth medium. The results of this study concurred with earlier observations that PSM can be effective in increasing soil P availability in soils that have a low P fixing capacity (P0.2<100 mg kg-1) (e.g., Mollisols and sandy soils), as reported by many authors (Osorio and Habte, 2013, 2015; Osorio, 2008; Peix et al., 2001). In the presence of soils with a very high P sorbing capacity (P0.2>1000 mg kg-1), most of the P released from the RP was removed from solution by the extremely high P adsorption activity of the soils.
The in vitro approach employed in the present study represented a simple, rapid, and relatively inexpensive approach for predicting whether or not a PSM can release P into solution in sufficient quantities for plant growth. This approach made it abundantly clear that the P released by a PSM may not remain in solution long enough to be taken up by plants in soils characterized as having a high to very high P fixing capacity. It is probable that the presence of arbuscular mycorrhizal fungi might considerably change the picture if the fungi succeed in capturing the P solubilized by a PSM before it is re-immobilized by P adsorbing sites.
ACKNOWLEDGMENTS
We are grateful to COLCIENCIAS for providing financial support to N.W. Osorio during his stay at the University of Hawaii.
REFERENCES
Atlas, R. and R. Bartha. 1997. Microbial Ecology. Addison Wesley Longman, New York, USA. 704 p. [ Links ]
Barea, J.M., M. Toro, M. Orozco, E. Campos and R. Azcon. 2002. The Application of isotopic (32P and 15N) dilution technique to evaluate the interactive effect of phosphate-solubilizing-rhizobacteria, mycorrhizal fungi and Rhizobium to improve the agronomic efficiency of rock phosphate form legume crops. Nutrient Cycling in Agroecosystems 63: 35-42. DOI: 10.1023/A:1020589732436. [ Links ]
Bolan, N.S., R. Naidu, J.K. Syers and R.W. Tillman. 1999. Surface charge and solute interactions in soils. Advances in Agronomy 67: 87-140. DOI: 10.1016/S0065-2113(08)60514-3. [ Links ]
Bolan, N.S., R. Naidu, S. Mahimairaja and S. Baskaran. 1994. Influence of low-molecular-weight organic acids on the solubilization of phosphates. Biology and Fertility of Soils 18: 311-319. DOI: 10.1007/BF00570634. [ Links ]
Cerezine, P.C., E. Nahas and D.A. Banzatto. 1988. Soluble phosphate accumulation by Aspergillus niger from fluorapatite. Applied Microbiology Biotechnology 29: 501-505. DOI: 10.1007/BF00269076. [ Links ]
Chen, Y.P., P.D. Rekha, A.B. Arun, F.T. Shen, W.A. Lai and C.C. Young. 2006. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Applied Soil Ecology 34: 33-41. DOI: 10.1016/j.apsoil.2005.12.002. [ Links ]
Delvasto, P., A. Valverde, A. Ballester, J.M. Igual, J.A. Muñoz, F. Gonzalez, M.L. Blázquez and C. Garcia. 2006. Characterization of brushite as a re-crystallization product formed during bacterial solubilization of hydroxyapatite in batch cultures. Soil Biology and Biochemistry 38: 2645-2654. DOI: 10.1016/j.soilbio.2006.03.020. [ Links ]
Gyaneshwar, P., G.N. Kumar, L.J. Parekh and P.S. Poole. 2002. Role of soil microorganisms in improving P nutrition of plants. Plant Soil 245: 83-93. DOI: 10.1023/A:1020663916259. [ Links ]
He, Z.L. and J. Zhu. 1997. Transformation and bioavailability of specifically sorbed phosphate on variable-charge mineral soils. Biology and Fertility of Soils 25: 175-181. DOI: 10.1007/s003740050300. [ Links ]
Hoberg, E., P. Marschner and R. Lieberei. 2005. Organic acid exudation and pH changes by Gordonia sp. and Pseudomonas fluorescens grown with P adsorbed to goethite. Microbiology Research 160: 177-187. DOI: 10.1016/j.micres.2005.01.003. [ Links ]
Illmer, P. and F. Schinner. 1995. Solubilization of inorganic calcium phosphates-solubilization mechanisms. Soil Biology and Biochemistry 27: 257-263. DOI: 10.1016/0038-0717(94)00190-C. [ Links ]
Jackman, J.M., R.C. Jones, R.S. Yost and C.J. Babcock. 1997. Rietveld estimates of mineral percentages to predict phosphate sorption by selected Hawaiian soils. Soil Science Society of America Journal 61: 618-625. DOI: 10.2136/sssaj1997.03615995006100020035x. [ Links ]
Juo, A.S.R. and R. L. Fox. 1977. Phosphate sorption characteristics of some bench-mark soils of West Africa. Soil Science 124: 370-376. DOI: 10.1097/00010694-197712000-00010. [ Links ]
León, J.D. and N.W. Osorio. 2014. Role of litter turnover in soil quality in tropical degraded lands of Colombia. The Scientific World Journal 2014: 1-11. DOI: 10.1155/2014/693981. [ Links ]
Loaiza-Usuga, J.C., J.D. León-Peláez, J.A Ramirez, M.I. González-Hernández, J.F. Gallardo-Lancho, W. Osorio-Vega and G. Correa-Londoño. 2013. Alterations in litter decomposition patterns in tropical montane forests of Colombia: a comparison of oak forests and coniferous plantations. Canadian Journal of Forest Research 43: 528-533. DOI: 10.1139/cjfr-2012-0438. [ Links ]
Murphy, J. and J.P. Riley. 1962. A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27: 31-35. DOI: 10.1016/S0003-2670(00)88444-5. [ Links ]
Ojo, O.D., A.A. Kintomo, E.A. Akinride and M.O. Akoroda. 2007. Comparative effect of phosphorus sources for grain amaranth production. Communications in Soil Science and Plant Analysis 38: 35-55. DOI: 10.1080/00103620601093611. [ Links ]
Osorio, N.W. 2008. Effectiveness of microbial solubilization of phosphate in enhancing plant phosphate uptake in tropical soils and assessment of the mechanisms of solubilization, Doctoral thesis. University of Hawaii, Honolulu, USA. [ Links ]
Osorio, N.W. and J.D. León. 2013. Roles of arbuscular mycorrizal association in plant nutrition and growth of tropical forestry and agroforestry in degraded soil reclamation. pp. 127-154. In: Hai, R. (ed.). Plantations Biodiversity, Carbon Sequestration and Restoration. Nova Science, New York, USA. [ Links ]
Osorio, N.W. and M. Habte. 2001. Synergistic influence of an arbuscular mycorrhizal fungus and P solubilizing fungus on growth and plant P uptake of Leucaena leucocephala in an Oxisol. Arid Land Research and Management 15: 263-274. DOI: 10.1080/15324980152119810. [ Links ]
Osorio, N.W. and M. Habte. 2013. Phosphate desorption from the surface of soil mineral particles by a phosphate solubilizing fungus. Biology and Fertility of Soils 49:481-486. DOI: 10.1007/s00374-012-0763-5. [ Links ]
Osorio, N.W. and M. Habte. 2013. Synergistic effect of a phosphate solubilizing fungus and an arbuscular mycorrhizal fungus on leucaena seedlings in an oxisol fertilized with rock phosphate. Botany 91: 274-281. DOI: 10.1139/cjb-2012-0226. [ Links ]
Osorio, N.W. and M. Habte. 2014. Soil phosphate desorption induced by a phosphate solubilizing fungus. Communications in Soil Science and Plant Analysis 45(4):451-460. DOI: 10.1080/00103624.2013.870190. [ Links ]
Osorio, N.W. and M. Habte. 2015. Effect of a phosphate-solubilizing fungus and an arbuscular mycorrhizal fungus on leucaena seedlings in tropical soils with contrasting phosphate sorption capacity. Plant and Soil 389:375-385. DOI: 10.1007/s11104-014-2357-5. [ Links ]
Osorno, L. and N.W. Osorio. 2014. Effect of carbon and nitrogen source and concentration on rock phospahte dissolution induced by fungi. Journal of Applied Biotechnology 2: 32-42. http://dx.doi.org/10.5296/jab.v2i2.5475. [ Links ]
Pandey, A., P. Trivedi, B. Kumar and L.M.S. Palni. 2006. Characterization of a phosphate solubilizing microorganism and antagonistic strain of Pseudomonas putida (B0) isolated from a sub-alpine location in the Indian Central Himalaya. Current Microbiology 53: 102-107. DOI: 10.1007/s00284-006-4590-5. [ Links ]
Peix, A., A.A. Rivas-Boyero, P.F. Mateos, C. Rodriguez-Barrueco, E. Martinez-Molina and E. Velasquez. 2001. Growth promotion of chickpea and barley by a phosphate solubilizing strain of Mesorhizobium mediterraneum under growth chamber conditions. Soil Biology and Biochemistry 33: 103-110. DOI: 10.1016/S0038-0717(00)00120-6. [ Links ]
Prabhakaran, K.P.N. 1996. The buffering power on plant nutrients and effects on availability. Advances in Agronomy 57: 237-287. DOI: 10.1016/S0065-2113(08)60926-8. [ Links ]
Radersma, S. and P. Grierson. 2004. Phosphorus mobilization in agroforestry: organic anions, phosphatase activity and phosphorus fractions in the rhizosphere. Plant Soil 259: 209-219. DOI: 10.1023/B:PLSO.0000020970.40167.40. [ Links ]
Shoji, S., M. Nanzyo and R.A. Dahlgren. 1993. Volcanic Ash Soils-Genesis, Properties, and Utilization. Elsevier Publishing, Amsterdam. 277 p. [ Links ]
Shrivastava, M., B.M. Bhujbal and S.F. D'Souza. 2007. Agronomic efficiency of Indian rock phosphate in acidic soils employing radiotracer A-value technique. Communications in Soil Science and Plant Analysis 38: 461-471. DOI: 10.1080/00103620601174288. [ Links ]
Singh, H. and S. Reddy. 2011. Effect of inoculation with phosphate solubilizing fungus on growth and nutrient uptake of wheat and maize plants fertilized with rock phosphate in alkaline soils. European Journal of Soil Biology 47: 30-34. DOI: 10.1016/j.ejsobi.2010.10.005. [ Links ]
Tinker, P.B. 1980. Role of rhizosphere microorganisms in phosphorus uptake by plants. pp. 617-654. In: Khasawneh, F.E., E.C. Sample and E.J. Kamprath (eds.). The Role of Phosphorus in Agriculture. Soil Science Society of America, Madison, USA. 910 p. [ Links ]
Turner, B.L., Frossard, E. and Oberson, A. 2006. Enhancing phosphorus availability in low- fertility soils. pp. 191-206. In: Uphoff, N. (ed.). Biological approaches to sustainable soil systems. CRC Press, Boca Raton, Fla, USA. 749 p. [ Links ]
Vassilev, N., M. Vassileva, M. Fenice and F. Federici. 2001. Immobilized cell technology applied in solubilization of insoluble inorganic (rock) phosphates and P plant acquisition. Bioresearch Technology 79: 263-271. http://dx.doi.org/10.1016/S0960-8524(01)00017-7. [ Links ]
Vyas, P., P. Rahi, A. Chauhan and A. Gulati. 2007. Phosphate solubilization potential and stress tolerance of Eupenicilium parvum from tea soil. Mycological Research 111: 931-938. DOI: 10.1016/j.mycres.2007.06.003. [ Links ]
Welch, S., A.E. Taunton and J.F. Banfiled. 2002. Effect of microorganisms and microbial metabolites on apatite dissolution. Geomicrobiology Journal 19: 343-367. DOI: 10.1080/01490450290098414. [ Links ]
Whitelaw, M.A. 2000. Growth Promotion of plants inoculated with phosphate-solubilizing fungi. Advances in Agronomy 69: 99-151. DOI: 10.1016/S0065-2113(08)60948-7. [ Links ]
Whitelaw, M.A., T.J. Harden and K.R. Helyar. 1999. Phosphate solubilisation in solution culture by the soil fungus Penicillium radicum. Soil Biology and Biochemistry 31: 655-665. DOI: 10.1016/S0038-0717(98)00130-8. [ Links ]