Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Facultad Nacional de Agronomía Medellín
versão impressa ISSN 0304-2847
Rev. Fac. Nac. Agron. Medellín vol.68 no.2 Medellín jul./dez. 2015
https://doi.org/10.15446/rfnam.v68n2.50991
Antagonistic action of Lactobacillus spp. against Staphylococcus aureus in cheese from Mompox - Colombia
Acción antagónica de Lactobacillus spp. frente a Staphylococcus aureus encontrados en queso de capa de Mompox - Colombia
Piedad M. Montero Castillo1; Antonio Díaz Caballero2 and Marlene Durán Lengua3
1 Associate Professor. Universidad de Cartagena - Facultad de Ingenierías - Programa Ingeniería de Alimentos - Grupo Nusca. Campus Piedra de Bolívar. Cartagena, Colombia. <pmonteroc@unicartagena.edu.co>
2 Full Professor. Universidad de Cartagena - Facultad de Odontología - Grupo Gitou. Campus de Zaragocilla, Cartagena, Colombia. <adiazc1@unicartagena.edu.co>
3 Associate Professor. Universidad de Cartagena - Facultad de Medicina - Grupo Farmabac. Campus de Zaragocilla. Cartagena, Colombia. <mduranl@unicartagena.edu.co>
Received: September 12, 2013; Accepted: October 5, 2014
DOI: http://dx.doi.org/10.15446/rfnam.v68n2.50991
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Abstract. In the food industry, food preservation techniques that do not use chemical products are becoming more common. Therefore, the aim of this research was to evaluate the antagonistic activity (antibiosis) of lactic-acid bacterial strains against pathogenic microorganisms. Lactic-acid bacterial strains were isolated from layered cheese and a commercial product (yogurt); and the same was done with pathogenic bacteria solely from layered cheese. The lactic-acid bacterial strains were identified as species from the Lactobacilli family, while the pathogenic bacteria from layered cheese were identified as Micrococcaceae family species (Staphylococcus aureus). Subsequently, in the same culture medium, bacteria of each species were sowed in order to determine the inhibitory activity ability of the Lactic Acid Bacteria (BAL) As a result, the highly antagonistic activity of the Lactobacilli (inhibition halos were larger than 0.5 centimeters in diameter) against isolated pathogenic microorganisms was demonstrated.
Key words: Antibiosis, bacteriocins, dairy products, Lactobacillus, Staphylococcus aureus, (Mesh Database).
Resumen. En la industria alimentaria, cada vez es más frecuente el uso de técnicas que permiten la conservación de alimentos, sin el uso de productos químicos tradicionales. Por lo tanto, el objetivo de este trabajo fue evaluar la actividad antagonista (antibiosis) de las cepas de bacterias ácido lácticas contra microorganismos patógenos. Las cepas de bacterias acido lácticas fueron aisladas de queso de capa y un producto comercial (yogurt), y lo mismo se hizo con las bacterias patógenas, las cuales fueron aislada del queso capa. Las cepas de bacterias ácido lácticas se identificaron como especies de la familia lactobacillaceae, mientras que las bacterias patógenas del queso de capa se identificaron como especies de la familia Micrococcaceae (Staphylococcus aureus). Posteriormente, las bacterias de cada especie fueron dispuestas con el fin de determinar la inhibición del crecimiento de las bacterias patógenas por Bacterias Ácido Lácticas (BAL). Estos resultados mostraron que los lactobacilos inhibieron el crecimiento de Staphylococcus aureus, evidenciándose la actividad antagónica (halos de inhibición más grandes de 0,5 centímetros de diámetro) contra microorganismos patógenos aislados de este productos.
Palabras clave: Antibiosis, bacteriocinas, productos lácteos, Lactobacillus, Staphylococcus aureus, (Decs Bireme).
Cheese is a good culture medium for microorganism growth because of its high water content, its pH, and the variety of compounds it contains (Settanni et al., 2012). One of the higher proportion ingredients in cheese is cow milk, which, due to rudimentary milking processes and transport, gains microbiota that include beneficial, saprophytic and pathogenic microorganisms (Bernardeau et al., 2008).
Traditionally, the pathogenic bacteria of most concern to food microbiologists are, among others: Staphylococcus aureus, Salmonella spp., and Clostridium botulinum. Nowadays, Staphylococcus aureus is one of the bacteria frequently linked to food poisoning outbreaks (Rodriguez et al., 2009) due to its wide distribution in the environment and its ability to produce toxins (Feutry et al., 2012). Nevertheless, in the last few years, certain microorganisms have gained relevance as food poisoning causative agents, for example: Listeria monocytogenes, Bacillus cereus, Aeromonas hydrophila, Yersinia enterocolitica, Escherichia coli verotoxigenic (ECVT), and Campylobacter jejuni (Ladero et al., 2010; Carraro et al., 2011).
Likewise, as a consequence of the abuse of antibiotics in medicine and farm animals, there has been a significant increase in multi-antibiotic resistant food pathogens (Call et al., 2008; Walsh and Fanning, 2008).
Currently, Lactic-acid bacteria present a substantial biotechnological potential due to its presence in several fermentative processes for human and animal food production, specifically those related with dairy products, vegetables, meat, baked goods, alcoholic beverages, and silages (Monteagudo et al., 2011).
These bacteria not only contribute to the development of organoleptic and rheological characteristics in the food, but also generates environments that were only slightly favorable for the development of pathogenic microorganisms due to their marked antagonistic capacity, which inhibits their proliferation in food over any other group of microorganisms that is present in the preliminary material (raw food) or that contaminates the subsequent product (Samelis et al., 2014).
The beneficial effects are caused by the antagonist ability, also known as antibiosis, which is based on organic acids and the production of other inhibitory metabolites. Among these metabolites, it is important to mention oxygen peroxide (H2O2) and others that are derived from oxygen metabolism, aromatic compounds (diacetyl, acetaldehyde), dehydrated derivates of glycerol (reuterin), bacteriolytic enzymes, bacteriocins and so forth (Madureira et al., 2011; Georgalaki et al., 2013).
In bacteriocin inhibitory mechanisms, the pore formation in the cytoplasmic membrane of sensitive cells seems to be a common action mechanism of substances produced by lactic acid bacteria. The structure of these peptides, α-helixes or β-laminates has two sides, an hydrophilic one and an hydrophobic one, which create oligomers that go through the membranes, forming pores; the hydrophobic side places itself close to the lipids of the membrane, while the hydrophilic side goes to the center of the pore (Nissen et al., 2010). This action produces losses in several essential compounds, such as K+ ions, ATP and, in some cases, amino acids and small molecules simultaneously, these losses trigger a membrane potential reduction, consumption of energy reserves in cells, and a decrease in the synthesis of DNA, RNA and proteins, which could ultimately lead to cell death (Reder and Bendas, 2011).
The bacteriocins produced by lactic acid bacteria are an innovative tool for the control of pathogens in food products as well as those pathogens that spoil food (Nielsen et al., 2010). Likewise, several strains can contribute to the preservation of fermented food through the production of bacteriocins (Castro et al., 2011). Studies on bacteriocins have increased during recent decades, including on bacteriocin use, or the microorganisms that produce them, as a natural preservative (Tolinački et al., 2012).
The objective of the present study, was to investigate the possible antagonism between BAL bacteria and pathogenic bacteria isolated from artisan layered cheese manufactured in Mompox, Colombia.
MATERIALS AND METHODS
Sampling and initial isolation of microorganisms. The layered cheese samples were taken in different places in Mompox. They were taken in triplicate and collected at a sufficient quantity and from sterile conditions. The samples were transported in Styrofoam boxes with ice to the Universidad of Cartagena Microbiology Laboratory (Engineering Program-Pilot Plants). The samples were processed under sterile conditions in DIES SEGURA bs 100 flow cabinets, and the incubation was performed in an incubator. Esco, IFA -54-8 USA.
Lactobacilli were isolated from a yogurt commercialized in Cartagena. This yogurt contained L. acidophilus and L. casei. In order to isolate and identify these microorganisms, Man-Rogosa Sharp-agar was used (MRS) while Brain Heart Infusion agar was chosen for the S. aureus isolation (Pereira et al., 2011).
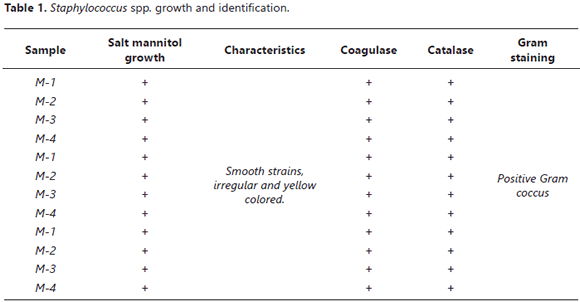
Staphylococcus aureus isolation and identification was done using the Imadi, Tavakoli and Naderi method (Imani et al., 2010). 10 g from the sample were weighed in sterilized bottles with 90 mL BHI broth (Brain Heart Infusion), homogenized and incubated for 37°C/24 h. After that, sowing was done in Petri dishes with salt mannitol agar, a S. aureus selective culture medium (Onoue et al., 2002). The dishes were incubated for 37oC/24 h. Strain confirmation was done with catalase and coagulase tests.
Lactic-acid bacteria isolation from commercial product (yogurt) and layered cheese. The isolation method of Lin was followed (Lin et al., 2006). In order to isolate strains from layered cheese, 10 g were weighed and homogenized with peptoned water 0,1% p/v. With these homogenized samples, sowing was done in Petri dishes using MRS agar, with incubation at 37°C for 48 hours.
Isolation from the commercial product was done with MRS agar, following the same method for layered cheese. After the isolation of the lactic acid bacterial (LAB), the following tests were performed: lactose fermentation, glucose, Gram coloration and catalase.
Layered cheese samples that contained S. aureus were obtained during several periods of the year and from different handmade-producers in Mompox. The method of Todorov was carried out to determine the active bacteriocins in the cheese (Todorov et al., 2011). 5 g of homogenized cheese were taken at 50°C with 10 mL of sterilized HCl 0.02 N and centrifuged at 12000 rpm at 4°C for 20 minutes. The supernatant was neutralized with NaOH 1 N until a pH of 6 was reached and then frozen at 20°C for 24 hours. Afterwards, the neutralized sample was inoculated with S. aureus in BHI broth.
The antimicrobial activity was verified by the agar well diffusion technique using a matrix with five little, stainless-steel rods (Abo-Amer, 2007). In each well, 100 μL of extract were dispensed, and each one was sealed with a thin agar layer; then, they were kept at refrigerated temperatures in order to facilitate the agar diffusion. After some time, 15 μL of 6x108 S. aureus suspension, according to the McFarland scale, were mixed with 12 mL of 0.8% BHI broth. This mixture was added to the agar with the refrigerated extracts and incubated at 37°C for 48 hours. Antimicrobial activity was evidenced by inhibition halos (Haroun et al., 2011).
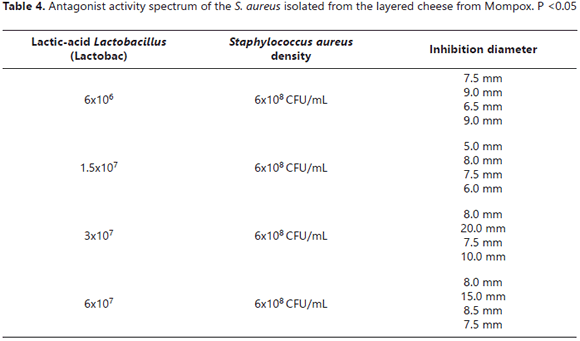
Antagonist activity spectrum. The method described by agg and McGiven in 1971 was used. The agar well diffusion technique was used with fluent MRS agar (15 mL) at 45°C. The little, stainless-steel rods were positioned. Everything was kept at room temperature until the culture medium solidified; at this point, the little, steel rods were removed, leaving the Petri dish with small wells ready for sowing. In the wells, quantities of 6x106, 1.5x107, 3x107, and 6x107 of the comercial Lactobacilli from the essay sample were deposited and an additional layer of MRS agar was added to seal the wells. After solidification, the Petri dishes were incubated for 12 hours at refrigerated temperatures, then 15 μL of 6x108 S. aureus, according to the McFarlan scale, isolated from the layered cheese from Mompox, were inoculated in 12 mL of BHI agar thawed at 45°C. Subsequent to the homogenization, the final mixture was added to MRS Rogosa agar-Petri dishes with the commercial Lactobacilli, resulting in a double agar layer. A period was allocated for solidification, followed by incubation at 37°C for 24 hours. The inhibition halos were then measured around the wells.
The results are shown as mean ± standar error of the three independent experiments. The significance level for the total coliformes was P<0.05.
RESULTS AND DISCUSSION
Staphylococcus aureus identification and count. The isolation was done using salt mannitol agar, as can be seen in Table 1. The S. aureus identification was positive in all of the analyzed samples. Confirmation was carried out with catalase and coagulase tests, which were also positive for S. aureus.
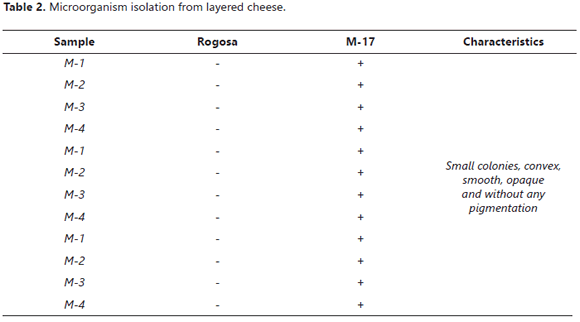
Lactic-acid bacteria isolation from commercial product (yogurt) and cheese. After isolation with MRS agar and M17 agar, a significant growth was found exclusively in the second culture medium, indicating a Streptococci lactic presence M17 Agar is used for isolating and enumerating lactic streptococci in yogurt, cheese starters and other dairy products Difco™ & BBL™ as can be seen in Table 2.
Active bacteriocin determination in the cheese. The evaluation for this substance was negative (Table 3). The determination of Active bacteriocins in the cheese by the agar well diffusion technique did not show any inhibition halos for S. aureus; this is due to the proteic nature of these substances which are inactivated, totally or partially, by proteolitic enzymes (Leonardo et al., 2001; Tuchilus et al., 2007).
Antagonist activity spectrum. After all the tests with Lactobacilli (wells) and the different strains of S. aureus using several microbial densities according to the McFarland scale were done, it was found that the Lactobacilli isolated from the commercial products inhibited growth of the pathogens, as can be seen in Table 4, which can be considered as an antagonic activity because previous essays showed that this kind of activity exists when halos are larger than 2 mm, according to Todorov et al. (2011).
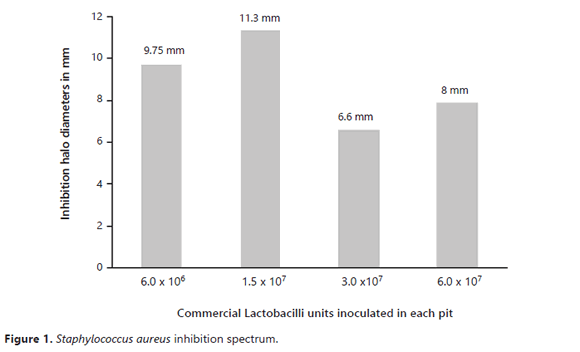
Inhibition spectrum averages related to S. aureus show that 1.5 x 107 was the quantity that produced the most significant inhibition, as compared to the other evaluated densities, as can be seen in Figure 1.
CONCLUSIONS
The results obtained in vitro show that commercial BAL possess biological characteristics compatible with potential use as a probiotics.
The determination of the antimicrobial activity in the layered cheese was negative, as was the Lactobacilli determination, showing that the addition of BAL during processing is important.
The results of the Inhibitory spectrum demonstrate the sensitivity of pathogenic microorganisms, specifically S aureus, to lactic-acid bacteria.
The presence of undesirable microorganisms was confirmed during the cheese production process.
The use of BAL at concentrations equal to 1.5x107 had the most relevant inhibitory effect against S. aureus.
The use of BAL is considered as other conservation methods which can be an interesting option to diminish the addition of chemical preservatives, providing safe foods naturally preserved.
REFERENCES
Abo-Amer, A. E. 2007. Molecular characterization of antimicrobial compound produced by Lactobacillus acidophilus AA11. Acta Microbiol Immunol Hung 54(2): 107-119. DOI: 10.1556/AMicr.54.2007.2.2 [ Links ]
Bernardeau, M., J. Vernoux, S. Henri-Dubernet and M. Guéguen 2008. Safety assessment of dairy microorganisms: the Lactobacillus genus. International journal of food microbiology 126(3): 278-285. DOI: 10.1016/j.ijfoodmicro.2007.08.015 [ Links ]
Call, D., M. Davis and A. Sawant (2008). Antimicrobial resistance in beef and dairy cattle production. Conference of Research Workers in Animal Diseases. Animal Health Research Reviews 9: 159-167. DOI: 10.1017/S1466252308001515 [ Links ]
Carraro, L., M. Maifreni, I. Bartolomeoli, M. E. Martino, E. Novelli, F. Frigo, M. Marino and B. Cardazzo 2011. Comparison of culture-dependent and-independent methods for bacterial community monitoring during Montasio cheese manufacturing. Research in microbiology 162(3): 231-239. DOI: 10.1016/j.resmic.2011.01.002. [ Links ]
Castro, M., N. Palavecino, C. Herman, O. Garro and C. Campos 2011. Lactic acid bacteria isolated from artisanal dry sausages: characterization of antibacterial compounds and study of the factors affecting bacteriocin production. Meat science 87(4): 321-329. DOI: 10.1016/j.meatsci.2010.11.006. [ Links ]
Feutry, F., M. Oneca, F. Berthier and P. Torre 2012. Biodiversity and growth dynamics of lactic acid bacteria in artisanal PDO Ossau-Iraty cheeses made from raw ewe's milk with different starters. Food microbiology 29(1): 33-42. DOI: 10.1016/j.fm.2011.08.011 [ Links ]
Georgalaki, M., K. Papadimitriou, R. Anastasiou, B. Pot, G. Van Driessche, B. Devreese and E. Tsakalidou 2013. Macedovicin, the second food-grade lantibiotic produced by Streptococcus macedonicus ACA-DC 198. Food microbiology 33(1): 124-130. DOI: 10.1016/j.fm.2012.09.008 [ Links ]
Haroun, A. A., E. Ahmed and M. A. Abd El-Ghaffar 2011. Preparation and antimicrobial activity of poly (vinyl chloride)/gelatin/montmorillonite biocomposite films. J Mater Sci Mater Med 22(11): 2545-2553. DOI: 10.1007/s10856-011-4437-x [ Links ]
Imani Fooladi, A., H. Tavakoli and A. Naderi 2010. Detection of enterotoxigenic Staphylococcus aureus isolates in domestic dairy products." Iran J Microbiol 2(3): 137-142. [ Links ]
Ladero, V., M. Fernández, I. Cuesta and M. A. Alvarez 2010. Quantitative detection and identification of tyramine-producing enterococci and lactobacilli in cheese by multiplex qPCR. Food microbiology 27(7): 933-939. DOI: 10.1016/j.fm.2010.05.026 [ Links ]
Leonardo, M. R., L. A. da Silva, M. T. Filho, K. C. Bonifacio and I. Y. Ito 2001. In vitro evaluation of the antimicrobial activity of a castor oil-based irrigant. J Endod 27(12): 717-719. DOI: 10.1097/00004770-200112000-00001 [ Links ]
Lin, W. H., C. F. Hwang, L. W. Chen and H. Y. Tsen 2006. Viable counts, characteristic evaluation for commercial lactic acid bacteria products. Food Microbiol 23(1): 74-81. DOI: 10.1016/j.fm.2005.01.013 [ Links ]
Madureira, A. R., M. E. Pintado, A. M. Gomes and F. X. Malcata 2011. Incorporation of probiotic bacteria in whey cheese: decreasing the risk of microbial contamination. Journal of Food Protection® 74(7): 1194-1199. DOI: 10.4315/0362-028X.JFP-10-217. [ Links ]
Monteagudo-Mera, A., I. Caro, L. Rodríguez-Aparicio, J. Rúa, M. Ferrero and M. García-Armesto 2011. Characterization of certain bacterial strains for potential use as starter or probiotic cultures in dairy products. Journal of food protection 74(8): 1379-1386. DOI: 10.4315/0362-028X.JFP-10 392 [ Links ]
Nielsen, D., G. Cho, A. Hanak, M. Huch, C. Franz and N. Arneborg 2010. The effect of bacteriocin-producing Lactobacillus plantarum strains on the intracellular pH of sessile and planktonic Listeria monocytogenes single cells. International journal of food microbiology 141: S53-59. DOI: 10.1016/j.ijfoodmicro.2010.03.040 [ Links ]
Nissen-Meyer, J., C. Oppegård, P. Rogne, H. S. Haugen and P. E. Kristiansen 2010. Structure and mode-of-action of the two-peptide (class-IIb) bacteriocins. Probiotics and Antimicrobial Proteins 2(1): 52-60. DOI: 10.1007/s12602-009-9021-z [ Links ]
Onoue, Y., R. Shindo, H. Teranishi, I. Furukawa, Y. Hasegawa and T. Maruyama 2002. Evaluation of selective plating media for the detection of heat- or freeze-injured Staphylococcus aureus. Shokuhin Eiseigaku Zasshi 43(4): 239-242. [ Links ]
Pereira, C. A., R. L. Romeiro, A. C. Costa, A. K. Machado, J. C. Junqueira and A. O. Jorge 2011. Susceptibility of Candida albicans, Staphylococcus aureus, and Streptococcus mutans biofilms to photodynamic inactivation: an in vitro study. Lasers Med Sci 26(3): 341-348. DOI: 10.1007/s10103-010-0852-3 [ Links ]
Reder-Christ, K. and G. Bendas 2011. Biosensor applications in the field of antibiotic research-A review of recent developments. Sensors (Basel, Switzerland) 11(10): 9450-9466. DOI: 10.3390/s111009450 [ Links ]
Rodríguez-Alonso, P., C. Fernández-Otero, J. Centeno and J. Garabal 2009. Antibiotic resistance in lactic acid bacteria and Micrococcaceae/Staphylococcaceae isolates from artisanal raw milk cheeses, and potential implications on cheese making. Journal of food science 74(6): M284-293. DOI: 10.1111/j.1750-3841.2009.01217.x [ Links ]
Samelis, J., A. Lianou, E. C. Pappa, B. Bogovič-Matijašić, M. Parapouli, A. Kakouri and I. Rogelj 2014. Behavior of Staphylococcus aureus in culture broth, in raw and thermized milk, and during processing and storage of traditional Greek Graviera cheese in the presence or absence of Lactococcus lactis subsp. cremoris M104, a Wild, Novel Nisin A-producing raw milk isolate. Journal of Food Protection 77(10): 1703-1714. DOI: 10.4315/0362-028X.JFP-14-105
Settanni, L., A. Di Grigoli, G. Tornambé, V. Bellina, N. Francesca, G. Moschetti and A. Bonanno 2012. Persistence of wild Streptococcus thermophilus strains on wooden vat and during the manufacture of a traditional Caciocavallo type cheese. International Journal of Food Microbiology 155(1-2): 73-81 DOI: 10.1016/j.ijfoodmicro.2012.01.022. [ Links ]
Todorov, S. D., D. N. Furtado, S. M. Saad and B. D. Gombossy de Melo Franco 2011. Bacteriocin production and resistance to drugs are advantageous features for Lactobacillus acidophilus La-14, a potential probiotic strain. New Microbiol 34(4): 357-370. [ Links ]
Tolinački, M., J. Lozo, K. Veljović, M. Kojić, Đ. Fira and L. Topisirović 2012. Examination of antimicrobial potential in natural isolates of Lactobacillus casei/paracasei group. Genetika 44(3): 661-677. DOI: 10.2298/GENSR1203661T. [ Links ]
Tuchilus, C., I. Goldu and A. Poiata 2007. In vitro activity of Ertapenem against staphylococci. Rev Med Chir Soc Med Nat Iasi 111(4): 1035-1039. [ Links ]
Walsh, C. and S. Fanning 2008. Antimicrobial resistance in foodborne pathogens-a cause for concern? Current Drug Targets 9(9): 808-815. [ Links ]