Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad Nacional de Agronomía Medellín
Print version ISSN 0304-2847
Rev. Fac. Nac. Agron. Medellín vol.69 no.1 Medellín Jan./June 2016
https://doi.org/10.15446/rfna.v69n1.54744
DOI: http://dx.doi.org/10.15446/rfna.v69n1.54744
Life-cycle parameters of Copitarsia uncilata (Lepidoptera: Noctuidae) on three natural diets
Parámetros del ciclo biológico de Copitarsia uncilata (Lepidoptera: Noctuidae) en tres dietas naturales
Ana Milena Castro Márquez1, and Daniel Rodríguez Caicedo1
1 Laboratorio de Control Biológico - Facultad de Ciencias Básicas y Aplicadas. Universidad Militar Nueva Granada. A.A. 49300, Cajicá, Cundinamarca, Colombia. <ecología@unimilitar.edu.co>
Received: May 14, 2015; Accepted: August 31, 2015
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ABSTRACT
This study describes the life cycle of Copitarsia uncilata Burgos & Leiva (Lepidoptera: Noctuidae) under laboratory conditions without photophase and a second experiment with photophase of 12 hours on three natural diets. The life cycle of C. uncilata was significantly shorter for females (76.46 ± 1.01 days, p=0.033) reared on alstroemeria (Alstroemeria sp.) diet without photophase, and for males (79.78 ± 0.36 days, p=0.046) reared on broccoli (Brassica oleracea italica), with photophase. The emergence of the adults was 100% and 73.33% from larvae fed on alstroemeria, 90.9% and 88.88% for individuals fed on broccoli, 86.2% and 50% for those fed on cauliflower (Brassica oleracea var. botrytis), without and with photophase respectively. The sex ratio (male:female) of individuals reared without photophase, evidenced a higher rate of females on alstroemeria (1:1.3), followed by cauliflower (1:0.6) and broccoli (1:0.5). In the experiment with photophase, the sex ratio was higher on alstroemeria (1:1.5), followed by cauliflower (1:0.9) and broccoli (1:0.6). As a conclusion, the most suitable diet for laboratory mass rearing in terms of life cycle parameters of C. uncilata is broccoli followed by alstroemeria and cauliflower.
Key words: Mass rearing, Brassica spp., Alstroemeria sp., Insect development, Insect stages, Moth
RESUMEN
Este estudio describe el ciclo biológico de Copitarsia uncilata Burgos & Leiva (Lepidoptera: Noctuidae) en condiciones de laboratorio, sin fotofase y 12 horas de fotofase, criado bajo tres dietas naturales. El ciclo de vida de C. uncilata fue significativamente más corto (76,46 ± 1,01 días, p=0,033) en hembras criadas en la dieta de alstroemeria (Alstroemeria sp.) sin fotofase, y el ciclo más corto (79,78 ± 0,36 días, p=0,046) en machos criados con broccoli (Brassica oleracea italica) con fotofase. La emergencia de los adultos fue 100% y 73.33% de larvas alimentadas con alstroemeria, 90,9% y 88,88% alimentadas con broccoli, 86,2% y 50% alimentadas con coliflor (Brassica oleracea var. botrytis), sin fotofase y con exposición a fotofase respectivamente. La proporción sexual (macho:hembra) de individuos sin fotofase, fue más alta en alstroemeria (1:1,3), seguido por coliflor (1:0.6) y broccoli (1:0,5). En el experimento con fotofase, la proporción sexual fue más alta en alstroemeria (1:1,5), seguido por coliflor (1:0,9) y broccoli (1:0,6). Como conclusión, la dieta más apropiada para cría en masa bajo condiciones de laboratorio en términos de los parámetros de ciclo biológico de C. uncilata es broccoli seguido por alstroemeria y coliflor.
Palabras claves: Cría en masa, Brassica spp., Alstroemeria sp., Desarrollo de insectos, Estado de insectos, Polilla.
Selection of suitable diet is an important key factor to establish a rearing system, in order to maintain a high number of insects under a diet easy of handling, and providing all the nutritional requirements at low cost of maintenance. The ideal host must provide a well development of the population in all the biological parameters as reproduction, survival, and completed life cycle (Singh, 1982). Natural diets are the host of a given organism under natural conditions and usually provide a complete source of nutrients. Because of this, natural diets tend to be successful for rearing insects. Contrary to artificial diets, which are widely used for mass rearing insects and use materials that require knowing the nutritional components to establish the correct conditions that the insects need (Carson, 2003). As a result, evaluation of artificial diets for growth of pests demands higher times than natural diets (Singh, 1982).
Copitarsia genus includes polyphagous species that affect a variety of fresh commodities causing high economic damage in the products. In the United States of America Copitarsia species are considered a quarantine pest and a risk to agriculture (Gould and Maldonado, 2006) and are commonly intercepted in commodities from Latin America, mainly originated from Colombia. Interceptions by Copitarsia spp. reported between the years 2000 and 2010 in commodities from Colombia were nearby of 6,577 that represents 60.92% of the total of interceptions (Gould et al., 2013). The most common products intercepted arriving from Colombia include Alstromeria sp. (Angulo and Olivares, 2003) and Brassica spp. (Pogue, 2014).
In Colombia, Cundinamarca is a department that produces a great variety of commodities to export which can be intercepted at US border crossing. The Copitarsia species associated with local crops are possibly a complex (Angulo and Olivares, 2010), from which C. unicalata (Lepidoptera: Noctuidae) and C. decolora (Lepidoptera: Noctuidae) are part. C. uncilata has been associated to cut flowers and species of Brassica oleraceae in Colombia and Mexico, it has an external morphology and alar pattern similar to those present in Copitarsia decolora and has been characterized as a new species, based principally on differences in the adult genitalia (Burgos et al., 2010).
C. uncilata has not been reported in studies of biology or rearing in laboratory whereas C. decolora has been well studied in field and laboratory, and it has been reared on different natural hosts such as alfalfa (Medicago sativa) (Urra and Apablaza, 2005), broccoli (Brassica oleracea var. italica), cauliflower (Brassica oleracea var. botrytis), cabbage (Brassica oleracea var. viridis) (Acatitla, 2010; Moreno and Serna, 2006; Peraza, 2011), corn (Zea mays), alstroemeria (Alstroemeria sp.) potato (Solanum tuberosum), dutch clover (Trifolium repens), rosemary (Rosmarinus officinalis), chili (Capsicum annuum), onion (Allium cepa) and physalis (Physalis peruviana). The highest survival rates of C. decolora have been found in cabbage, alstroemeria, broccoli and asparagus (Huaman, 2007; Gould and Maldonado, 2006). Some other species of the genus have been studied under different natural diets. C. turbata has been reared on onion (Velázquez, 1987), and C. incomoda has been reared on broccoli, cauliflower, and cabbage (Flores et al., 2004)
Mass insect rearing is an important strategy to know the basic biology of pest species, in turn essential to design integrated pest management programs (Jesper et al., 2012) using a continuous colony of insects in the laboratory under a suitable host easy to handle with a minimum time and labor. As a result, this research was made to identify the best of three diets for feeding of C. uncilata, in terms of the life cycle of the pest.
MATERIALS AND METHODS
Insect Collection
A small laboratory colony was started from field-collected eggs and larvae of C. uncilata from the youngest leaves of alstroemeria in a commercial crop located in El Rosal, Cundinamarca, Colombia (04º 86.03' 31" N and 74º 22.41' 12"). Individuals were kept in plastic transparent containers (15 mL) with alstroemeria leaves inside and covered with fine mesh. The specimens were transported to the biological control laboratory of Nueva Granada Military University and placed in a rearing room under similar conditions to those found in field (19.7 ± 0.4 °C and 58.4 ± 5.6% RH and without photophase).
Insect rearing
The experiments were carried between October 2013 and November of 2014 and began after establishing a continuous breeding colony on each diet. Couples (1:1 male to female ratio) were placed in mating chambers (plastic containers of 14 x 17 x 9.5 cm), leaves of alstroemeria were placed inside the chamber as an oviposition substrate. Food source for the adult moths was provided using cotton flakes moistened with honeydew. The chambers were covered with fine mesh on the top for ventilation. Eggs (n= 90) were collected with a fine brush and deposited individually in cylindrical container (9 cm diameter x 5 cm height) covered with fine mesh cloth on each container for ventilation. Hatched eggs and neonate larvae were reared on each treatment: 1. Leaves, stems and flowers of alstroemeria (Control) (Alstroemeria sp.), n=30; 2. Leaves and flower head of broccoli, n=30; and 3. Leaves and flowers head of Cauliflower, n=30. After 24 h, excreta were removed from the containers and fresh food was provided daily until pupation stage.
The experimental units were arranged on a completely random design, making daily individual observations on each individual as a repetition. The life cycle was evaluated by the duration of each biological stage at 19.7 ± 0.4 °C and 58.4 ± 5.6% RH without photophase during the first generation of the experiment. Since temperature and photoperiod can influence the end of the larval stage for pupation (Kollberg et al., 2013) and in order to obtain synchronization in the adult emergence, previous studies of biology of Copitarsia have included photoperiod (Acatitla et al., 2004; Gould and Maldonado, 2006; Muñoz et al., 2007). Considering this aspect, the second generation was reared at 19.5 ± 0.1 °C, 63.8 ± 0.4% RH, and photophase 12 h using fluorescent light tubes and the experiment started with 90 eggs from the first experiment.
Larval stage
Larvae fed on each natural diet were individually placed with a fine hairbrush in petri dishes to captured images of head capsule and body using a camera Sony Cyber-Shot DSC-W180. After observations each larvae was returned to the containers of rearing. The images obtained were analyzed in the ImageJ 1.38e program to measure body length of individuals and width of cephalic capsule, which allows identify the larval instar. Daily observations of the presence of exuviae were done to confirm the change of instar.
Pupal stage
The prepupae period was assumed occurring when the larvae decreased in size and stopped feeding. Pupae were maintained in the same rearing containers with a moistened piece of filter paper inside until the adult emergence. The pupae were sexed locating the specific genitalia in the last abdominal segments according to Moreno and Serna (2006) in a stereoscope (Motic SMZ-168). The pupae duration stage was recorded by daily observations.
Adult stage
Adults were kept in plastic containers individualized and separated by sex. As soon as the adults emerged, they were organized in couples (male:female) (N=45) in the mating chambers previously described. Longevity, emergence percentage and sex ratio were recorded on each treatment.
Identification
The specimens were identified by the genitalia of male adults. The morphology of the genitalia was studied in abdomens placed in KOH 10% during 30 min at 90 °C for clearing (Suarez et al., 2006; Brambila, 2009).
Genitalia were placed in petri dishes to capture images with a camera Sony Cyber-Shot DSC-W180 in a stereoscope (Motic SMZ-168) and preserved in alcohol at 70%. Morphological traits were compared with the description of the genus Copitarsia (Castillo y Angulo, 1991; Angulo y Olivares, 2003; Simmons and Pogue, 2004; Angulo et al., 2008; Quimbayo et al., 2010). Additionally, photographs were sent to the Department of Zoology, Faculty of Natural and Oceanographic Sciences, Universidad de Concepción. Results confirmed the species C. uncilata by having male genitalia with a broad spatulated uncus, apex of the digitus concave and ampulla elongated and recurved in the apex. Although Pogue (2014) described Copitarsia uncilata Burgos & Leiva 2010 as a synonymy of Copitarsia decolora Gunné, the authors of C. uncilata submitted a paper for publication to the Journal SHILAP-Revista de Lepidopterología to revalidate the species1. According to the previous lines the species evaluated in the present study was referred as C. uncilata.
Statistical analyses
The collected data were analyzed using the R 3.1.2 software. Shapiro-Wilk normality tests were made to check normality of residuals and Bartlett's test to evaluate homoscedasticity. Data of width of the cephalic capsule and length of the larval body were analyzed by one-way ANOVA. Comparisons of averages between the treatments were made with Duncan's multiple range test. Generalized Linear Models (GLM) employing Poisson distribution were used to analyze differences in the duration of the egg, larvae, prepupae, pupae and adult stage on the diets (broccoli and cauliflower) compared with the control (alstroemeria) (Mengistu et al., 2009). Differences between treatments were assumed when p-values were less than 0.05 (P< 0.05).
RESULTS AND DISCUSSION
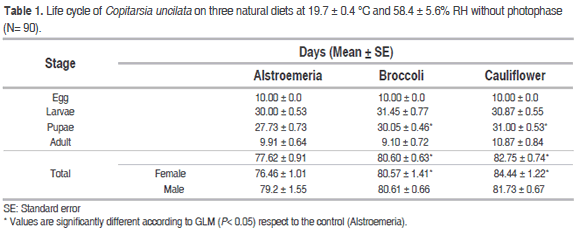
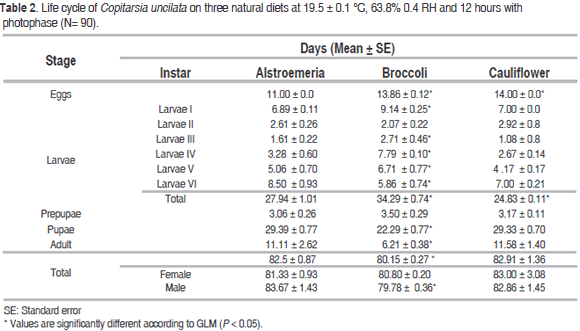
Effect of diets on duration of the developmental stages of C. uncilata without photophase is shown in Table 1 and photophase in Table 2. Individuals of the first generation without photophase had not statistical differences in the duration of egg stage (10 days) on the three diets. The second generation with 12 h of photophase presented a longer duration of the egg stage on cauliflower (14.00 ± 0.0 days, p=0.021) and broccoli (13.86 ± 0.12 days, p=0.022) than the control alstroemeria. Incubation period of C. uncilata reported here was longer compared with other studies of Copitarsia spp.: the average duration of egg stage of C. decolora on alstroemeria was 6 days at 17.7 ± 0.7 °C and 4 days at 23.72 ± 1.56 °C (Moreno and Serna, 2006), 3 to 4 days on broccoli and cauliflower for C. incomoda at 25 ± 3 °C (Flores et al., 2004), and 5.037 ± 0.21 days in Copitarsia spp. reared on brassicas in laboratory conditions (Cardona et al., 2004).
The average duration of the larval stage of C. uncilata in the cohorts without photophase was not statistically different respect to alstroemeria on broccoli (31.45 ± 0.77; p=0.366) and cauliflower (30.87 ± 0.55; p=0.420). For the cohorts with photophase, larval stage duration was significantly longer on broccoli (34.29 ± 0.74; p=0.0094) respect to alstroemeria, but shorter in Cauliflower. These results are close to the reported for duration of the larval stage of C. incomoda on cauliflower (30 days) and on broccoli (33 days) at a higher temperature (Flores et al., 2004), and of C. decolora on alstroemeria (35.10 days) (Moreno and Serna, 2006).
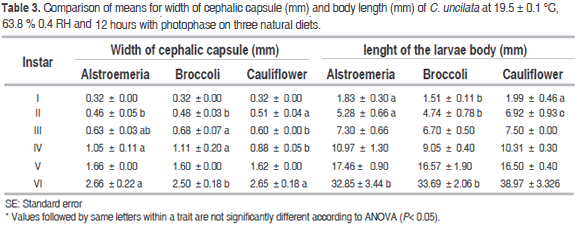
According to the measures of cephalic capsule, six larval instars were present on the three diets, which agrees with the work of Cardona et al. (2004): Copitarsia sp. fed on cauliflower and cabbage goes through six instars, but differs from the report of Moreno and Serna (2006) who found five larval instars in C. decolora reared on alstroemeria.
The diets evaluated had an effect on the width of cephalic capsule and length body of C. uncilata larvae (Table 3). In the first instar, the width of cephalic capsule had not significant difference among the diets but the length of the body was shortest on broccoli (1.51 ± 0.11, f=47.47 p= 3.2e-12). In the second instar, the width of the capsule cephalic was significant greater on cauliflower (0.51 ± 0.04, f = 4.34, p= 0.024), the length of the body had significant differences between the three diets (5.28 ± 0.66 mm, 4.74 ± 0.78, 6.92 ± 0.93, f= p= 3.23e-12 on alstroemeria, broccoli and cauliflower respectively). In the third instar, the width of cephalic capsule was significant different between broccoli and cauliflower (0.68 ± 0.07 and 0.60 ± 0.00, f= 4.34, p= 0.0246) although the length of the larvae had not significant differences among the diets. In the four instars, the width of the capsule was significant shortest on cauliflower (0.88 ± 0.05, f=19.93, p=1.87e-07) and the length of the larvae was not different among the diets. For the fifth instar, width of cephalic capsule and body length of larvae had not significant differences among the diets. The cephalic capsule was significantly shorter for broccoli (2.50 ± 0.18, f= 4.136, p= 0.0199) and the length had not significant difference in the sixth instar. According to results of Moreno and Serna (2006), C. decolora on alstroemeria reached a maximum width of Cephalic capsule and body length of 2.47 mm and 21.07 mm respectively in the maximum instar, such measures were smaller than the obtained for C. uncilata in this study.
The duration of the pupal stage without photophase was significantly longer respect to alstroemeria on cauliflower (31.00 ± 0.53 days, p=0.001) followed by broccoli (30.05 ± 0.46 days; p=0.001). The Duration of pupal stage with photophase was significantly shorter on broccoli (22.29 ± 0.77, p = 0.0001) respected to the control. Other study showed that the duration of larval stage of C. incommoda was 45 days on cauliflower and 49 days on broccoli at a higher temperature 25 ± 3 °C (Flores et al., 2004). Duration of the pupae stage of C. decolora reared on alstroemeria (Moreno and Serna, 2006) was 30.4 days at a lower temperature 17.72 ± 1.56 °C than the employed for rearing of C. uncilata in this study.
Longevities of adult females and males were not different on the three diets without photophase, however for individuals of the second generation reared with photophase, the longevity was significantly shorter on broccoli (6.21 ± 0.38; p=0.00005) than on alstroemeria and cauliflower. Studies of adult longevity of C. decolora on alstroemeria at 17.72 ± 1.56 °C reported longer longevity (18.44 days for females and 15 days for males) (Moreno and Serna, 2006) than the results obtained here for C. uncilata.
The life cycle of C. uncilata without photophase was significantly shorter on females reared on asltroemeria (76.46 ± 1.01, p=0.033), while differences were not found for males. The duration of the life cycle with photophase was significantly shorter on males reared on broccoli (79.78 ± 0.36, p=0.046), although averages for females were not different. These results are close for males (77.1 days) and females (80 days) of C. decolora reared on alstroemeria (Moreno and Serna, 2006). The life cycle span of the entire cohort of C. incomoda was 75 days on cauliflower and 74 days on broccoli at a higher temperature than the used in this work (25 ± 3 °C) (Flores et al., 2004).
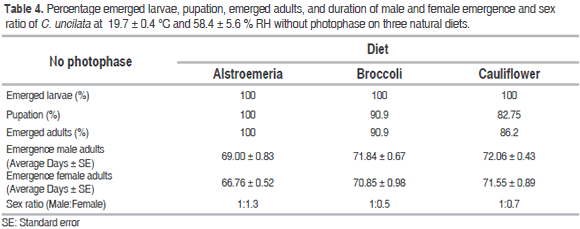
For cohorts evaluated without photophase, the emergence of larvae was 100% on the three diets (Table 4). Percentage of pupation was highest from larvae fed on alstroemeria (100%), followed by broccoli (90.9%) and cauliflower (82.75%). The greatest percentage of emerged adults was from larvae fed on alstroemeria (100%), followed by broccoli (90.9%) and cauliflower (86.2%). The longest values for durations of male and female adults emergence were obtained from larvae reared on cauliflower (71.84 ± 0.67 days and 70.85 ± 0.98 days). Such parameters were minor on broccoli (71.84 ± 0.67 days and 70.85 ± 0.98 days) and the control (69.00 ± 0.83 days and 66.76 ± 0.52 days) respectively.
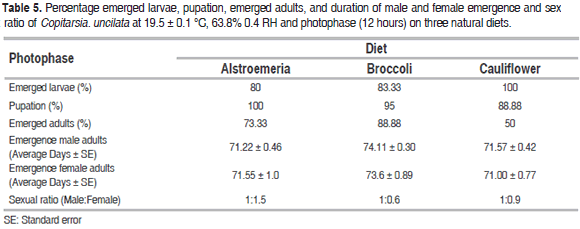
Individuals reared with photophase showed a larval emergence of 100% on cauliflower, 83.33% on broccoli and 80% on alstroemeria (Table 5). Percentage of pupation was greater in larvae fed on alstroemeria (100%) than on broccoli (95%) and cauliflower (88.8%). The greatest percentage of emerged adults was from larvae fed on broccoli (88.8%), followed by alstroemeria (73.33%) and cauliflower (50%). The emergence of male and female adults was longer from larvae reared on cauliflower (72.06 ± 0.43 days and 71.55 ± 0.89 days) than those reared on broccoli (71.84 ± 0.67 days and 70.85 ± 0.98 days) and the control (69.00 ± 0.83 days and 66.76 ± 0.52 days) respectively.
According to the results, the male and female emergence times were similar in C. uncilata reared with photophase, which could be attributed to the importance of photoperiod for the normal behavior of individuals (Kostal and Hodek, 1997), since insects must go through each developmental phase at a specific synchronized time (Fitz-Earle and Barclay, 1989): particularly for mating it is important that the adults emerge at the same time (Elsey, 1982).
Regarding the sexual ratio without photophase, for each male there are 1.3 females on alstroemeria, 0.5 females on broccoli and 0.7 females on cauliflower. Rearing with photophase for each male there are 1.5 females on alstroemeria, 0.5 females on broccoli and 0.8 females on cauliflower. Cardona et al. (2004) reported a sex ratio of 1:1 for copitarsia sp. fed on brassicas. The predominance of females over males on the alstroemeria diet allow to predict the dynamic of the population in the mass rearing of C. uncilata, because of females can increase the population in the next generation to study pest control in laboratory conditions (Pereira et al., 2013).
In addition to the conditions of rearing (temperature, humidity and photophase), the quality and nutrition of the diet affect the behavior of insects (Grenier and Clercq, 2003; Carson, 2004; Genç, 2006; Farahani et al., 2011), explaining the differences in the estimates of C. uncilata on the three diets evaluated in this study. The effect on the biological parameters of C. uncilata of the three diets could be explained by differences in the quality of the food offered: characteristics as thickness affect that larvae in the first instars avoiding feeding (Peraza, 2011; Flores et al., 2004), in our research, cauliflower and broccoli have thicker leaves and stems than alstroemeria and the first instars preferred to feed of young leaves which are softer. Additionally, secondary metabolites of brassicales, which are produced by the plants as defense of phytophagous insects (Bohinc et al., 2012), can affect biological parameters. Flores et al. (2004) and Peraza (2011) found differences in the life cycle of C. decolora on brassicales that might be attributed to secondary metabolites.
CONCLUSIONS
As a conclusion alstroemeria, broccoli and cauliflower are suitable hosts for laboratory mass rearing of C. uncilata. Alstroemeria and broccoli might be better suitable hosts than cauliflower due to the higher percentage of adults emerged. Individuals reared on broccoli presented the shortest duration of the life cycle respect to the control alstroemeria independently of other rearing conditions. However, broccoli is not the best host regarding to handling facility for a rearing process compared with alstroemeria and cauliflower, because broccoli heads dehydrated quickly to the temperature of rearing, therefore, the diet requires replacing each 12 h, and remove the first instars larval from the spaces from each small flower takes a long time. In any case, the behavior showed of the moth allowing to decide the suitable host for providing individuals in the quantities required in the laboratory and understand the feeding habits if C. unicilata on those crops for future evaluations on pest control.
ACKNOWLEDGEMENTS
This research was supported by CIAS-1465 Project, funded by the research vicerectory of Universidad Militar Nueva Granada. Dr. Andrés Angulo O. from the Faculty of Natural and Oceanographic Sciences of the Universidad de Concepción kindly supported us with the identification of specimens of C. uncilata.
REFERENCES
Acatila, C., N. Bautista., N. Vergara., J. Romero y C. Calyecac. 2004. Ciclo biológico y tasas de supervivencia y reproducción de Copitarsia incommoda Walker (Lepidoptera:Noctuidae) en cinco dietas artificiales. Agrociencia 38: 355-363. [ Links ]
Acatitla, C. 2010. Copitarsia decolora Guenée: Su preferencia por brócoli, col y coliflor, su caracterización molecular y de tres de sus himenópteros parasitoides. Tesis de Doctorado en Ciencias, Postgrado de Fitosanidad, Entomología y Acaralogía. Colegio de Postgrados, Texoco, México. 68 p. [ Links ]
Angulo, A.O. and T.S. Olivares. 2003. Taxonomic update of the species of Copitarsia Hampson 1906, (Lepidoptera: Noctuidae: Cuculliinae). Gayana 67(1): 33-38. doi: 10.4067/S0717- 65382003000100005. [ Links ]
Angulo, A. y T. Olivares 2010. La polilla Copitarsia decolora: revisión del complejo de especies con base en la morfología genital masculina y de los huevos (Lepidoptera: Noctuidae). Revista de Biologia Tropical 58: 769-776. doi: 10.15517/rbt.v58i2.5244. [ Links ]
Bohinc, T., G. Ban., D. Ban and S. Trdan. 2012. Glucosinolates in plant protection strategies: A review. Archives of Biological Sciences 64(3): 821-828. doi: 10.2298/ABS1203821B. 821. [ Links ]
Brambila, J. 2009. Steps for the dissection of male Spodoptera moths (Lepidoptera : Noctuidae) and notes on distinguishing S. litura and S. littoralis from native Spodoptera species. USDA-APHIS-PPQ. [ Links ]
Burgos, R., R. Leiva-Rifo., A. Angulo y T. Olivares. 2010. Copitarsia uncilata Burgos & Leiva sp. nov. de Cuculliinae para Colombia y Mexico (Lepidoptera, Noctuidae). Revista Brasileira de Entomologia 54(3): 372-375. doi: 10.1590/S0085-56262010000300005. [ Links ]
Callado, M. 2013. Effect of Age, Body Weight and Multiple Mating on Copitarsia decolora (Lepidoptera: Noctuidae) Reproductive Potential and Longevity. Journal of Insect Behavior 26(6): 860-872. doi: 10.1007/s10905-013-9401-9. [ Links ]
Cardona, D., M. Londoño y J. Jaramillo. 2004. Estudios biologicos de Copitarsia sp. (Lepidoptera: Noctuidae) bajo condiciones de Insectario. Revista Colombiana de Entomologia 30(2): 205-209. [ Links ]
Carson, A. 2003. Insect Diets: Science and Technology. CRC Press., Arizona. 312 p. [ Links ]
Elsey, K. 1982. Photoperiod and temperature effects on the occurrence and periodicity of mating in Pickleworm moths (Lepidoptera: Pyralidae). Florida Entomologist 65(4): 466-471. [ Links ]
Farahani, S., A.A. Talebi and Y. Fathipour. 2011. Life cycle and fecundity of Spodoptera exigua (Lepidoptera: Noctuidae) on five soybean varieties. Journal of Entomological Society of Iran 30(2): 1-12. [ Links ]
Fitz-Earle, M and H. Barclay. 1989. Is there an optimal sex ratio for insect mass rearing? Ecological Modelling 45(3): 205-220. doi: 10.1016/0304-3800(89)90082-3. [ Links ]
Flores, L., N. Bautista., J. Vera., J. Valdez y A. Angulo. 2004. Ciclo de vida y tasas de supervivencia y reproducción de Copitarsia incommoda Walker (Lepidoptera: Noctuidae) en tres cultivares de Brassica oleracea L. Agrociencia 38: 517-523. [ Links ]
Flores, R., M. Bautista., J. Carrasco., O. Morales y S. Quiñones. 2005. Comparación de dos técnicas de medición de cápsulas cefálicas para separar estadios larvales de Copitarsia incomoda (Walker) (Lepidoptera: Noctuidae). Acta Zoologica Mexicana 21(2): 109-113. [ Links ]
Genç, H. 2006. General principles of insect nutritional ecology. Trakya University Journal of Social Science 7(1): 53-57. [ Links ]
Gould, J. and H. Maldonado. 2006. Copitarsia decolora (Lepidoptera: Noctuidae) larvae escaping from discarded asparagus: data in support of a pathway risk analysis. Journal of Economic Entomology 99(5): 1605-1609. doi: 10.1603/0022-0493-99.5.1605. [ Links ]
Gould, J., R. Simmons and R. Venette. 2013. Copitarsia spp.: Biology and risk posed by potentially invasive Lepidoptera from South and Central America. Chapter 9. pp. 60-182. In: Peña, J. (ed.). Potential invasive pests of agricultural crops. CABI, London, UK. 412 p. [ Links ]
Grenier, S. y Clercq, P. 2003. Comparison of Artificially vs. Naturally Reared Natural Enemies and Their Potential for Use in Biological Control. Chapter 9. pp. 115-127. In: Lenteren, J. (ed). Quality control and production of biological control agents. CABI Publishing, Netherlands. 305 p. [ Links ]
Huaman, M. 2007. Uso de la irradiación gamma como tratamiento cuarentenario para el control de Copitarsia decolora (Guenée, 1852) Lepidoptera. Tesis de Pregrado de Biología. Facultad de Ciencias Biológicas. Universidad Nacional Mayor de San Marcos, Lima, Perú. 131 p. [ Links ]
Jesper, S., A. Matthew and T. John. 2012. Mass-rearing of insects for pest management: challenges, synergies and advances from evolutionary physiology. Crop Protection 38: 87-94. doi: 10.1016/j.cropro.2012.03.023. [ Links ]
Kollberg, I., H. Bylund., A. Schmidt., J. Gershenzon and C, Björkman. 2013. Multiple effects of temperature, photoperiod and food quality on the performance of a pine sawfly. Ecological Entomology 38(2): 201-208. doi: 10.1111/een.12005. [ Links ]
Kostal, V. and I. Hodek. 1997. Photoperiodism and control of summer diapause in the Mediterranean Tiger Moth Cymbalophora pudica. Journal of Insect Physiology 43(8): 767-777. [ Links ]
Mengistu, L., T. Tefera., Y. Assefa and F. Yirefu. 2009. Biology of Sesamia calamistis Hampson (Lepidoptera: Noctuidae) at Metahara. Proceeding of Ethiopa Sugar Industry Biennial 44: 35-44. [ Links ]
Muñiz, E., J. Cibrian., J. Rojas., O. Diaz., J. Valdes and N. Buatista. 2007. Capturas de Copitarsia decolora (Lepidoptera: Noctuidae) en trampas cebadas con diferentes proporciones de feromona sexual. Agrociencia 41: 575-581. [ Links ]
Moreno, O. y F. Serna. 2006. Biología de Copitarsia decolora (Lepidoptera: Noctuidae: Cuculliinae), en flores cultivadas del híbrido comercial de Alstromeria spp. Revista Facultad Nacional de Agronomía Medellín 59(1): 3257-3270. [ Links ]
Peraza, A. 2011. Preferencia de hospedero y parámetros de desarrollo de Copitarsia decolora sobre plantas seleccionadas para la diversificación del cultivo de uchuva (Physalis peruviana). Tesis de Pregrado Ingeniería Agrónomica. Facultad de Agronomía. Universidad Nacional de Colombia, Bogotá, D.C. 40 p. [ Links ]
Pereira, F.F., J.C. Zanuncio., S.O. Kassab., P.L. Pastori., R.H. Barbosa., and C. Rossoni. 2013. Biological characteristics of Palmistichus elaeisis Delvare & La Salle (Hymenoptera: Eulophidae) on refrigerated pupae of Anticarsia gemmatalis Hubner (Lepidoptera: Noctuidae. Chilean Journal of Agricultural Research. 73(2): 117-121. doi: 10.4067/S0718-58392013000200005. [ Links ]
Pogue, M.G. 2014. Review of the Copitarsia decolora (Guenée) (Lepidoptera: Noctuidae) Species complex with the description of a new species from Chile and Argentina. Neotropical Entomology 43(2): 143-153. doi: 10.1007/s13744-013-0190-9. [ Links ]
R Core Team: A language and environment for statistical computing. R foundation for Statistical Computing, Vienna, Austria, 2013. In: http://www.R-project.org/; accessed: December 2014. [ Links ]
Singh, P. 1982. The rearing of beneficial insects. New Zealand Entomologist 7(3): 304-310. doi: 10.1080/ 00779962.1982.9722404. [ Links ]
Suarez, D., N. Bautista., J. Valdez, A. Angulo., R. Alatorre., J. Vera., A. Equihua., and V. Pinto. 2006. Fluctuación poblacional de Copitarsia decolora (Gueéne) y su asociación con crucíferas comerciales. Agrociencia 40(4): 501-509. [ Links ]
Urra, F. y J. Apablaza. 2005. Temperatura base y constante térmica de desarrollo de Copitarsia decolora (Lepidoptera: Noctuidae). Ciencia e Investigación Agraria 32(1): 19-26. [ Links ]
Velázquez, L. 1987. Ciclo biológico de Copitarsia turbata (Lep.: Noctuidae) sobre cebolla, en Arequipa. Revista Peruana de Entomología 30: 108-110. [ Links ]