Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad Nacional de Agronomía Medellín
Print version ISSN 0304-2847
Rev. Fac. Nac. Agron. Medellín vol.68 no.2 Medellín July/Dec. 2015
https://doi.org/10.15446/rfnam.v68n2.50993
DOI: http://dx.doi.org/10.15446/rfnam.v68n2.50993
Agro-industrial fruit co-products in Colombia, their sources and potential uses in processed food industries: a review
Co-productos de origen agroindustrial fruticola en Colombia, procedencia y sus usos potencialesen matrices alimenticias procesadas
Miguel Ángel Alarcón García1; Jairo Humberto López Vargas2 and Diego Alonso Restrepo Molina3
1 Zootecnista, M.Sc. en Ciencia y Tecnología de Alimentos. Universidad Nacional de Colombia - Sede Bogotá - Instituto de Ciencia y Tecnología de Alimentos - ICTA, Cra 30 No. 45-03, Bogotá, Colombia. <maalarcong@unal.edu.co>
2 Profesor Asociado. Universidad Nacional de Colombia - Sede Bogotá - Instituto de Ciencia y Tecnología de Alimentos - ICTA. Cra 30 No. 45-03, Bogotá, Colombia. <jhlopezv@unal.edu.co>
3 Profesor Asociado. Universidad Nacional - Sede Medellín- Facultad de Ciencias Agrarias -Departamento de Ingeniería Agrícola y de Alimentos. A.A. 1779, Medellín, Colombia. <darestre@unal.edu.co>
Received: October 08, 2013; Accepted: April 18, 2015
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Abstract. Fruit agribusinesses generate large amounts of by-products with diverse characteristics that are inherent to the fruits from which they come, which are a source of great use potential because their compositions include molecules that are currently of high interest (antioxidants and dietary fiber). It is clear that, without correct handling and disposal, theses fruits present a problem due to the environmental pollution that large quantities of residues can generate. Although there are varied uses for agro-industrial co-products, this review focused on the potential uses that co-products could have in different processed food matrices. In this sense, this paper led to the revelation that one of the principal objectives of the reviewed research was to condition co-products for use in processed foods in an attempt to take advantage of the bio-active compounds they contain, principally the natural antioxidant activity, which especially enjoys acceptance by consumers of processed foods.
Key words: Agro-industrial by-products, environmental pollution, food industry branches, fruit agribusiness, bioactive compounds.
Resumen. La agroindustria frutícola genera grandes cantidades de subproductos con características muy diversas inherentes a los frutos de los cuales provienen y así como son una fuente con un potencial de aprovechamiento muy grande debido a que en su composición se pueden hallar moléculas en las cuales se tiene un alto interés hoy en día (antioxidantes y fibra dietaria), es claro que sin una correcta manipulación y disposición de los subproductos estos corren un alto riesgo de volverse un problema debido a la polución ambiental que grandes cantidades de subproductos pueden generar. Aunque podrían existir usos muy variados para los subproductos agroindustriales éste documento hace una revisión enfocada en los potenciales usos que los co-productos podrían tener en diferentes matrices alimenticias procesadas; en este orden de ideas este documento permitió reconocer que uno de los principales objetivos en muchas de las investigaciones consultadas es el de poder acondicionar los co-productos para poder hacer la inclusión de éstos en alimentos procesados, buscando aprovechar los compuestos bio-activos presentes, principalmente por su actividad antioxidante de origen natural la cual goza de especial aceptación entre los consumidores de alimentos procesados.
Palabras claves: Subproductos agroindustriales, contaminación ambiental, tipos de industrias alimenticias, agronegocios frutícolas, compuestos bioactivos.
In Colombia, there are nearly 48 perennial and non-perennial fruit species with different degrees of importance to the fruit sector, depending on their economic and social impact (Reyes et al., 2006). Within the perennial category, the first 5 species that cover the largest cultivated area are the orange (Citrus sinensis), mango (Mangifera indica), avocado (Persea americana), guava (Psidium spp.) and tangerine (Citrus spp.), comprising 44.8% of the cultivated area (or 98,837 ha) intended for fruit production, not including domestic plantain and banana production; furthermore, these fruit species had production equaling 330,000, 222,000, 225,000, 80,000, and 104,000 t per year, respectively (Agronet, 2013). On the other hand, the transient crops or non-perennial species had a greater area of production (ha) (without taking into account species such as the plantain and banana); for example, the pineapple (Ananas comosus), blackberry (Rubus ulmifolius), tamarillo fruit (Solanum betaceum) and lulo (Solanum quitoense), which comprise 23.48% of the cultivated area, or 51,805 ha, with production that reached 480,000; 88,000; 131,750 and 58,000 t per year, respectively (Agronet, 2013). The remaining 31.72% ha, dedicated to fruit production were for other transient and perennial crops of lesser importance (Reyes et al., 2006). The production of Musa genus fruits in Colombia, according to the FAO (2013), reached 2,815,050 and 2,034,340 t per year for plantain and banana with the use of 345,109 and 80,518 ha, respectively.
Within the perennial category, the first 5 species that cover the largest cultivated area are the orange (Citrus sinensis), mango (Mangifera indica), avocado (Persea americana), guava (Psidium spp.) and tangerine (Citrus spp.), comprising 44.8% of the cultivated area (or 98,837 ha) intended for fruit production, not including domestic plantain and banana production; furthermore, these fruit species had production equaling 330,000; 222,000; 225,000; 80,000 and 104,000 t per year, respectively (Agronet, 2013). On the other hand, the transient crops or non-perennial species had a greater area of production (without taking into account species such as the plantain and banana); for example, the pineapple (Ananas comosus), blackberry (Rubus ulmifolius), tamarillo fruit (Solanum betaceum), lulo (Solanum quitoense), papaya (Carica papaya) and watermelon (Citrullus lanatus) which comprise 23.48% of the cultivated area, or 51,805 ha, with production that reached 480,000, 88,000, 131,750; 58,000; 170,000 and 150,000 t per year, respectively (Agronet, 2013). The remaining 31.72% of the ha dedicated to fruit production were for other transient and perennial crops of lesser importance (Reyes et al., 2006). The production of Musa genus fruits in Colombia, according to the FAO (2013), reached 2,815,050 and 2,034,340 t per year for plantain and banana with the use of 345,109 and 80,518 ha respectively. The fruit species mentioned above generate large amounts of waste, both at the harvest and the agro-industrial levels in Colombia and worldwide, because a lot of these fruit species correspond to fruit crops with high global production, as is the case of the banana, plantain, watermelon, orange, mango, pineapple, papaya, and avocado FAO (2013). Other fruits recognized as important crops in the global production as apple (Malus domestica), grape (Vitis spp), peach (Prunus persica) and lemon (Citrus limon) wich are reported 69,511975; 67,16,255; 20,528,283 and 13,933,864 t per year, respectively (FAO, 2012); in Colombia these fruits are produced in low quantities in respect to global production, in this case 1,200; 21,400; 20,800 and 81,000 for apple, grape, pech and lemon respectively (Agronet, 2013).
There are also fruit crops, such as the apple (Malus domestica), grape (Vitis spp.), peach (Prunus persica), and lemon (Citrus lemon), that are recognized around the world for their high production, for which the FAO (2013) reports 69,511,975, 67,116,255, 20,528,283, and 13,933,864 t, respectively. Colombia produces low quantities of these fruits, representing a very small percentage of global production, with 1,200, 21,400, 20,800, and 81,000 t for the apple, grape, peach and lemon, respectively (Agronet, 2013). These numbers demonstrated the fact that cultivated fruit production in Colombia reaches several million t and that it has been reported that up to 70% of a fruit's weight can correspond to the fractions composed of the seeds, peels and pulp remnants (Ayala et al., 2011). In this sense, this review aimed to discover how the co-products of some of the more representative fruit agro-industries of Colombia are generated along with uses that have been proposed by different research from around the world, which has mainly focused on revaluing and taking advantage of co-products, searching not only for a better economic yield specifically for the links of value agro-chins that correspond to the production and industrialization sectors but also for an offering of different types of solutions for other industries, such as chemical-pharmaceutical and processed food industries, in which phytochemicals have been developing a continuously more important role in recent years due to the fact that they correspond to natural molecules that are highly appreciated by consumers who are increasingly more conscious of products that can be found in different markets (Silva et al., 2014).
Generation of agro-industrial by-products
Due to the fact that many harvest products are not directly consumed by consumers without being submitted to processing to remove the non-edible parts, they generate a bulky amount of byproducts, such as peels and seeds (Ayala et al., 2010) and, in some cases, the excess residues are more than the product itself (Ayala et al., 2011; Lousada et al., 2007), as seen in pineapple, papaya, mango, plantain, banana, apple, grapes, citrus fruits, and guava, among others; of which natural or preserved pulps and derivatives, such as juices, are in high demand (Ayala et al., 2011; Schieber et al., 2001b).
The use of byproducts is difficult due to the bacterial spoilage of these materials, thereby limiting their potential, as well as the high cost of drying or storage (Schieber et al., 2001b). However, several authors still argue that the use of co-products as raw materials is a very inexpensive source of resources and/or substrates for biotechnological products, such as antioxidants, enzymes, etc. (Dhillon et al., 2011; Elleuch et al., 2011; Grigorevski De Lima et al., 2005; Schieber et al., 2001a).
The fruit and vege production of various countries has seen, over the last few years, an increasing trend, generating more waste and by-products at harvest or when selling them in different markets. For example, in the case of Kampala, Uganda (a city of just 1,200,000 inhabitants), up to 18,000 t of waste are produced per year (including, mainly from the banana (Musa acuminata), potato (Ipomoea batatas), and nakati (garden eggs) (Solanum aethiopicum)), of which only a small portion is used for compost and animal feed, while most is not collected for different reasons, causing serious environmental problems and missed opportunities for potential uses due to the probability of higher contamination with other solid wastes (Katongole et al., 2008).
The waste and byproducts of fruits and vegetables are highly heterogeneous materials because they come from different plants with different botanical origins and, additionally, during the processing of the different parts of the fruits, these byproducts might be exposed to different physical and chemical treatments that seek to remove the "economically important part". These operations affect the matrix of waste and byproducts, wherein non-starch polysaccharides, plus lignin, constitute a significant portion of the weight on a dry basis. The out-coming froth of vegetables is rich in dietary fiber and other components (Serena and Knudsen, 2007).
The aforementioned upticks in the production of fruits and vegetables are mainly attributed to the use of better techniques for handling raw materials, such as preservation, marketing and distribution systems. Furthermore, consumers have increased their demand for better sensory properties and the awareness of the benefits of consuming fruits and/or vegetables and their derivatives has also increased (Gonzalez et al., 2010; Schieber et al., 2001b).
The production of large amounts of waste and byproducts in agro-industries leads to great economic losses due to environmental requirements, which exacerbate the low-margin of profitability seen in the food industry and the high cost of raw materials, which should make the prudent use of residues attractive to the agro-industry in order to increase profitability (Akkerman and Van Donk, 2008).
This review took into account some of the more important fruits at the Colombian and global levels, considering the production and traditional data of the Colombian market along with reports from other countries because, currently, data on the domestic production of agro-industrial fruit co-products are practically nonexistent. In terms of the establishment and production of co-products for use in processed foods, all of the reviewed papers agreed with the use of processes that principally consist of a reduction in the particle size in order to facilitate manipulation, subsequent use, and drying in order to sufficiently decrease the moisture values to allow storage for extended periods of time (Ayala et al., 2011; Khalili et al., 2012; Schieber et al., 2001b; Serena and Knudsen, 2007; Silva et al., 2014; Viuda et al., 2010a).
Citrus fruits (Orange, lemon, tangerine)
Marín et al. (2007) considered citrus fruit crops to be the most abundant in the world because, when crops of orange, lemon, and tangerine are included, they can reach a global total of 88 x 106 t; but, there are fruit crops that can exceed this amount, such as the watermelon and banana, with global productions of up to 99 x 106 and 102 x 106 t, respectively, FAO (2013). However, the production of citrus fruits represents one of the largest worldwide and an enormous source of byproducts because 50% of the total amount of produced fruits are for processing and used primarily to get juice, jams and preserves, among others (Marín et al., 2007).
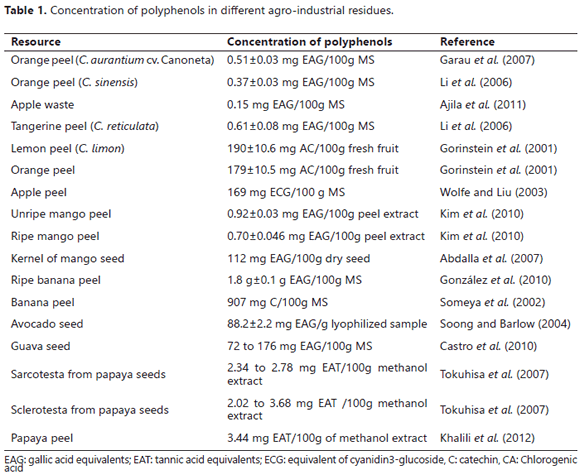
Fruits intended for processing (mainly for the production of juices, canned fruits, jams and extracts of essential oils and flavonoids) represent 98% of the total of harvested fruits worldwide. So, it can be inferred that the quantity of co-products generated from the agribusiness of citrus fruits would be a great source of potential resources for the pharmaceutical and food industries (Fernández et al., 2007; Izquierdo and Sendra, 2003; Romero et al., 2011; Viuda et al., 2010b). Other authors have reported the same (Annadurai et al., 2002; Benoit et al., 2006) for the residues of agro as potential low-cost substrates to get polyphenol-family molecules. Researchers have evaluated the antioxidant capacity of fruit residues, such as lemon, grapefruit and orange, in trapping free-radicals, revealing in all cases a better activity of the cellulose from the peels than the decorticated fruits and, also, pointing out that these types of resources are sources of polyphenols (Table 1). This same trend is also seen in fruits for highly valued components such as the total dietary fiber and the components: soluble and insoluble dietary fiber (Gorinstein et al., 2001).
The dietary fiber from the co-products of the citrus fruits (mainly from the orange juice industry) has also been subjected to extensive research on its inclusion in food matrixes, such as in meat products. Various meat products, such as mortadella (Viuda et al., 2010a), fermented salami (Fernández et al., 2008), dry cured sausage (Fernández et al., 2007), hamburger (Aleson et al., 2005; Turhan et al., 2005) and Bologna, a type of sausage, (Fernández et al., 2004b) among others, have been used with these co-products because they contain significant levels of dietary fiber, complex carbohydrates, or bioactive compounds, such as polyphenols.
Ingredients with significant levels of complex carbohydrates help consumers to increase their intake of dietary fiber, according to the quantities recommended by some international entities which range between 25 g and 38 g per day for women and men, respectively, or 14 g per 1,000 kcal consumed (American Dietetic Association, 2008; Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, 2005).
Apple
The use of waste and/or agro-industrial co-products is a major economic aspect that could mitigate environmental impacts (Dhillon et al., 2011) in countries such as Germany, where apple juice is one of the most popular fruit juices amongst the population and the production of juice reaches 700,000 t of apples per year and generates 250,000 t of co-products (Endreß, 2000) (mainly peels, seeds, stems, pulp and juice residue), which could be added-value products because some authors (Endreß, 2000; Rha et al., 2011; Schieber et al., 2003; Sudha et al., 2007) consider these types of residues as a source of fiber, polyphenols and pectin; of which pectin is used in food products as gelling agents, emulsifiers and stabilizers, while polyphenols have gained importance due to their antioxidant properties and can be found in co-products of the apple industry, a low-cost material (Lu and Foo, 2000).
Dietary fiber is also considered very useful due to its inclusion in food matrix processes to improve consumer perception as a "natural product" (Viuda et al., 2010a). In addition, dietary fiber is considered a functional ingredient (Fernández et al., 2004a) and has several benefits, such as the prevention of certain chronic diseases. In addition, the intake of dietary fiber can be increased without generating radical changes in eating habits in consumers (Viuda et al., 2010b) when mixed with other foods.
The composition of agro-industrial residues might be highly variable, although it is claimed that some co-products, for example apple residues, do not have an ideal use (Schieber et al., 2001b); even so, other authors have tried to come up with alternative solutions for the use of these wastes and to produce citric acid by fermentation with Aspergillus niger, using apple co-products as a substrate (Dhillon et al., 2011). The residues (mainly peels) from the apple juice industry are a rich source of polyphenols (Table 1), while the juice obtained in the processing stage also has levels of polyphenols, but in a lesser amount (Schieber et al., 2001a). Some phenolic compounds that have been identified in agro-industrial wastes are: procyanidins, quercetin, catechin, phloretin and hidroxycinnamatos (Schieber et al., 2001a). The phenolic compounds that have been identified in the co-products of apples, from the apple agro-industry, are considered similar to natural antioxidants, which can provide health benefits when included in human diets. Moreover, this type of molecule also has anti-allergic, anti-microbial and anti-inflammatory properties (Ajila et al., 2007), as well as preventive activities and/or modulations of chronic diseases, such as arthritis and cancer (Fernández et al., 2004c).
Plantain and banana
Bananas and plantains produce large amounts of co-products, although only a small part of bananas and plantains actually go into production lines. This is because the peel of these products, which includes the non-edible part, is between 30% and 40% of the fruit weight (Schieber et al., 2001b; Tchobanoglous et al., 1993). These fruits are also considered to be part of the larger harvests in the world, since they are produced in nearly 100 countries (Aurore et al., 2009). According to the Food and Agriculture Organization - FAO (2014), 102 million t of bananas (mainly of the Musa species Musa AA and AAA) and 36.5 million t of plantains (mainly species of Musa AAB, ABB Musa and Musa BBB) were produced worldwide according to reports from Aurore et al. (2009), (2014); Fao (2014). Some of the potential uses for residues generated from Musa genus fruit species are animal feed, methane production, pectin production, biomass production, adsorbents for water purification and ethanol production, among others (Annadurai et al., 2002; Clarke et al., 2008; Essien et al., 2005; Happi et al., 2008).
Someya et al. (2002) reported that residues from the fruits of the Musa genus are sources of polyphenol-type antioxidants (Table 1), such as gallocatechin found in concentrations of 907 mg g-1 100 of dried peel. These molecules have also been reported by Alarcón et al. (2014), who used the bio-active compounds found in an intermediate food product obtained from a plantain peel co-product (Musa AAB) as an ingredient in order to mitigate the lipid and protein degradation in the model system of a meat product and obtained positive results for the decrease of the protein degradation rate of the meat product. In previous studies, these authors evaluated technological functional properties in terms of the capacity to retain water, the capacity to absorb oil, the capacity to absorb water and the capacity to absorb organic molecules found in the intermediate food product obtained from plantain peels (Musa AAB) in order to determine the usefulness of including this raw material as an ingredient in the formulation of processed meat products, reporting that this raw material contains relatively high values for the measured values as compared to other sources of dietary fiber obtained from co-products of different fruit products (Alarcón et al., 2013). Other molecules found in and reported for this type of residue included phenols, such as dopamine (known as a neurotransmitter in humans that is involved in endocrine function) and L-dopa (a product of endocrine disorders that cause movement disorders in people (Oak et al., 2000)). These molecules also showed antioxidant activity and the ability to scavenge free-radicals (González et al., 2010).
Another important component found in the peels of plantains and bananas is dietary fiber, which, according to data reported by Happi et al. (2007), can range between 32.9% and 49.7% on a dry weight basis depending on the ripening state (taking into account the color of the peel, with green representing an immature state and yellow the ideal state of maturation for consumption) and the type of banana or plantain. Other components change concentrations during the ripening of the fruit, such as starch, which sees a reduction of between 0.1% and 39.3% on a dry weight basis due to the action of several endogenous enzymes, such as amylases, glycosidases, phosphorylases, sucrosesynthetase and invertase; this action is affected in the same way as dietary fiber with respect to the maturation and type of fruit (Adão and Glória, 2005). Similarly, Adão and Glória (2005) and Happi et al. (2007) also reported that the levels of soluble sugars presented a significant increase, mainly for monosaccharaides such as glucose and fructose; dimers, such as sucrose, may increase during maturation development up to Day 14 of the post-harvest period, but lowered afterwards, when the monosaccharaides found their highest points at 21 to 35 days of the post-harvest period.
During maturation, fruits also present a phenomenon of water migration from the peel to the pulp; this generates, along with starch changes, other desirable modifications in the appearance and the perception of softness in the pulp at the time of consumption (Mota et al., 1997).
Mango
The mango (Mangifera indica) enjoys worldwide popularity, especially in the European market where they are imported from developing countries (mainly Brazil and Peru) at an estimated 231,613 t (Food and Agriculture Organization, 2009). The mango is a fruit that generates one of the largest amounts of co-products because the peels and seeds constitute up to 60% of the fruit (Cerezal et al., 1995). Scientific studies have reported that mango co-products are a source of dietary fiber, carbohydrates, oil and phenolic compounds (Abdalla et al., 2007; Maisuthisakul and Gordon, 2009; Vergara et al., 2007). The co-products generated from processing mango (composed of peels (10-20%), seeds (10-25%) and pulp remnants) are a source of potential resources, but their usage depends on the mitigation of the environmental problems linked to poor disposal of this type of material. Besides the aforementioned aspects, about 20% of mango production goes to processing for the creation of products such as purées, nectars and canned products, among other things.
The use of mango co-products allows for the creation of different products; one report stated that mango peels have bioactive compounds such as polyphenols, carotenoids, and dietary fiber (Ajila et al., 2007), while the seed has a kernel in its interior and is a resource for oil production. A study performed by Abdalla et al. (2007) reported on the composition of the almond and discovered it had high levels of unsaturated fat (55%), mainly consisting of fatty acids such as oleic (46.1%), followed by linoleic (8.2%) and linolenic acid (1.2%).
The search for bioactive compounds in fruits, such as the mango, has led to the characterization of mango peels in order to assess the levels of polyphenols and carotenoids in the residues of the peels.
Based on the above, Ajila et al. (2007) reported that the levels of polyphenols in mango peels, for the raspuri and the badami varieties, have fluctuating figures, from 37.92 to 73.88 mg g-1 for dried, mature mango peels and 33.31 to 46.31 mg g-1 for dried, unripe mango peels; these quantities match the ethanol extracts of a mango peel. In terms of the carotenoids for the same varieties, the researchers found that, contrary to the polyphenol levels that showed lower figures when the fruit was in advanced stages of maturation, the carotenoids had their highest levels in the mature state; these data reflected between 365 and 493 µg g-1 for dry mango peels from unripe fruits and between 1,400 and 3,945 µg g-1 for dry mango peels from ripe fruits (Ajila et al., 2007).
As for the phenolic compound profile, Berardini et al. (2005) and Ajila et al. (2010) reported that the Tommy atkins mango presents a profile with components such as manguiferina, isomanguiferina, manguiferina gallate, isomanguiferina gallate, and quercetin (with residues of galactose, glucose, xylose, arabinopyranose, arabinofuranose, nammnosa, gallicacid and ellagicacid, among others).
There are different bioactive compounds in mango peels and the level of dietary fiber can fluctuate between 44% and 78% of the total dietary fiber, depending on the ripeness for the varieties raspuri and badami, which also have a higher level of total dietary fiber in their more mature states and vice versa (Ajila et al., 2007). There are studies that showed the potential use of mango residues in common food products, such as cookies.
Ajila et al. (2008) reported there were increased levels of dietary fiber, carotenoids and polyphenols in products such as biscuits when adding mango peel flour with 51.2% dietary fiber, 96 mg g-1of polyphenols and 3,092 µg g-1of carotenoids. Mango peel flour was added to the food matrix of biscuits at levels of up to 0.5, 7.5, 10, 15 and 20%. After these additions, the authors reported significant changes in the reduction of the diameter and thickness of the cookie type products when 15 and 20% were used, which were attributed to a dilution effect of the gluten; in the same way, these two addition levels presented a significant increase in resistance to breakage of the product structures and also contributed to the expansion of the structure formed by the gluten in the food matrix.
Avocado
The avocado is one of the most important perennial crops in Colombia, ranking third in production with 212,500 t (Reyes et al., 2006), and a representative crop worldwide with 3,891,626 t FAO (2013). This crop is ranked 21 for the most cultivated fruit and generates a great amount of co-products, regardless of the different forms of exploitation (for consumption in fresh and processed fruit). These residues generate a quantity equal to the 12 to 16% peel and 14 to 24% seeds, in terms of the fresh fruit weight (Bressani, 2009); thus, it is necessary to use this type of co-product. In accordance with the above, Bora et al. (2001) conducted a study to examine the seed of the avocado and identify the potential uses that its composition affords: 56.04 ± 2.58% humidity, 1.87 ± 0.31% fat, 1.95 ± 0.16% raw protein, 1.87 ± 0.24% ash, 5.10 ± 1.11% fiber and 33.17 ± 2.73% carbohydrates, all calculated by differences. Other studies for the description of the avocado seed showed that the polyphenol content has gallic acid at 88.2 ± 2.2 mg g-1 of a lyophilized sample, which would explain the antioxidant capacity that the water-ethanol extract (50:50) of an avocado seed has. These figures are similar to the antioxidant capacity of the ascorbic acid at 236.1 ± 45.1 µmol g-1 for a fresh sample and 725 ± 39.4 µmol g-1 for a lyophilized one (Soong and Barlow, 2004).
As for the avocado, Cano et al. (2007) conducted a study to identify the compounds presented in the peel of a Hass avocado, identifying essential oils, such as ciclohexasiloxanos, Copaene, caryophyllene, germacrenos D and B, and fatty acids, such as erucic, oleic, stearic and palmitic acids, among others. The authors also argued that some of these compounds are reliant on the maturation state, when, at day 21 after the harvest of the fruit, most of these compounds were identified.
One study on the use of co-products from avocado peels and seed extracts from Hass and Fuerte avocados showed that the existence of bioactive compounds in avocado co-products might be used in meat food matrixes, such as the Patty type, in order to prevent the deterioration of food products during cold storage. These bioactive compounds from the extracts of avocado peels and seeds allowed for a reduction with significant differences (p<0.05) for malonaldehyde in meat products, as compared to meat products that did not contain the extracts; this indicated greater stability of the fats (Rodríguez et al., 2011). Moreover, they inhibited protein degradation because a measurement of the carbonyl groups in the amino-acid residues indicated an oxidative change and they were much lower (p<0.05) in the meat products with avocado peels and seed extracts added than in the control treatment. Furthermore, the Hass avocado extracts showed a greater effect than the Fuerte avocado extracts in the inhibition of protein oxidation (Rodríguez et al., 2011).
Guava
The guava is a fruit grown in tropical and sub-tropical
areas such as Brazil, Colombia, South Africa, Peru, Ecuador and India, amongst other countries. It is also a fruit in high demand in North America, Europe and the Middle East (Corporacion Colombiana Internacional, 2013).
The consumption of fresh or processed guava in order to create products such as juice, nectar, purée, jam and jelly (Kashyap et al., 2001) generates residues such as peels, seeds, and small remnant pulp portions (Thongsombat et al., 2007). These co-products have been studied together because they are produced in the guava process industry without separating the components (waste of pulp, seeds and peels).
El-Deek et al. (2009a) reported bromatology type characteristics associated with guava residues, such as a humidity of 5.9%, raw protein of 9.08%, ethereal extract of 10%, raw fiber of 39.5%, ash of 2.55%, and 32.97% nitrogen-free extract on a dry weight basis. This research aimed to test these residues at levels of inclusion of 2%, 4%, 6% and 8% in a diet specifically formulated to feed broilers in the last phase of their growth cycle (from 30 to 42 days of age). They demonstrated that there was not a significant effect (p>0.05) from the guava co-products with the different levels of inclusion in the diet on the final weight of the animals or on the weight of the chicken carcasses; however, the abdominal fat in the broilers was effected when 8% guava co-products were used, generating lower levels of abdominal fat. Therefore, El-Deek et al. (2009a) concluded that the guava co-products used in the research were appropriate for the diet of chickens at the end of the growth cycle.
El-Deek et al. (2009b) also had similar results for the bromatological composition of guava residues and reported for the same material (waste of pulp, seeds and peels) the following data: moisture content of 6.94%, raw protein of 9.78%, ethereal extract of 4.52%, raw fiber of 40%, nitrogen-free extract of 33.14%, and ash of 5.62% on a dry basis. Furthermore, the guava co-products were used to fed animals, such as laying hens from 32 to 48 weeks of age. The authors reported that there were no adverse effects using this material in the diet of the hens; thus, the authors concluded that the dry residues could be included at up to 15% in the diet of animals with the same physiological and environment characteristics. In regards to guava seeds, several studies have reported that they could be a non-conventional source of fat, but the fat content is much lower than that of oilseeds, ranging between 4.2% and 14.1%, and depends on the type of solvent used for the extraction. In addition, the highest quantity of product was obtained with the use of a mixture of super critical CO2 and ethyl acetate. The report also stated that earlier methods of oil extraction had fatty acids such as palmitic, stearic and linoleic acid (Castro et al., 2010).
Moreover, there are other reports on the polyphenol content and the antioxidant capacity that are found in guava seeds: the level of polyphenols are equal to gallic acid figures, between 72 and 176 mg 100 g-1 of crushed and dry seeds, which are directly related to the antioxidant capacity because, with a greater quantity of polyphenols, there is a greater oxidant capacity, ranging from 0.3 to 1.3 mmol mg-1 trolox of gallic acid (estimation with the DPPH method), where the higher antioxidant capacity figures were associated with the higher levels of polyphenols and vice versa (Castro et al., 2010).
Packer et al. (2010) assessed the antioxidant capacity of guava co-products (material composed of peels and seeds) using ethanol extracts with residues; the solvent was removed through a rotary-evaporator at a temperature of 50°C and 2 g (equivalent of 2 g of polyphenols) of this product were mixed with 50 mL of water, called an aqueous extract of guava nuts and seeds by the authors. Then, they applied the aqueous extracts to chicken meat (thighs), without bones or skin, at dosages to generate 20, 40 and 60 mg kg-1 of polyphenols of meat to compare the three dosages of polyphenols and assess which might be more effective in preventing lipid oxidation.
Papaya
There is high production and consumption of this fruit worldwide, with 11,568,346 t FAO (2013), and Colombia contributes about 145,000 t (Agronet, 2013). This high production in turn generates a large amount of co-products, which are normally considered waste and used for animal feed, but most of the time, they are not well-managed or given adequate treatment and have become a focus of phytosanitary diseases that represent a risk to human health (Chaiwut et al., 2010).
Papaya co-products mainly consist of peels and seeds, the latter being a potential source of polyphenol compounds according to the research of Tokuhisa et al. (2007), who reported that the sarcotesta (outermost layer of integument with soft and fleshy consistency) and the esclerotesta (hard cover) of papaya seeds are rich in polyphenols. The sarcotesta presented between 2.34 and 2.78 mg tannic acid equivalent/100 g of methanol extract and the esclerotesta from 2.02 to 3.68 mg tannic acid equivalent/100 g of methanol extract. Moreover, the authors reported that, among the most abundant phenolic compounds found in papaya seeds, there are: caffeic, chlorogenic, dihidrocafeico, sinapic and p-hydroxybenzoic acids. In addition, Khalili et al. (2012) reported a polyphenol content of 3.44 mg for papaya peels, gallic acid equivalent/100 g of methanol extract, similar to the contents of the seed fractions mentioned above. Furthermore, the peel extract was compared in terms of percentage of antioxidant activity and the capacity to scavenge free-radicals with synthetic antioxidants, such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA), and a statistically significant difference (p<0.05) was found, with the extract of papaya peels having a lower percentage of antioxidant activity, as well as a lower capacity to scavenge free-radicals when compared to the synthetic antioxidants (BHT and BHA).
The use of papaya co-products has been reported in various fields for the extraction of protease from peels (Chaiwut et al., 2010). The proteases in papaya peels are highly appreciated by different fields, such as health fields (for medical applications in the areas of ophthalmology, urology, neurosurgery, etc.), the beer industry and cosmetics industry (Caygill, 1979; Salas et al., 2008), among others. Therefore, Chaiwut et al. (2010) evaluated the three-phase extraction method and argued this method was more effective than the normal two-phase method of purification (upper phase and aqueous phase) since the third phase formed by t-butanol (solvent) allows for the removal of low molecular weight compounds, such as lipids and phenols, in contrast to the traditional method.
Potential problems associated with the consumption of molecules found in co-products
Considering the above content, agro-industrial co-products offer a natural source of molecules of catalogued antioxidants, such as polyphenols, that show reported benefits when included in the diets of people (Soobrattee et al. , 2005), but other authors have suggested that an excess of polyphenols can cause damage to human health rather than provide beneficial effects. For example, in the case of the tannins, which are associated with the inhibition of vasoconstriction, the benefit is only seen when consumed in moderate amounts (Taiz and Zeiger, 2006), while excesses are associated with possible carcinogenic roles and toxicity to the thyroid gland, among others (Balasundram et al. , 2006); this coincides with a widely known concept wherein polyphenols are a heterogeneous group of molecules and part of the secondary metabolism of plants used to protect themselves not only from pathogen agents, but also from potential predators, causing digestive problems when plant tissues are consumed because, in some cases, tannins generate a protein precipitation related to the covalent bonds between them (Taiz and Zeiger, 2006).
CONCLUSIONS
The use of agro-industrial co-products allows for the mitigation of environmental problems and the nutritional improvement of food products, among others. Because these co-products can comprise up to 70% of the fresh fruit, depending on the fruit species, and due to the poor disposal of these materials as simply trash, pollution problems are generated everywhere they are deposited. However, with appropriate processing, it is possible to exploit the content of bioactive compounds, such as dietary fiber, polyphenols, and proteases, which are currently highly appreciated by consumers and the health and pharmaceutical sectors because their nutritional properties go beyond simply providing simple calories and other components, such as proteins, carbohydrates and fats, and may come to be recommended for ingestion with processed products in order to avoid the appearance of chronic diseases. Considering the above, along with the elevated quantity that is produced by some of the mentioned fruit species, this review evidenced the large industrial, chemical-pharmaceutical and new product development potential for processed foods that exists in Colombia, which can be realized through the discovery, appropriate manipulation, and processing of agro-industrial fruit co-products.
REFERENCES
Abdalla, A., S.M. Darwish, E.H.E. Ayad and R.M. El-Hamahmy. 2007. Egyptian mango by-product 1. Compositional quality of mango seed kernel. Food Chemistry 103(4): 1134-1140. DOI: http://dx.doi.org/10.1016/j.foodchem.2006.10.017. [ Links ]
Adão, R.C. and M.A. Glória. 2005. Bioactive amines and carbohydrate changes during ripening of 'Prata' banana (Musa acuminata x Musa balbisiana). Food Chemistry 90(4): 705-711. DOI: 10.1016/j.foodchem.2004.05.020. [ Links ]
Agronet. 2013. Red de Informacion y Comunicacion del Sector Agropecuario. Ministerio de Agricultura y Desarrollo Rural, Republica de Colombia, http://www.agronet.gov.co [ Links ]
Ajila, C.M., S.G. Bhat and U.J.S. Prasada. 2007. Valuable components of raw and ripe peels from two Indian mango varieties. Food Chemistry 102(4): 1006-1011. DOI: 10.1016/j.foodchem.2006.06.036. [ Links ]
Ajila, C.M., S.K. Brar, M. Verma, R.D. Tyagi and J.R. Valéro. 2011. Solid-state fermentation of apple pomace using Phanerocheate chrysosporium - Liberation and extraction of phenolic antioxidants. Food Chemistry 126(3): 1071-1080. DOI: 10.1016/j.foodchem.2010.11.129. [ Links ]
Ajila, C.M., L. Jaganmohan and U.J.S. Prasada. 2010. Characterization of bioactive compounds from raw and ripe Mangifera indica L. peel extracts. Food and Chemical Toxicology 48(12): 3406-3411. DOI: 10.1016/j.fct.2010.09.012. [ Links ]
Ajila, C.M., K. Leelavathi and U.J.S. Prasada. 2008. Improvement of dietary fiber content and antioxidant properties in soft dough biscuits with the incorporation of mango peel powder. Journal of Cereal Science 48(2): 319-326. DOI: 10.1016/j.jcs.2007.10.001. [ Links ]
Akkerman, R. and D.P. Van Donk. 2008. Development and application of a decision support tool for reduction of product losses in the food-processing industry. Journal of Cleaner Production 16(3): 335-342. DOI: 10.1016/j.jclepro.2006.07.046. [ Links ]
Alarcón, M.A., J.H. López y D.A. Restrepo. 2013. Caracterizacion de las propiedades funcionales tecnologicas de una fuente rica en fibra dietaria obtenida a partir de cáscara de plátano. Revista Facultad Nacional de Agronomía 66(1): 6959 - 6968. [ Links ]
Alarcón, M.Á., J.H. López y D.A. Restrepo. 2014. Efecto de la inclusión de una fuente de fibra dietaria sobre la degradación lipídica y proteica de un producto cárnico tipo hamburguesa. Revista Chilena de Nutrición 41: 77-84. DOI: http://dx.doi.org/10.4067/S0717-75182014000100011. [ Links ]
Aleson, L., J. Fernández, J.A. Pérez and V. Kuri. 2005. Characteristics of beef burger as influenced by various types of lemon albedo. Innovative Food Science and Emerging Technologies 6(2): 247-255. DOI: http://dx.doi.org/10.1016/j.ifset.2005.01.002. [ Links ]
American Dietetic Association. 2008. Position of the American Dietetic Association: Health implications of dietary fiber. Journal of the American Dietetic Association 108(10): 1716-1731. DOI: 10.1016/j.jada.2008.08.007. [ Links ]
Annadurai, G., R. Juang and D. Lee. 2002. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. Journal of Hazardous Materials 92(3): 263-274. DOI: 10.1016/s0304-3894(02)00017-1. [ Links ]
Aurore, G., B. Parfait and L. Fahrasmane. 2009. Bananas, raw materials for making processed food products. Trends in Food Science and Technology 20(2): 78-91. DOI: 10.1016/j.tifs.2008.10.003. [ Links ]
Ayala, J.F., C. Rosas, V. Vega and G.A. González. 2010. Antioxidant enrichment and antimicrobial protection of fresh-cut fruits using their own byproducts: Looking for Aurore, G., B. Parfait and L. Fahrasmane. 2009. Bananas, raw materials for making processed food productsn. Journal of Food Science 75(8): 175 - 181. [ Links ]
Ayala, J.F., V. Vega, C. Rosas, H. Palafox, J.A. Villa, M. Siddiqui, J.E. Dávila and G.A. González. 2011. Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Research International 44(7): 1866-1874. DOI: 10.1016/j.foodres.2011.02.021. [ Links ]
Balasundram, N., K. Sundram and S. Samman. 2006. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chemistry 99(1): 191-203. DOI: 10.1016/j.foodchem.2005.07.042. [ Links ]
Benoit, I., D. Navarro, N. Marnet, N. Rakotomanomana, L. Lesage-Meessen, J.-C. Sigoillot, M. Asther and M. Asther. 2006. Feruloyl esterases as a tool for the release of phenolic compounds from agro-industrial by-products. Carbohydrate Research 341(11): 1820-1827. DOI: 10.1016/j.carres.2006.04.020. [ Links ]
Berardini, N., M. Knödler, A. Schieber and R. Carle. 2005. Utilization of mango peels as a source of pectin and polyphenolics. Innovative Food Science and Emerging Technologies 6(4): 442-452. DOI: 10.1016/j.ifset.2005.06.004. [ Links ]
Bora, P.S., N. Narain, R.V. Rocha and M.Q. Paulo. 2001. Characterization of the oils from the pulp and seeds of avocado (cultivar fuerte) fruits. Grasas y Aceites 52: 17-174. [ Links ]
Bressani, R. 2009. La composición química, capacidad antioxidativa y valor nutritivo de la semilla de variedades de aguacate. Proyecto Fodecyt 02-2006, Centro de Ciencia y Tecnología de Alimentos. Universidad del Valle de Guatemala. 56 p. [ Links ]
Cano, F., S. Jiménez, A. Villagómez, T. Sánchez, R. Guevara and R. Miranda. 2007. Identificación de compuestos presentes en cáscara de aguacate por espectrometría de masas. IX Congreso de Ciencia de los Alimentos y V Foro de Ciencia y tecnología de Alimentos. [ Links ]
Castro, H., L.P. Restrepo and F. Parada. 2010. Semillas de Guayaba: ¿Residuo o Subproducto? In Morales, A.L. and L.M. Melgarejo (Eds.): Desarrollo de Productos Funcionales Promisorios a PArtir de la Guayaba (Psidium guajava L.) para el Fortalecimiento de la Cadena Productiva (Vol. 1, pp. 187-198). Colombia: Facultad de Ciencias, Universidad Nacional de Colombia. [ Links ]
Caygill, J.C. 1979. Sulphydryl plant proteases. Enzyme and Microbial Technology 1(4): 233-242. DOI: http://dx.doi.org/10.1016/0141-0229(79)90042-5. [ Links ]
Cerezal, P., J.A. Larrauri and R.M. Piñera. 1995. Influencia de factores en el aprovechamiento de subproductos de la industria de frutas y vegetales en Cuba. Alimentaria: Revista de Tecnología e Higiene de los Alimentos 268: 101-105. [ Links ]
Clarke, W.P., P. Radnidge, T.E. Lai, P.D. Jensen and M.T. Hardin. 2008. Digestion of waste bananas to generate energy in Australia. Waste Management 28(3): 527-533. DOI: 10.1016/j.wasman.2007.01.012. [ Links ]
Corporacion Colombiana Internacional. 2013. Manual del exportador de frutas, hortalizas y tubérculos en Colombia. from http://www.cci.org.co/cci/cci_x/Sim/Manuales/Productos/Frutas/Guayaba/guayaba02.htm [ Links ]
Chaiwut, P., P. Pintathong and S. Rawdkuen. 2010. Extraction and three-phase partitioning behavior of proteases from papaya peels. Process Biochemistry 45(7): 1172-1175. DOI: http://dx.doi.org/10.1016/j.procbio.2010.03.019. [ Links ]
Dhillon, G.S., S.K. Brar, M. Verma and R.D. Tyagi. 2011. Utilization of different agro-industrial wastes for sustainable bioproduction of citric acid by Aspergillus niger. Biochemical Engineering Journal 54(2): 83-92. DOI: 10.1016/j.bej.2011.02.002. [ Links ]
El-Deek, A., M. Asar, S. Hamdy and A. Abdalla. 2009a. Utilization of guava by products in broiler finisher diets. Egyptian Poultry Science 29(1): 53 - 75. [ Links ]
El-Deek, A., S. Hamdy, Y. Attia and A. El-Shahat. 2009b. Guava by-product meal processed in various ways and fed in differing amounts as a component in laying hen diets. International Journal of Poultry Science 8(9): 866-874. [ Links ]
Elleuch, M., D. Bedigian, O. Roiseux, S. Besbes, C. Blecker and H. Attia. 2011. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chemistry 124(2): 411-421. DOI: 10.1016/j.foodchem.2010.06.077. [ Links ]
Endreß, H.U. 2000. High quality resulting from product integrated environment protection-PIUS. Fruit Processing 10: 273 -276. [ Links ]
Essien, J.P., E.J. Akpan and E.P. Essien. 2005. Studies on mould growth and biomass production using waste banana peel. Bioresource Technology 96(13): 1451-1456. DOI: 10.1016/j.biortech.2004.12.004. [ Links ]
Fao. 2013. Food and Agriculture Organization, FAOSTAT. Food and Agriculture Organization of the United Nations - FAO, Rome, Italy. [ Links ]
Fao. 2014. Food and Agriculture Organization, FAOSTAT. Food and Agriculture Organization of the United Nations - FAO, Rome, Italy. [ Links ]
Fernández, J., J.M. Fernández, L. Carbonell, E. Sendra, E. Sayas and J.A. Pérez. 2004a. Application of functional citrus by-products to meat products. Trends in Food Science and Technology 15(3-4): 176-185. DOI: 10.1016/j.tifs.2003.08.007. [ Links ]
Fernández, J.M., J. Fernández, E. Sayas, E. Sendra and J.A. Pérez. 2004b. Lemon albedo as a new source of dietary fiber: Application to bologna sausages. Meat Science 67(1): 7-13. DOI: 10.1016/j.meatsci.2003.08.017. [ Links ]
Fernández, L.J., E. Sendra, E. Sayas-Barberá, C. Navarro and J.A. Pérez-Alvarez. 2008. Physico-chemical and microbiological profiles of "salchichón" (Spanish dry-fermented sausage) enriched with orange fiber. Meat Science 80(2): 410-417. DOI: 10.1016/j.meatsci.2008.01.010. [ Links ]
Fernández, L.J., M.M. Viuda, E. Sendra, E. Sayas, C. Navarro and J.A. Pérez. 2007. Orange fibre as potential functional ingredient for dry-cured sausages. European Food Research and Technology 226(1): 1-6. DOI: 10.1007/s00217-006-0501-z. [ Links ]
Fernández, M.S., D. Villaño, M.C. García and A.M. Troncoso. 2004c. Antioxidant activity of wines and relation with their polyphenolic composition. Analytica Chimica Acta 513(1): 113-118. DOI: 10.1016/j.aca.2004.02.028. [ Links ]
Food and Agriculture Organization. 2009. The market for organic and fair-trade mangoes and pineapples. Food and Agriculture Organization of the United Nations - FAO, http://www.fao.org/fileadmin/templates/organicexports/docs/Market_Organic_FT_Pineapple_Mango.pdf [ Links ]
Garau, M.C., S. Simal, C. Rosselló and A. Femenia. 2007. Effect of air-drying temperature on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. Food Chemistry 104(3): 1014-1024. DOI: 10.1016/j.foodchem.2007.01.009. [ Links ]
Gonzalez, G.A., J.A. Villa, J.F. Ayala and E.M. Yahia. 2010. Improvement of the antioxidant status of tropical fruits as a secondary response to some postharvest treatments. Trends in Food Science andTechnology 21(10): 475-482. DOI: 10.1016/j.tifs.2010.07.004. [ Links ]
González, R., M. Lobo and M. González. 2010. Antioxidant activity in banana peel extracts: Testing extraction conditions and related bioactive compounds. Food Chemistry 119(3): 1030-1039. DOI: 10.1016/j.foodchem.2009.08.012. [ Links ]
Gorinstein, S., O. Martín -Belloso, Y.-S. Park, R. Haruenkit, A. Lojek, M. ĈıiŽ, A. Caspi, I. Libman and S. Trakhtenberg. 2001. Comparison of some biochemical characteristics of different citrus fruits. Food Chemistry 74(3): 309-315. DOI: 10.1016/s0308-8146(01)00157-1. [ Links ]
Grigorevski De Lima, A.L., R. Pires Do Nascimento, E.P. Da Silva Bon and R.R.R. Coelho. 2005. Streptomyces drozdowiczii cellulase production using agro-industrial by-products and its potential use in the detergent and textile industries. Enzyme and Microbial Technology 37(2): 272-277. DOI: 10.1016/j.enzmictec.2005.03.016. [ Links ]
Happi, T., R. Andrianaivo, B. Wathelet, J. Tchango and M. Paquot. 2007. Effects of the stage of maturation and varieties on the chemical composition of banana and plantain peels. Food Chemistry 103(2): 590-600. DOI: 10.1016/j.foodchem.2006.09.006. [ Links ]
Happi, T., S.N. Ronkart, C. Robert, B. Wathelet and M. Paquot. 2008. Characterisation of pectins extracted from banana peels (Musa AAA) under different conditions using an experimental design. Food Chemistry 108(2): 463-471. DOI: 10.1016/j.foodchem.2007.10.078. [ Links ]
Izquierdo, L. and J.M. Sendra. 2003. CITRUS FRUITS | Composition and Characterization. In Editor-in-Chief: Benjamin, C. (Ed.): Encyclopedia of Food Sciences and Nutrition (Second Edition) (pp. 1335-1341). : Academic Press, Oxford. [ Links ]
Kashyap, D.R., P.K. Vohra, S. Chopra and R. Tewari. 2001. Applications of pectinases in the commercial sector: a review. Bioresource Technology 77(3): 215-227. DOI: http://dx.doi.org/10.1016/S0960-8524(00)00118-8. [ Links ]
Katongole, C.B., F.B. Bareeba, E.N. Sabiiti and I. Ledin. 2008. Nutritional characterization of some tropical urban market crop wastes. Animal Feed Science and Technology 142(3-4): 275-291. DOI: 10.1016/j.anifeedsci.2007.09.002. [ Links ]
Khalili, M., C. Abdullah and A. Manaf. 2012. Total antioxidant activity, total phenolic content and radical scavenging activity both flesh and peel of red pitaya, white pitaya and papaya. International Journal of Pharmacy and Pharmaceutical Sciences 4(2): 113 - 122. [ Links ]
Kim, H., J.Y. Moon, H. Kim, D.-S. Lee, M. Cho, H.-K. Choi, Y.S. Kim, A. Mosaddik and S.K. Cho. 2010. Antioxidant and antiproliferative activities of mango (Mangifera indica L.) flesh and peel. Food Chemistry 121(2): 429-436. DOI: 10.1016/j.foodchem.2009.12.060. [ Links ]
Li, Y., C. Guo, J. Yang, J. Wei, J. Xu and S. Cheng. 2006. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chemistry 96(2): 254-260. DOI: 10.1016/j.foodchem.2005.02.033. [ Links ]
Lousada, J.E., J.M. Costa, J.N. Neiva and N.M. Rodriguez. 2007. Physical-chemical characterization of tropical fruit by-products for use in animal feed. Revista Ciência Agronômica 37: 70 - 76. [ Links ]
Lu, Y. and L. Foo. 2000. Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chemistry 68(1): 81-85. DOI: http://dx.doi.org/10.1016/S0308-8146(99)00167-3. [ Links ]
Maisuthisakul, P. and M.H. Gordon. 2009. Antioxidant and tyrosinase inhibitory activity of mango seed kernel by product. Food Chemistry 117(2):332-341. DOI: http://dx.doi.org/10.1016/j.foodchem.2009.04.010. [ Links ]
Marín, F.R., C. Soler-Rivas, O. Benavente-García, J. Castillo and J.A. Pérez-Alvarez. 2007. By-products from different citrus processes as a source of customized functional fibres. Food Chemistry 100(2): 736-741. DOI: 10.1016/j.foodchem.2005.04.040. [ Links ]
Mota, R.V., F.M. Lajolo and B.R. Cordenunsi. 1997. Composição em carboidratos de algumas cultivares de banana (Musa spp.) durante o amadurecimento. Ciência e Tecnologia de Alimentos, Campinas 17(2): 94-97. [ Links ]
Oak, J.N., J. Oldenhof and H.H.M. Van Tol. 2000. The dopamine D4 receptor: one decade of research. European Journal of Pharmacology 405(1-3): 303-327. DOI: 10.1016/s0014-2999(00)00562-8. [ Links ]
Packer, V.G., M.M. Selani, F.G. Gomes, J.N. Ruiz, T.R. Augusto, C.J. Contreras and S.M. Alencar. 2010. Addition of Beet and Guava Agro-Industrial Residues in Cooked and Refrigerated Chicken Meat and its effect on lipid oxidation. Polytechinic University of Valencia, Spain. [ Links ]
Reyes, R.T., J.C. Toro, H. González, R. García, E. Reyes, A. Bolaños y A. Méndez. 2006. Plan Frutícola Nacional, Diagnóstico y Análisis de los Recursos para la Fruticultura en Colombia. Colombia: Ministerio de Agricultura y Desarrollo Rural. [ Links ]
Rha, H.J., Y. Bae, S. Lee, S. Yoo, P.S. Chang and H.G. Lee. 2011. Enhancement of anti-radical activity of pectin from apple pomace by hydroxamation. Food Hydrocolloids 25(3): 545-548. DOI: http://dx.doi.org/10.1016/j.foodhyd.2010.08.010. [ Links ]
Rodríguez, J.G., D. Morcuende and M. Estévez. 2011. Avocado by-products as inhibitors of color deterioration and lipid and protein oxidation in raw porcine patties subjected to chilled storage. Meat Science 89(2): 166-173. DOI: http://dx.doi.org/10.1016/j.meatsci.2011.04.013. [ Links ]
Romero, M.R., P. Osorio, L.A. Bello, J. Tovar and A. Bernardino. 2011. Fiber Concentrate from Orange (Citrus sinensis L.) Bagase: Characterization and Application as Bakery Product Ingredient. International Journal of Molecular Sciences 12(4): 2174-2186. [ Links ]
Salas, C.E., M.T.R. Gomes, M. Hernandez and M.T.P. Lopes. 2008. Plant cysteine proteinases: Evaluation of the pharmacological activity. Phytochemistry 69(12): 2263-2269. DOI: http://dx.doi.org/10.1016/j.phytochem.2008.05.016. [ Links ]
Schieber, A., P. Hilt, P. Streker, H.U. Endreß, C. Rentschler and R. Carle. 2003. A new process for the combined recovery of pectin and phenolic compounds from apple pomace. Innovative Food Science and Emerging Technologies 4(1): 99-107. DOI: http://dx.doi.org/10.1016/S1466-8564(02)00087-5. [ Links ]
Schieber, A., P. Keller and R. Carle. 2001a. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. Journal of Chromatography A 910(2): 265-273. DOI: 10.1016/s0021-9673(00)01217-6. [ Links ]
Schieber, A., F.C. Stintzing and R. Carle. 2001b. By-products of plant food processing as a source of functional compounds - recent developments. Trends in Food Science & Technology 12(11): 401-413. DOI: 10.1016/s0924-2244(02)00012-2. [ Links ]
Serena, A. and K.E.B. Knudsen. 2007. Chemical and physicochemical characterisation of co-products from the vegetable food and agro industries. Animal Feed Science and Technology 139(1-2): 109-124. DOI: 10.1016/j.anifeedsci.2006.12.003. [ Links ]
Silva, L.M., E.A. Figueiredo, N.M. Ricardo, I.G. Vieira, R. Figueiredo, I.M. Brasil and C.L. Gomes. 2014. Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chemistry 143(0): 398-404. DOI: http://dx.doi.org/10.1016/j.foodchem.2013.08.001. [ Links ]
Someya, S., Y. Yoshiki and K. Okubo. 2002. Antioxidant compounds from bananas (Musa Cavendish). Food Chemistry 79(3): 351-354. DOI: 10.1016/s0308-8146(02)00186-3. [ Links ]
Soobrattee, M.A., V.S. Neergheen, A. Luximon-Ramma, O.I. Aruoma and T. Bahorun. 2005. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 579(1-2): 200-213. DOI: 10.1016/j.mrfmmm.2005.03.023. [ Links ]
Soong, Y. and P.J. Barlow. 2004. Antioxidant activity and phenolic content of selected fruit seeds. Food Chemistry 88(3): 411-417. DOI: http://dx.doi.org/10.1016/j.foodchem.2004.02.003. [ Links ]
Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, T.N.A. 2005. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). A Report of the Panel on Macronutrients Subcommittees on Upper Reference Levels of Nutrients Interpretation Uses of Dietary Reference Intakes. NAP. [ Links ]
Sudha, M.L., V. Baskaran and K. Leelavathi. 2007. Apple pomace as a source of dietary fiber and polyphenols and its effect on the rheological characteristics and cake making. Food Chemistry 104(2): 686-692. DOI: http://dx.doi.org/10.1016/j.foodchem.2006.12.016. [ Links ]
Taiz, L. and E. Zeiger. 2006. Plant Physiology (Third Edition ed.). Google Books: Publicacions Universitat Jaume I. [ Links ]
Tchobanoglous, G., H. Theisen and S. Vigil. 1993. Integrated Solid Waste Management: Engineering Principles and Management Issues. Google books: MCGraw-Hill, New York. [ Links ]
Thongsombat, W., A. Sirichote and S. Chanthachum. 2007. The production of guava juice fortified with dietary fiber. Songklanakarin Journal Science and Technology 29(1): 187-196 [ Links ]
Tokuhisa, D., D. Fernandes Dos Santos, E. Mantovani, P. Hilst e A. Demuner. 2007. Compostos fenólicos inibidores da germinação em sementes de mamão (Carica papaya L.). Revista Brasileira de Sementes 29(3): 180-188. [ Links ]
Turhan, S., I. Sagir and U.N. Sule. 2005. Utilization of hazelnut pellicle in low-fat beef burgers. Meat Science 71(2): 312-316. DOI: 10.1016/j.meatsci.2005.03.027. [ Links ]
Vergara, N., E. Granados, E. Agama, J. Tovar, J. Ruales and L.A. Bello. 2007. Fibre concentrate from mango fruit: Characterization, associated antioxidant capacity and application as a bakery product ingredient. LWT - Food Science and Technology 40(4): 722-729. DOI: 10.1016/j.lwt.2006.02.028. [ Links ]
Viuda, M.M., N.Y. Ruiz, L.J. Fernández and A.J.A. Pérez. 2010a. Effect of added citrus fibre and spice essential oils on quality characteristics and shelf-life of mortadella. Meat Science 85(3): 568-576. DOI: 10.1016/j.meatsci.2010.03.007. [ Links ]
Viuda, M.M., N.Y. Ruiz, L.J. Fernández and A.J.A. Pérez. 2010b. Effect of orange dietary fibre, oregano essential oil and packaging conditions on shelf-life of bologna sausages. Food Control 21(4): 436-443. DOI: 10.1016/j.foodcont.2009.07.004. [ Links ]
Wolfe, K.L. and R.H. Liu. 2003. Apple peels as a value-added food ingredient. Journal of Agricultural and Food Chemistry 51(6): 1676-1683. DOI: 10.1021/jf025916z. [ Links ]