Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad Nacional de Agronomía Medellín
Print version ISSN 0304-2847
Rev. Fac. Nac. Agron. Medellín vol.68 no.2 Medellín July/Dec. 2015
https://doi.org/10.15446/rfnam.v68n2.50982
A coating of chitosan and propolis extract for the postharvest treatment of papaya (Carica papaya L. cv. Hawaiiana)
Un Recubrimiento de quitosano y extracto de propóleos para el manejo poscosecha de la papaya (Carica papaya L. cv. Hawaiiana)
Elizabeth Barrera1; Jesús Gil2; Ana Restrepo3; Kelly Mosquera4 and Diego Durango5
1Magister in Sciences and food technology. Universidad Nacional de Colombia - Facultad de Ciencias Agrarias. Calle 59A 63-20, Autopista Norte, Medellín-Colombia.<my_tiza@hotmail.com>
2 Associate Professor. Universidad Nacional de Colombia - Facultad de Ciencias Agrarias. Calle 59A 63-20, Autopista Norte, Medellín-Colombia.<jhgilg@unal.edu.co>
3 Chemical Engineer. Universidad Nacional de Colombia - Facultad de Minas. Carrera 80 No 65-223, Medellín-Colombia. <anamaria2416@hotmail.com>
4 Chemical Engineer. Universidad Nacional de Colombia - Facultad de Minas. Carrera 80 No 65-223, Medellín-Colombia.<kjmosque@gmail.com>
5 Associate Professor. Universidad Nacional de Colombia - Facultad de Ciencias. Calle 59A 63-20, Autopista Norte, Medellín-Colombia. <dldurango@unal.edu.co>
Received: January 30, 2015; Accepted: March 25, 2015.
DOI: http://dx.doi.org/10.15446/rfnam.v68n2.50982
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Abstract. Propolis is a natural antimicrobial that can be used as a bioadditive in coatings to control fruit quality losses. The effect of two coatings was evaluated, a control (chitosan, 1%) and a treatment (chitosan, 1%; containing propolisethanolic extract, 5%), on the microbiological and physicochemical properties of papaya fruits. The chemical profile of the propolis revealed the presence of fatty acids and their esters, carbohydrates, diterpenic acids, and pentacyclic triterpenes. The fruits covered with the treatment demonstrated a reduced deterioration index and infection diameter of the fungus Colletotrichum gloeosporioides, as compared to the control papayas, postponing the appearance of damage by two days. Additionally, the treatment did not significantly affect the physicochemical properties of the papaya, as compared to the control. In conclusion, the coating formulated with propolis exhibited an in situ fungicidal and bactericidal effect without altering the physiological changes of the papaya fruit during storage.
Key words: Chitosan, C. gloeosporioides, composition, decay rate, postharvest, propolis.
Resumen. El propóleo es un antimicrobiano natural que puede ser usado como bioaditivo en recubrimientos para controlar pérdidas en la calidad de frutas. Se evaluó el efecto de dos recubrimientos, control (quitosano, 1%) y tratamiento (quitosano, 1%; conteniendo extracto etanólico de propóleos, 5%), sobre las propiedades microbiológicas y fisicoquímicas de frutos de papaya. El perfil químico del propóleo reveló la presencia de ácidos grasos y sus ésteres, carbohidratos, ácidos diterpénicos, y triterpenos pentacíclicos. Los frutos recubiertos con el tratamiento mostraron un índice de deterioro y un diámetro de la infección del hongo Colletotrichum gloeosporioides inferior al de las papayas control, extendiéndose en dos días la aparición de daños. Adicionalmente, el tratamiento no afectó significativamente las propiedades fisicoquímicas de la papaya, en comparación con el control. En conclusión, el recubrimiento formulado con propóleos exhibió un efecto fungistático y bacteriostático in situ, sin alterar los cambios fisiológicos de los frutos de papaya durante el almacenamiento.
Palabras claves: Quitosano, C. gloeosporioides, composición, índice de decaimiento, poscosecha, propóleos.
Papaya (Carica papaya L.) is an important fruit for domestic and export markets in tropical and subtropical regions. Unfortunately, papaya is a climacteric fruit that is highly perishable and susceptible to attacks from pathogenic microorganisms, resulting in large losses during storage. The disease that causes the largest amount of deterioration in fruits during postharvest periods is anthracnose (a causal agent of the fungus Colletotrichum gloeosporioides). The symptom of the disease is manifested by sunken stains that are round and watery on the surface of mature fruits, affecting the appearance and causing considerable economic losses. Traditionally, this disease has been controlled through the application of synthetic chemical fungicides on the crop and during storage, and their repeated use has caused resistance in microorganisms and toxicity for humans.
In the postharvest period, the preservation of papaya can be maintained for a maximum period of 2 to 4 weeks at between 8-10ºC or 5 to 7 days at 22ºC (Paull et al., 1997). In order to prolong the shelf-life of papaya fruits, several preservation methods have been evaluated, such as thermal treatments (Jiménez, 2002), storage in modified atmospheres (González et al., 2003), treatments with watery plant extracts (Bautista et al., 2003) or sodium bicarbonate solutions (Gamagae et al., 2003), and edible coatings (Bautista et al., 2006; Hamzah et al., 2013). In the latter method, chitosan has recently been used for the preservation of papaya (Brasil et al., 2012; Ali et al., 2011). Chitosan (poly-ß(1,4)-2-amino-2-deoxy-D-glucopyranose) is a cationic biopolymer with the ability to form semi-permeable films that regulate the gaseous exchange, reduce water loss, lower the production of ethylene, and retard maturation (Bautista et al., 2003; 2006). Furthermore, these coatings are being used for the incorporation and/or controlled release of antioxidants, nutraceutics, and natural antimicrobial agents (Elsabee and Abdou, 2013).
In recent times, the use of biologically active natural products has received a lot of attention for controlling decay and prolonging the storage life of fruits and vegetables because they have the potential to replace synthetic fungicides and also possess a higher consumer acceptance (Tripathi and Dubey, 2004). Propolis, a resinous substance used by bees (Apismellifera L.) to protect the hive against pathogens (Kasote et al., 2015), has demonstrated a large potential for inhibiting the growth of phytopathogenic microorganisms in vitro, including B. cinerea, P. expansum (Tripathi and Dubey, 2004) and C. gloeosporioides (Mattiuz et al., 2015). However, studies that have used propolis as an active agent in the formulation of coatings for the preservation of fruits are scarce (Barrera et al., 2012; Pastor et al., 2011). Therefore, the objective of the present study was to evaluate the effect of a coating formulated with chitosan and containing a ethanolic extract of propolis (EEP) at 5% w/v on the microbiological and physicochemical quality attributes of Hawaiiana papaya fruits (Carica papaya L. cv. Hawaiana) stored at a temperature of 25±3ºC and a relative humidity of 65 to 70%.
MATERIALS AND METHODS
Propolis. The sample was obtained from the Llanta Azul apiary in the municipality of Zaragoza (Antioquia-Colombia). The EEP was produced following the procedure described by Rodríguez et al. (2012).
Chemical characterization of the EEP. The chemical characterization of the EEP was carried out by gas chromatography-mass spectrometry (GC-MS) with a Varain 3800 gas chromatographer equipped with a Saturn 2000 mass detector (ion trap type). For the separation, a HPS-MS column was employed (30 m x 0.25 mm x 0.25 μm, Agilent Technologies). The propolis sample was analyzed after its derivation (methylation) using the method described by Markham et al. (1996); an aliquot of 400 μL (10 mg mL-1) of the EEP was combined with a solution of CH2N2 (400 μL) in a glass vial and the resulting mixture was refrigerated for 4 hours to allow for complete methylation. A 1 μL volume of the sample was injected and analyzed through GC-MS. The initial temperature of the column was 100 °C, which was brought to 280°C and maintained for 15 min, for a total analysis time of 45 min. The injection was carried out in a splitless mode at 250°C. Helium was used as a carrier gas at a flow of 1 mL min-1. The peaks were identified through comparison with data in the literature and Nist02 database.
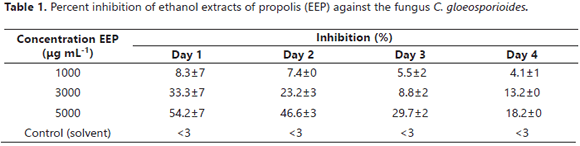
In vitro antifungal activity of the EEP. The method of contaminated food was used. The C. gloesporioides strain was donated and morphologically characterized by the Laboratorio de Sanidad Vegetal of the Universidad Nacional de Colombia. The methodology of Palomino et al. (2010) was used for the evaluations. The final EEP concentrations of 1000, 3000 and 5000 µg mL-1 were evaluated.
Vegetative material. The 240 analyzed papayas (Carica papaya L. cv. Hawaiana) were acquired from a local market; 120 papayas were selected for each treatment, which had a maturation index of 3 (green color with yellow traces in 50% of the total area), free of physical damage and fungal infections, with a uniform color and size. The fruits were washed (with water), disinfected (sodium hypochlorite solution at 150 ppm for 10 min) and dried (at room conditions) with subsequent coating (González et al., 2003; Ali et al., 2011).
Preparation and application of the coatings. Two coatings were formulated: the first treatment contained chitosan (1% w/v) dissolved in an acetic acid solution with the additives Span 60 and Tween 80; subsequently, the EEP was added to obtained a final concentration of 5% (w/v); the second coating (control) was prepared in the same manner except distilled water was used instead of EEP. The coatings were applied to the fruits manually and the fruits were left to dry at room temperature and then stored 8 or 9 days at a temperature of 25±3ºC and a relative humidity (RH) between 65 to 70%.
Evaluations of the effect of the coatings
Weight loss, changes in the color and firmness. The weight loss: seven fruits were registered at the start of storage and daily for 8 days. The obtained results were expressed as a percentage of weight loss in relation to the initial weight (Pérez et al., 2002). Changes in the color: seven fruits were used for each treatment. In each fruit, 3 reference points were marked on the equatorial axis. The measurements were taken with a spectrocolorimeter (XRITE) and the data were reported in values of L*a*b* of the CIELAB scale (Santamaría et al., 2009). Firmness: fruit firmness was measured on eight fruits per treatment. The test was carried out every three days in 3 equidistant points on the equatorial axis in order to establish the maximum rupture force of the peel and the mean force of the pulp. The data were obtained using a TA.XT2 texturometer (Stable Micro Systems) with a 2 mm diameter cylinder that was 3 cm in length, a penetration speed of 2 mm s-1, a penetration depth of 10 mm and a cell of 50 kg (Hamzah et al., 2013); the values were reported in Newton (N).
Soluble solids, titratable acidity and pH. Every 3 days, during 9 days of storage, pulp was taken from five fruits, which was analyzed for the soluble solids content (SSC), pH, and titratable acidity (TA), expressed as a percentage of citric acid (AOAC 932.12, 2000; AOAC 942.15, 2000).
Content of total polyphenols, monomeric anthocyanins, caroteniods, chlorophyll a and b. The contents of the compounds were quantified every 3 days, with two replications with three repetitions each. All of the readings were obtained in a double-beam spectrophotometer (Shimadzu UV-1800). Extraction: 15.0 g of papaya peel were extracted with cold acetone at 80% (50 mL) for 5 min. The suspension was centrifuged at 9000 rpm at 4 °C for 15 min (HettichZentrifugen 320 R) and the supernatant was filtered using Whatman Nº1 filter paper. The precipitant was diluted with 35 mL of cold acetone at 80%, homogenized (Ultra-Turrax IKA T25 D S1) for 3 min, centrifuged and filtered. The solvent was evaporated in a rotaevaporator (IKA RV 10D) at 38±2 °C for a final volume of 25 mL (Wolfe et al., 2003). Total polyphenols: the total polyphenol content in the acetone extract was determined by the Folin-Ciocalteau colorimetric method (Pavan et al., 2014) and expressed as equivalents of gallic acid (mg gallic acid (GA) g-1 of sample). Monomeric anthocyanins: the concentration of anthocyanins in the acetone extract was determined by the pH differential method (Wrolstad et al., 2005). The content of anthocyanins was expressed as the total of monomeric anthocyanins (mg 100 g-1). Carotenoids and chlorophyll a and b: the readings were taken at 662 nm for chlorophyll a (Ca), 645 nm for chlorophyll b (Cb) and 470 nm for the total carotenoids (TC). The equations of Lichtentaler and Wellburn were employed to calculate the compounds concentration (Dere et al., 1998).
Deterioration index. 20 fruits were used per treatment for the qualitative analysis using daily visual inspection during eight days of storage. For the deterioration grade, the peel hydration and damage, mechanical and/or caused by fungi, were considered. A hedonic scale was used: 1=No damage, 2=low. 3=moderate, and 4=severe. The scores were considered as follows: a) low: area of the damaged surface was less than 10%; b) moderate: area of the damaged surface was 15%; and c) severe: affected area over 20%. Subsequently, the results were quantified using the following equation: 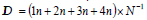 ,where n is the number classified in each level of the hedonic scale and N is the total of the analyzed fruits in each treatment per day (Pérez et al., 2002).
,where n is the number classified in each level of the hedonic scale and N is the total of the analyzed fruits in each treatment per day (Pérez et al., 2002).
Microbiological tests. PetrifilmTM plates were used as a culture medium for the count of the aerobic mesophilic (AM), mold and yeast (MY) at 3, 6, and 9 days of storage (AOAC 990.12, 2000).
Fruit Inoculation. A Colletotrichum gloeosporioides strain was incubated at 26±2°C for 8 days. Next, the Petri dish was washed with a sterile saline solution that contained a tensoactive agent; the spore count was carried out for the suspension in a hemocytometer, obtaining a concentration of the inoculum of 2.6 x 106 spores mL-1 (López et al., 2006). The washed, disinfected, and dried papayas were treated with the respective coating (control and treatment); subsequently, the fruits were artificially inoculated through a puncture made using a sterile 2 mm diameter needle at a depth of 2 mm with 100 µL of the spore solution and the surface of the fruits was left to dry at room conditions. The papayas were stored at 25±3ºC and a RH of 65 to 70% for 8 days. The diameter of the injury caused by the mold was measured daily (Bolívar et al., 2009).
Statistical analysis. The program Statgraphics Centurion V.I was used, carrying out a multifactor variance analysis, ANOVA, and Tukey test with a confidence level of 95%; the statistical differences were determined with P<0.05.
RESULTS AND DISCUSSION
Chemical characterization of the EEP. The chemical composition of propolis is complex and varies in accordance with its botanical and geographical origin. The analysis of the chemical composition of the EEP collected in Bajo Cauca antioqueño (Colombia) was carried out with GC-MS. A total of 55 components were tentatively identified based on mass fragmentation spectra. The principal compounds of the propolis were: fatty acids (~62.8%; lauric, myristic, pentadecanoic, oleic, heptadecanoic, palmitic, stearic, linoleic), carbohydrates and derivatives (~19.2%; D-glucose, D-ribose, D-galactose, D-manitol), tetra- and pentacyclic triterpenes (~5.1%; lanosterol, lupeol, α-amyrin, lupeol acetate), cycloartane-type triterpenes (~0.5%; cycloartenol, cycloartenol acetate), mono- (~1.7%; p-cymene, trans-pinane), sesqui- (~0.5%; spathulenol), and diterpenes (~0.5%; torulosol, abietic, agathic, and agatholic acids), aromatic acids and esters (~1.5%; benzoic acid and derivatives, cinnamic acid and derivatives), anacardic acids (~0.4%; methyl ester of the acid 2-(12-heptadecenyl)-6-methoxybenzoic, 4-methoxy 2-stearoyl phenol, 2-methoxy-6-(8-pentadecenyl) benzoic), phytol (0.1%), and flavonoids (0.4%; retusin), among others.
The chemical composition analysis of the propolis revealed a high content and diversity of long-chain aliphatic acids and a low content of aromatic acids. It has been generally accepted that propolis from temperate zones (North America, Europe, and non-tropical regions of Asia) and whose botanical source are principally poplar trees (Populus spp., Salicaceae) is rich in aromatic acids and their esters and flavonoids (Bankova et al., 2002). Propolis from tropical regions, on the other hand, is rich in another class of metabolites. As such, green propolis from Brazil (from Baccharis dracunculifolia) and yellow propolis from Cuba have an elevated proportion of terpenes (Márquez et al., 2010). The results showed that the analyzed propolis presented an aliphatic acid content that was much higher than that reported in other propolis from tropical countries (Custodio et al., 2003). The high proportion of long-chain aliphatic acid and their esters could come from a bad collection of propolis (combined with bee wax). Furthermore, an elevated content of carbohydrates was seen. Similar to that seen in propolis from the Canary Islands, the high proportion and diversity of sugars, including disaccharides, indicate a mucilage origin (Bankova et al., 1998).
In green propolis from Brazil, the presence of labdane and cycloartane diterpenes has been detected, with the botanical origins of Baccharis dracunculifolia (Asteraceae) and Mangifera indica L. (Anacardiaceae) (Park et al., 2004), respectively. These metabolites have also been found in propolis in Greece, Croatia, Malta, China, and Colombia (Melliou and Chinou, 2004; Meneses et al., 2009; Popova et al., 2009; Popova et al., 2010; Miguel and Antunes, 2011). Diterpenic acids (abietic, dehydroabietic) have been reported in propolis from Turkey and Greece (Popova et al., 2005; Miguel and Antunes, 2011). In propolis from Brazil, Thailand, and Cameroon, cardanols (alquil phenols) and anacardic acids, with plant origins from the Anacardiaceae family, have been encountered (Boonsai et al., 2014). Pentacyclic triterpenes have been reported for propolis from Cuba, Cameroon, and Egypt (Cuesta et al., 2007; Sakava et al., 2014).
The antimicrobial activity of propolis has been attributed to the presence of different classes of chemical compounds. Among the identified contributors in EEP, there are anacardic acids, diterpenic acids (Bankova et al., 1996; Trusheva et al., 2011), and pentacyclic triterpenes. Anacardic acids have not been previously reported in Colombian propolis and their presence may have been due to the existence of M. indica L. in the area surrounding the apiary. These compounds possess recognized antimicrobial properties (Trusheva et al., 2011). Terpenic acids (abietic) can come from conifer species (especially Pinus sp.), as has been proposed for samples from Greece (Melliou and Chinou, 2004). The labdane type (agathic and agatholic) has been reported in green propolis from Brazil and the Mediterranean and the botanical origins are Araucaria species and conifers from the Cupressaceae family, respectively (Bankova et al., 1996; Popova et al., 2010). This class of diterpenes possesses an antimicrobial activity (Bankova et al., 1996; Meneses et al., 2009). The activity of pentacyclic triterpenes has also been demonstrated (Kardar et al., 2014). In our analysis, the presence of amirine, lupeol, cycloartenol and their respective acetates was detected.
In vitro antifungal activity of the EEP. The EEP presented fungicidal activity against the mold C. gloeosporioides. Table 1 demonstrates that the inhibition increased with the concentration of the EEP and decreased over the time of the evaluation. The highest inhibition was seen during the first day with 5000 µg mL-1 with a decrease to 18.2% on the fourth day. The in vitro antifungal activity agreed with the results of Barrera et al. (2012) and Meneses et al. (2009), who established the potential of EEP as an antimicrobial agent against the mold of the Colletotrichum sp genus. This behavior reflects the potential of the extract as an antimicrobial ingredient, despite the apparent mechanism of detoxification by the microorganism.
Microbiological and physicochemical characterization of the coated fruits
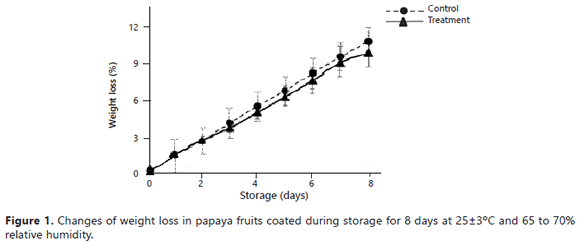
Physicochemical parameters of coated fruits. The fruits presented a weight loss throughout the storage period (Figure 1), reaching maximum values of 9.75% (treatment) and 10.68% (control).
Nevertheless, significant differences were not observed between the control and treatment (P>0.05).
The weight loss appeared to be the principal determining factor for the storage life and the quality of the papaya (Ali et al., 2011).The weight change was principally due to the elimination of water caused by the processes of transpiration and respiration of the fruits (Hernández et al., 2006). Possibly, the hydrophobic characteristic of the propolis resulted in a lower water reduction through transpiration. The obtained values agreed with the results reported by Ali et al. (2011), who reported weight losses in papaya cv Eksotika II coated with chitosan at 1%, with values close to 8% for 5 weeks of storage. In a previous study, papaya fruits coated with wax and propolis extract showed weight losses of 15% after 8 days of storage (Barrera et al., 2012).
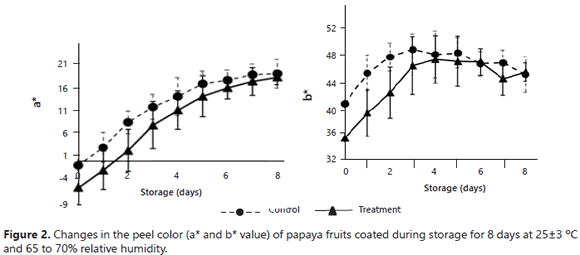
Color is one of the more important sensorial attributes in papaya fruits. In the epidermis of the fruits, color changes were observed from green to yellow, independent of the applied coating (Figure 2).
On day 0, the a*/b* values for the treatment and control fruits were -5.42/35.48 and -0.80/41.38, respectively; at the end of the storage (day 8), similar a*/b* values were observed (17.46/46.80: treatment and 18.20/46.37: control), indicating a complete maturation of the fruits in both cases. However, significant differences (P<0.05) were observed in the first 3 days of storage, revealing a slower development in the treatment fruits. The color changes (green to yellow) occurred with the degradation of the green chlorophyll pigments, which, when disappearing, uncovered the characteristic pigments (carotenoids, anthocyanins, among others) present in the tissue of mature fruits (orangish and reddish yellow colors) (Barreiro and Sandoval, 2006). The color changes obtained in this study are comparable with those seen by Santamaría et al. (2009), who observed a*/b* values (15 days after harvest) of 15/55 in mature fruits when evaluating the quality of maradol papayas in the postharvest period.
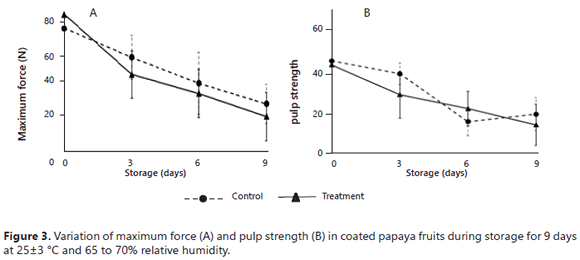
Figure 3 shows the firmness changes of the papaya fruits. There were no significant differences between the applied coatings (P>0.05). During the beginning of the storage period, the fruits presented sufficiently smooth, uniform, and firm peels (75.14 control and 83.65 N treatment); subsequently, the fruits acquired a wrinkled and bland texture (28.90 control and 21.13 N treatment). The pulp also changed texture during storage, with firmness values changing from 44.92 to 19.54 N (control) and from 43.02 to 14.42 N (treatment), indicating that the pulp went from having a firm and hard texture to having a smooth and bland texture. The changes of texture of the papaya fruits were due to the fact that chitosan-based coatings have selective barriers for O2 and CO2, producing an increase in the total soluble solids content and a decrease in the respiration rate of fresh fruits. These facts are closely related to water loss and, consequently, to reductions in the firmness of the papayas (Hernández et al., 2006).The texture loss of the papayas was related to the dehydration of the fruits, decreasing the turgency and producing blandness and dryness. Furthermore, it can be explained by the action of the enzyme pectinase on the pectin that is found in the papaya peel and by the hydrolysis of starch or fats, generating a bland and smooth texture.
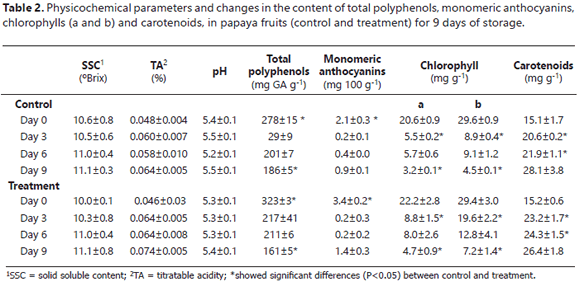
Table 2 contains the results of the physicochemical parameters. The SSC, TA and pH values did not present significant differences (P>0.05) between the control and the treatment. The SSC and the acidity of the fruits increased over time for both coatings. Nevertheless, the papaya fruits had low acidity contents. The pH values confirmed that the papaya had a low degree of acidity, independent of the applied coating.
The variation in the SSC was possibly due to the increase in the metabolism of starch, which produced mono- and disaccharides, leading to the fruits having a sweeter flavor in the state of higher maturation and a disappearance of the astringent compounds, as well as tannins (Barreiro and Sandoval, 2006). The SSC values agreed with those reported by Santamaría et al. (2009), who, when evaluating the quality of maradol papaya, found values between 10.47 and 12.45 °Brix for the two maturation states. Similarly, Ali et al. (2011) found soluble solids contents close to 11°Brix in Eksotika II papaya coated with chitosan at 0.5% and stored in a refrigerator for 4 weeks. The TA and pH values were similar to those reported by Barrera et al. (2012), who evaluated physicochemical characteristics of papaya fruits coated with wax + EEP and reported a TA between 0.07 and 0.09% and a pH between 5.23 and 5.35. In addition, a slight decrease was observed in the percentage of acidity in the papaya on the sixth day of the storage period for the control and the treatment, resulting from the reduction of the organic acid content in the maximum maturation state (Hernández et al., 2006).
The reports in the literature are scare for the content of polyphenols in papaya fruit peel in relation to maturation. In the present study, for both coatings, higher total polyphenol contents were seen in the fruits with a maturation state of 2 (day 0) in comparison with the mature fruit (day 9). Some authors have reported that caffeic, ferulic and p-coumaric acids, free and esterificated, are generally the most abundant phenolic acids in fruits and vegetables and represent between 75% and 100% of the total hydroxycinnamic acids of the majority of fruits, including papaya (Schweiggert et al., 2011). Although phenolic acids are found in all of fruit, the highest concentration has been observed in the external tissue, protecting the plant from solar radiation. The other hand, the lowest concentration of polyphenols is found in mature fruits (186 mg of gallic acid g-1 of the control, 161 mg of gallic acid g-1 of the treatment). Nevertheless, these values were higher than those reported in mature papaya pulp, 54 mg of gallic acid g-1 of pulp (Patthamakanokporn et al., 2008). On day 0, the fruits coated with EEP presented the highest concentration of polyphenols, which are related to antimicrobial activity (Santos et al., 2003).
During storage, the fruits decreased their contents of chlorophyll a and b (pigments of green fruits) and of anthocyanins due to the maturation process of the fruits. On the same day, the treatment fruits presented a higher content of anthocyanins in comparison with the control, indicating a possible retardation of maturity. In addition, the treatment fruits demonstrated a higher concentration of chlorophylls at the end of storage, compared to the control fruits, confirming that the fruits covered with EEP presented a slower pigmentation metabolism. The content of carotenoids presented a growth behavior during the storage period with values of 15.1 (control) and 15.2 mg g-1 (treatment) for the green fruits and 28.1 (control) and 26.4 mg g-1 (treatment) for the mature fruits. Santamaría et al. (2009) found chlorophyll contents of 6.5 mg g-1 and total carotenoid contents of 13.1 mg g-1 (in green papaya peels) and 67 mg g-1 (in mature fruits).
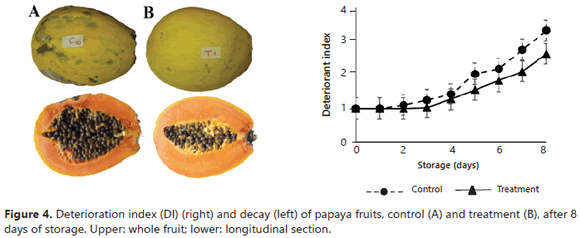
Deterioration index (DI). The principal observed damage in the coated fruits were: black stains, stem and fruit rot, fungus formation, surface lesions, dehydration, and coffee-colored stains that are characteristic of anthracnose. Figure 4 (left) contains two fruits from day 8 of the storage with their respective cuts, evidencing a higher damage in the control fruits; for example, upper peel dehydration. Further development of fungi was also evident. On the stem, symptoms of the development of Colletotrichum gloeosporioides (causal agent of anthracnose) were observed, along with a higher deformation of the pulp. Additionally, in Figure 4 (right), the results obtained for the DI of the control and treatment fruits stored for 8 days can be seen. An increase in the DI was observed during the storage period. The control fruits presented a more rapid decay and more severe damage; in addition, the treatment fruits were damage-free until day 4, while the control was only damage-free until day 2. At the end of the storage, the treatment fruits presented moderate damage, evidencing a higher resistance to deterioration compared to the control fruits. Besides, during the entire storage period, the DI of the treatment fruits was lower than the calculated values for the control, demonstrating the fungicidal and bactericidal effect of propolis. Similarly, starting at day 5, there were significant differences (P<0.05) between the treatments, with higher deterioration in the control fruits. These results were better than those previously found for papayas coated with waxes and EEP under the same conditions (Barrera et al., 2012); probably, there is a synergistic antimicrobial effect between EEP and chitosan. Torlak and Sert (2013) demonstrated that chitosan-coated films exhibited a broad-spectrum antibacterial activity and the incorporation of EPP to coatings at 10% (propolis resin/chitosan) enhanced the antibacterial activity against all of the tested pathogens.
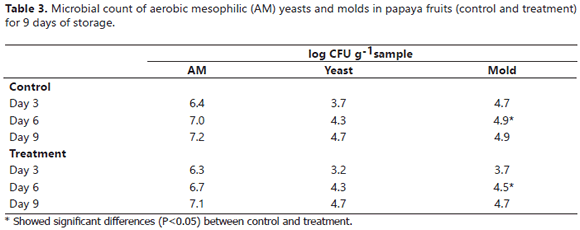
Table 3 represents the variation of the aerobic mesophyll, yeast and mold counts. A growth behavior was observed for the microbial load during the storage period (9 days); however, there were no significant differences (P>0.05) between the two coatings, except on day 6 when a significant reduction was observed for the number of the CFU of molds in the treatment fruits, evidencing the fungicidal effect of EEP. For the other microorganisms, the CFU count was always lower in the treatment fruits. These results agreed with those reported for the DI (Figure 4), in which a higher microbiological damage was observed in the control fruits during the storage period.
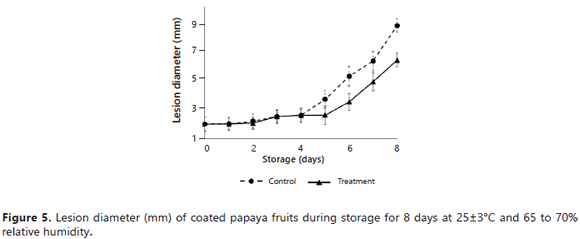
Inoculation. The diameters of the lesions of the fruits inoculated with spores of the fungus (2.6 x 106 CFU mL-1) C. gloeosporioides are presented in Figure 5. There were no significant differences during the first 5 days of storage. The control fruits, on day 3, evidenced the presence of the fungus with small gray points, which grew rapidly. In contrast, the treatment fruits demonstrated a growth incidence of the fungus only starting on day 5. Subsequently, in the control and the treatment, an accelerated-growth lesion was observed, reaching diameters on the eight day of 8.6 and 6.4 mm, respectively. These results were related with the values obtained in the decay index; wherein, starting on day 6, the EEP effect was considerably reduced, leaving the fruits exposed to microbial damage again.
Unlike the control fruits, the fungus in the treatment fruits did not completely development; in particular, sporulation did not occur. However, over time, growth was seen for the fungus and the lesion. This fact was possibly due to the fungicidal action of the propolis in combination with chitosan, despite the fact that the latter was found at low concentrations (Bautista et al., 2006). The inoculation results were related with those for DI, wherein the control fruits presented decay starting on day 2 and the deterioration was higher in the treatment fruits. According to the obtained microbiological results, EEP can be considered viable for use as an active principal for incorporation in food coatings or in natural agrochemical products for the preservation of papaya fruits because it inhibits the development of microorganisms during the first days of storage.
CONCLUSIONS
The coating formulated with the propolis extract exhibited in situ fungicidal and bactericidal effects without altering the physicochemical properties of the papaya fruits. This suggests the possibility of using this class of coatings for the improvement of sanitary characteristics of papaya fruits in the postharvest period. However, additional optimization studies are needed for chitosan coatings that contain propolis as a natural preservative.
ACKNOWLEDGEMENTS
The authors acknowledge the Dirección Nacional de Investigaciones of the Universidad Nacional de Colombia and the Ministerio de Agricultura y Desarrollo Rural de Colombia for grant project 003-2008C3783-3453.
REFERENCES
Ali, A., M.T.M. Muhammad, S. Kamaruzaman and S. Yasmeen. 2011. Effect of chitosan coating on the physicochemical characteristics of Eksotika II papaya (Carica papaya L) fruit during cold storage. Food chemistry 124(2): 620-626. DOI: 10.1016/j.foodchem.2010.06.085 [ Links ]
AOAC, 2000. Official Methods of Analysis, 17th ed. Association of Official Analytical Chemists, Washington DC, USA. [ Links ]
Bankova, V., M.C. Marcucci, S. Simova, N. Nikolova, A. Kujumgiev and S. Popov. 1996. Antibacterial diterpenic acids from Brazilian propolis. ZeitschriftfürNaturforsch C 51(5-6): 277-280. DOI: 10.1016/S0031-9422(98)00108-3
Bankova, V., R.S. Christov and A.D. Tejera. 1998. Lignans and other constituents of propolis from the Canary Islands. Phytochemistry 49(5): 1411-1415. [ Links ]
Bankova, V., M. Popova, S. Bogdanov and A.G Sabatini. 2002. Chemical composition of European propolis: expected and unexpected results. Zeitschriftfür Naturforsch C 57(5-6): 530-533. [ Links ]
Barreiro, J.A. y A. Sandoval. 2006. Operaciones de conservación de alimentos por bajas temperaturas. Ed. Equinoccio, Baruta, Miranda, Venezuela. 365 p. [ Links ]
Barrera, E., M. Gil, C.M García, D.L. Durango y J.H. Gil. 2012. Empleo de un Recubrimiento Formulado con Propóleos para el Manejo Poscosecha de Frutos de Papaya (Carica papaya L. cv. Hawaiana. Revista Facultad Nacional de Agronomía Medellín 65(1): 6497-6506. [ Links ]
Bautista, S., A.N. Hernández, M.G. Velázquez del Valle, M. Hernández, E. AitBarka, E. Bosquez and C.L Wilson. 2006. Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Protection 25(2): 108-118. DOI: 10.1016/j.cropro.2005.03.010 [ Links ]
Bautista, S., M. Hernández, E. Bosquez and C.L. Wilson. 2003. Effects of chitosan and plant extracts on growth of Colletotrichum gloeosporioides, anthracnose levels and quality of papaya fruit. Crop Protection 22(9): 1087-1092. DOI: 10.1016/S0261-2194(03)00117-0 [ Links ]
Bolívar, K., M.E. Sanabria, D. Rodríguez, D. Ulacio, M. Camacaro, M. Pérez de Camacaro, L.J. Cumana y O. Crescente. 2009. Calidad poscosecha en frutos de mango (Mangifera indica L.) inoculados con Colletotrichum gloeosporioides y tratados con extractos vegetales. Revista científica UDO Agrícola 98(1): 41-50. [ Links ]
Boonsai, P., P. Phuwapraisirisan and C. Chanchao. 2014. Antibacterial activity of a cardanol from Thai Apis mellifera propolis. International Journal of Medical Sciences 11(4): 327-336. DOI: 10.7150/ijms.7373 [ Links ]
Brasil, I.M., C. Gomes, A. Puerta, M.E. Castell and R.G. Moreira. 2012. Polysaccharide-based multilayered antimicrobial edible coating enhances quality of fresh-cut papaya. Food Science and Technology 47(1): 39-45. DOI: 10.1016/j.lwt.2012.01.005 [ Links ]
Cuesta, O., A.L. Piccinelli, M.C. Fernandez, I.M. Hernández, A. Rosado and L. Rastrelli. 2007. Chemical characterization of Cuban propolis by HPLC-PDA, HPLC-MS, and NMR: the brown, red and yellow Cuban varieties of propolis. Journal of Agricultural and Food Chemistry 55(18): 7502-7509. DOI: 10.1021/jf071296w [ Links ]
Custodio, A.R., M.M.C. Ferreira, G. Negri and A. Salatino. 2003. Clustering of comb and propolis waxes based on the distribution of aliphatic constituents. Journal of the Brazilian Chemical Society 14(3): 354-357. DOI: 10.1590/S0103-50532003000300003 [ Links ]
Dere, S., T. Günes and R. Sivaci. 1998. Spectrophotometric determination of chlorophyll - a, b and total carotenoid contents of some algae species using different solvents. Turkish Journal of Botany 22(1): 13-17. [ Links ]
Elsabee, M.Z. and E.S. Abdou. 2013. Chitosan based edible films and coatings: A review. Materials Science and Engineering C 33(4): 1819-1841. DOI: 10.1016/j.msec.2013.01.010 [ Links ]
Gamagae, S.U., D. Sivakumar, R.S. Wilson Wijeratnam and R.L.C. Wijesundera. 2003. Use of sodium bicarbonate and Candida oleophila to control anthracnose in papaya during storage. Crop Protection 22(5): 775-779. DOI: 10.1016/S0261-2194(03)00046-2 [ Links ]
González, G.A., J.G. Buta and C.Y Wang. 2003. Methyl jasmonate and modified atmosphere packaging (MAP) reduce decay and maintain postharvest quality of papaya 'Sunrise'. Postharvest Biology and Technology 28(3): 361-370. DOI: 10.1016/S0925-5214(02)00200-4 [ Links ]
Hamzah, H.M., A. Osmana, C.P. Tan and F.M. Ghazali. 2013. Carrageenan as an alternative coating for papaya (Carica papaya L. cv. Eksotika). Postharvest Biology and Technology 75(1): 142-146. DOI: 10.1016/j.postharvbio.2012.08.012 [ Links ]
Hernández, P., E. Almenar, M.J. Ocio and R. Gavara. 2006. Effect of calcium dips and chitosan coatings on postharvest life of strawberries (Fragaria x ananassa). Postharvest Biology and Technology 39(3): 247-253. DOI: 10.1016/j.postharvbio.2005.11.006 [ Links ]
Jiménez, J. 2002. Manual práctico para el cultivo de la papaya hawaiana. Ed. Earth, Guácimo, Limon, Costa Rica. [ Links ]
Kardar, M.N., T. Zhang, G.D. Coxon, D.G. Watson, J. Fearnley and V. Saidel. 2014. Characterisation of triterpenes and new phenolic lipids in Cameroonian propolis. Phytochemistry 106(1): 156-163. DOI: 10.1016/j.phytochem.2014.07.016 [ Links ]
Kasote, D., A. Ahmad, W. Chen, S. Combrinck and A. Viljoen. 2015. HPTLC-MS as an efficient hyphenated technique for the rapid identification of antimicrobial compounds from propolis. Phytochemistry Letters 11(1): 326-331. DOI: 10.1016/j.phytol.2014.08.017 [ Links ]
López, A., M. Vélez, M. Sánchez, C. Bonilla y P. Gallo. 2006. Evaluación de extractos vegetales para manejo de hongos patógenos en banano y fresa almacenados. Acta Agronómica 55(4): 39-44. [ Links ]
Markham, K.R., K.A. Mitchell, A.L. Wilkins, J.A. Daldy and Y. Lu. 1996. HPLC and GC-MS identification of the major organic constituents in New Zealand propolis. Phytochemistry 42(1): 205-211. DOI: 10.1016/0031-9422(96)83286-9 [ Links ]
Márquez, I., O. Cuesta, M. Campo, A. Rosado, R. Montes-de Oca Porto, A.L. Piccineli and L. Rastrelli. 2010. Studies on the constituents of yellow Cuban propolis: GC-MS determination of triterpenoids and flavonoids. Journal Agricultural and Food Chemistry 58(8): 4725-4730. DOI: 10.1021/jf904527n [ Links ]
Melliou, E. and I. Chinou. 2004. Chemical analysis and antimicrobial activity of Greek propolis. Planta Medica 70(6): 515-519. DOI: 10.1186/1752-153X-5-33 [ Links ]
Mattiuza, B.H., M.N. Ducamp, C.F. Machado, C. Vigneault, K. Magalhães, W. Sagouab and D. Montet. 2015. Effect of propolis on postharvest control of anthracnose and quality parameters of 'Kent' mango. Scientia Horticulturae 184(1): 160-168. DOI: 10.1016/j.scienta.2014.12.035 [ Links ]
Meneses, E.A., D. Durango and C.M. García. 2009. Antifungal activity against postharvest fungi by extracts from Colombian propolis. Quimica Nova 32(8): 2011-2017. DOI: 10.1590/S0100-40422009000800006 [ Links ]
Miguel, M.G.and M.D. Antunes. 2011. Is propolis safe as an alternative medicine? Journal of Pharmacy and Bioallied Sciences 3(4): 479-495. DOI: 10.4103/0975-7406.90101 [ Links ]
Palomino, L.R., J.P. Martínez, C.M. García, J.H. Gil y D.L. Durango. 2010. Caracterización fisicoquímica y actividad antimicrobiana del propóleos en el municipio de La Unión (Antioquia, Colombia). Revista Facultad Nacional de Agronomía Medellín 63(1): 5373-5383. [ Links ]
Park, Y.K., J.F. Paredes, C.L. Aguiar, S.M. Alencar and F.Y. Fujiwara. 2004. Chemical constituents in Baccharis dracunculifolia as the main botanical origin of southeastern Brazilian propolis. Journal Agricultural and Food Chemistry 52(5): 1100-1103. DOI: 10.1021/jf021060m [ Links ]
Pastor, C., L. Sánchez, A. Marcilla, A. Chiralt, M. Cháfer and C. González. 2011. Quality and safety of table grapes coated with hydroxypropylmethylcellulose edible coatings containing propolis extract. Postharvest Biology and Technology 60(1): 64-70. DOI: 10.1016/j.postharvbio.2010.11.003 [ Links ]
Patthamakanokporn, O., P. Puwastien, A. Nitithamyong and P.P. Sirichakwal. 2008. Changes of antioxidant activity and total phenolic compounds during storage of selected fruits. Journal of Food and Composition Analysis 21(3): 241-248. DOI: 10.1016/j.jfca.2007.10.002 [ Links ]
Paull, R., W. Nishijama, M. Reyes and C. Cavaletto. 1997. Postharvest handling and losses during marketing of papaya (Carica papaya L.). Postharvest Biology and Technology 11(3):165-179. DOI: 10.1016/S0925-5214(97)00028-8 [ Links ]
Pavan, V., R.A. Soriano and G.M. Pastore. 2014. The effect of in vitro digestion on the antioxidant activity of fruit extracts (Carica papaya, Artocarpus heterophillus and Annona marcgravii). Food Science and Technology 59(2): 1247-1251. DOI: 10.1016/j.lwt.2014.05.040 [ Links ]
Pérez, M.B., C. Rojas and M.A. Del Rio. 2002. Effect of lípid type and amount of edible hidroxypropyl methylcellulose lípid composited coating used to protect posthasrvest quality of mandarins cv. Fortune. Journal of Food Science 67(8): 2903-2910. DOI: 10.1111/j.1365-2621.2002.tb08836.x [ Links ]
Popova, M.P., I.B. Chinou, I.N. Marekov and V.S. Bankova. 2009. Terpenes with antimicrobial activity from Cretan propolis. Phytochemistry 70(10): 1262-1271. DOI: 10.1016/j.phytochem.2009.07.025 [ Links ]
Popova, M.P., K. Graikou, I. Chinou and V.S. Bankova. 2010. GC-MS profiling of diterpene compounds in Mediterranean propolis from Greece. Journal Agricultural and Food Chemistry 58(5): 3167-3176. DOI: 10.1021/jf903841k [ Links ]
Rodríguez, Y., F. Sánchez, B. Rojano, D. Durango, J. Gil y J. Marín. 2012. Caracterización fisicoquímica y evaluación de la actividad antioxidante de propóleos recolectados en el departamento del Atlántico, Colombia. Revista U.D.C.A Actualidades & Divulgación Científica 15(2): 303-311. [ Links ]
Sakava, P., E. Talla, M. Chelea, T.A. Tchinda, M.E. Zeuko'o, S. Laurent, E.L. Vander, F.M. Tagatsing, G.A.B. Yaya, D.T.A. Atchade and T.J. Mbafor. 2014. Pentacyclictriterpenes and crude extracts with antimicrobial activity from Cameroonian Brown propolis samples. Journal of Applied Pharmaceutical Science 4(7): 1-9. DOI: 10.7324/JAPS.2014.40701 [ Links ]
Santamaría, F., R. Díaz, E. Sauri, F. Espadas, J.M. Santamaría y A. Larqué. 2009. Características de calidad de frutos de papaya Maradol en la madurez de consumo. Agricultura Técnica en México 35(3): 347-353. [ Links ]
Santos, F.A., E.M. Bastos, A.B. Maia, M. Uzeda, M.A. Carvalho, L.M. Farias and E.S. Moreira. 2003. Brazilian propolis: physicochemical properties, Planta origin and antibacterial activity on periodonto pathogens. PytotherapyResearch 17(3): 285-289.: 10.1002/ptr.1117 [ Links ]
Schweiggert, R.M., C.B. Steingass, E. Mora, P. Esquivel and R. Carle. 2011. Carotenogenesis and physico-chemical characteristics during maturation of red fleshed papaya fruit (Carica papaya L.). Food Research International 44(5): 1373-1380. DOI: 10.1016/j.foodres.2011.01.029 [ Links ]
Torlak, E. and D. Sert. 2013. Antibacterial effectiveness of chitosan-propolis coated polypropylene films against foodborne pathogens. International Journal of Biological Macromolecules 60(1): 52- 55. DOI: 10.1016/j.ijbiomac.2013.05.013 [ Links ]
Tripathi, P. and N. Dubey. 2004. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biology and Technology 32(3): 235-245. DOI: 10.1016/j.postharvbio.2003.11.005 [ Links ]
Trusheva, B., M. Popova, E. Budi Koendhori, I. Tsvetkova, C. Naydenski and V. Bankova. 2011. Indonesian propolis: chemical composition, biological activity and botanical origin. Natural Products Research 25(6): 606-613. DOI: 10.1080/14786419.2010.488235 [ Links ]
Wolfe, K., X. Wu and R.H. Liu. 2003. Antioxidant activity of apple peels. Journal Agricultural and Food Chemistry 51(3): 609-614. DOI: 10.1021/jf020782a [ Links ]
Wrolstad, R.E., R.W. Durts and J. Lee. 2005. Tracking color and pigment changes in anthocyanin products. Trends in Food Science and Technology 16(9): 423-428. DOI: 10.1016/j.tifs.2005.03.019 [ Links ]