Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad Nacional de Agronomía Medellín
Print version ISSN 0304-2847
Rev. Fac. Nac. Agron. Medellín vol.69 no.1 Medellín Jan./June 2016
https://doi.org/10.15446/rfna.v69n1.54751
DOI: http://dx.doi.org/10.15446/rfna.v69n1.54751
Biotransformation of ferulic acid by the phytopathogenic fungi Colletotrichum acutatum and Lasiodiplodia theobromae
Biotransformación del ácido ferúlico con los hongos fitopatógenos Colletotrichum acutatum y Lasiodiplodia theobromae
Manuel Alejandro Numpaque1, Jesús Humberto Gil González1 and Diego Luis Durango Restrepo1
1 Facultad de Ciencias Agrarias - Universidad Nacional de Colombia - A.A. 1779, Medellín, Colombia. <jhgilg@unal.edu.co>
Received: October 19, 2015; Accepted: October 30, 2015
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ABSTRACT
The microbial transformation of ferulic acid (FA) offers a cleaner, more economical alternative for the natural production of flavorings and fragrances. In the present study, the biotransformation of FA using the filamentous phytopathogenic fungi Colletotrichum acutatum and Lasiodiplodia theobromae was researched. Initially, the toxicity of FA against both fungi was evaluated; the FA displayed a moderate toxicity (total inhibition at concentrations ≥ 2000 mg L-1) and apparently a detoxification mechanism was present. Afterwards, the microorganisms were incubated with the substrate at room conditions using a Czapek-Dox culture medium. The results demonstrated that the FA was mainly converted to 4-vinylguaiacol, reaching the highest abundance within the first 48 hours. To a lesser extent, acetovanillone, ethylguaiacol, and vanillin, among others, were produced. Interestingly, the compounds generated in the biotransformation of FA with C. acutatum and L. theobromae have been used as flavorings. Based on the identified metabolites, a possible metabolic pathway was proposed.
Key words: Microbial transformation, Flavoring 4-vinylguaiacol, Metabolic pathway
RESUMEN
La transformación microbiana de ácido ferúlico (AF) puede ofrecer una alternativa más limpia y económica para la producción natural de algunos saborizantes y aromas. En el presente estudio, se investigó la biotransformación de AF usando los hongos filamentosos fitopatógenos Colletotrichum acutatum y Lasiodiplodia theobromae. Inicialmente, se evaluó la toxicidad del AF contra ambos hongos; el sustrato exhibió una toxicidad moderada (inhibición total a concentraciones ≥ 2000 mg L-1) y aparentemente se presentó un mecanismo de desintoxicación. Luego, los microorganismos se incubaron con el sustrato a condiciones ambientales, usando el medio de cultivo Czapek-Dox. Los resultados muestran que el AF es convertido principalmente en 4-vinilguayacol, alcanzando su mayor abundancia dentro de las primeras 48 horas. En menor proporción se producen acetovainillona, etilguayacol, vainillina, entre otros. Interesantemente, los compuestos generados en la biotransformación de AF con C. acutatum y L. theobromae se han empleado como agentes saborizantes. Con base en los metabolitos identificados, se propone una posible ruta metabólica.
Palabras claves: Transformación microbiana, Saborizantes 4-vinilguayacol, Ruta metabólica
Flavorings and fragrances are widely used in the food, drink, and cosmetic industries; however, the majority of flavorings in the global market are obtained through chemical synthesis and less than 5% have a natural origin, generally obtained through plant extraction (Bicas et al., 2010). Currently, chemical synthesis dominates the production of flavorings, using methods that often become incompatible with the environment; furthermore, the low selectivity of the reactions may cause the formation of undesirable product mixtures, which reduces the efficiency of the process and increases the costs of production. According to the European Union Legislature (Xu, et al., 2007), the use of synthetic flavorings is becoming increasingly restricted in food, drinks, and cosmetics; therefore, natural flavorings are appealing to the global market despite their high price. Nevertheless, the production of natural flavorings from direct extractions of botanical materials suffers several problems. Firstly, the concentration of compounds with flavoring or fragrant characteristics can be low in plants, making extraction a costly stage. In addition, agricultural production takes time and the quality of the harvest is influenced by climatic conditions, the availability of soils and plant diseases.
According to the FDA and the European Union Legislature, the products obtained by biotechnological methods can be considered natural when the substrate that is used is natural (Serra et al., 2005). Therefore, biotransformation processes have been suggested as a new environmentally friendly alternative for the production of natural fragrances that are in demand. Ferulic acid (FA), a hydroxycinnamic acid found in the cellular wall of plants, is a natural precursor that plays an important role in the formation of fragrances such as vanillin and some vinylphenols (Hu et al., 2015). The industrial demand for 4-vinylphenols such as 4-vinylcatechol, 4-vinylguaiacol and 2,6-dimethoxy-4-vinylphenol is not met by the availability provided by natural sources (Bernini et al., 2007).This makes FA a highly interesting compound as a substrate for the production of natural flavorings with a commercial value through biotransformation processes. In the literature, there is a significant number of reports on the microorganisms and biotechnical process for the production of flavorings from FA. Some bacterium and yeast genera, such as Pseudomonas, Bacillus, Streptomyces, Lactobacillus, Enterobacter, Streptomyces, Cupriavidus, Candida, Debaryomyces, and Saccharomyces, among others, have developed multiple routes for the bioconversion of FA into vanillin, vanillic acid, protocatechuic acid, and vanillic alcohol (Mishra et al., 2014; Gallage and Møller, 2015). Nevertheless, the biotransformation of FA by fungi has been little-studied; in particular, the fungi Schizophyllum commune, Aspergillus niger, Rhizopus oryzae, Sporotrichum thermophile, and Pycnoporus cinnabarinus have been evaluated for the biotransformation of FA (Baqueiro-Peña et al., 2010; Tsujiyama and Ueno, 2008; Shanker et al, 2007; Topakas et al., 2003: Bonnin et al., 1999). For their part, the phytopathogenic fungi Lasiodiplodia theobromae and Colletotrichum acutatum have demonstrated high metabolic versatility because they have transformed different phenylpropanoid substrates, resulting in compounds with an aggregate value (Velasco et al., 2007; Velasco et al., 2009; Velasco et al., 2010; Velasco et al., 2012). However, the capacity of both fungi to produce flavoring compounds through biotransformation has been scarcely studied. This article reports for the first time the ability of the fungi C. acutatum and L. theobromae to transform FA into compounds with an aggregate value with flavoring attributes, such as 4-vinylguaiacol, 4-ethylguaiacol, vanillin, vanillic acid and acetovanillone.
MATERIALS AND METHODS
Materials
Analytical grade solvents: ethyl acetate (EtOAc), methanol (MeOH), n-hexane and CHCl3were obtained from Merck. The FA standard was acquired from Sigma-Aldrich. In addition, silica gel 60 (0.040-0.063 mm) and Sephadex® LH-20 from Merck and Sigma-Aldrich, respectively, were used. For the culture media, this study employed casein peptone (Merck), alpha-D(+)-glucose anhydrous (Acros Organics), yeast extracts (Oxoid), K2HPO4 (Ma-llinckrodt Chemical), NaNO3 (Merck); MgSO4.7H2O (Protokimica), and FeSO4.7H2O (Carlos Erba). The extracts used for the liquid-liquid extractions were bidistilled before their use.
Separation and analytical methods
Thin layer chromatography (TLC) was carried out using chromatoplates Merck Kiesegel 60 F254 0.25 mm thick and as the mobile phase a n-hexane:EtOAc (8:2) mixture. The compounds were visualized under UV radiation at 254 and 365 nm, and spraying with the acetic acid:H2SO4:H2O mixture (143:28:30) followed by heating (~100 °C, 1 min). Column chromatography (CC) was performed using silica gel 60 or Sephadex® LH-20 as stationary phase. The gas chromatography (GC) analysis used a Hewlett-Packard 6890 chromatograph (Agilent Technologies) interfaced to Agilent HP 5973 Mass Selective Detector in the electronic ionization mode. A DB-35MS column (30 m x 0.25 mm i.d. x 0.25 µm coating thickness) was employed. The chromatographic conditions included the following: column temperature, 50-250 °C at 10 °C min-1; injector temperature, 230 °C; detector temperature, 280 °C; and carrier gas, N2 at 1 mL min-1. The relative composition of the individual components was determined from the peaks average area. Identification of some metabolites was based on interpretation of their mass spectra, comparison with authentic compounds, and by contrast with the NIST 02 Mass Spectral Library. The nuclear magnetic resonance (NMR) spectra were obtained using deuterated chloroform as a solvent with a Bruker AMX 300 model. The multiplicities were established with the JMOD pulse sequence. The chemical displacements (d) were expressed in values of ppm and the coupling constant (J) in hertz (Hz).
Toxicity of the ferulic acid (FA) substrate
The toxicity evaluations, referred to as FA's antifungal activity against the filamentous fungi C. acutatum and L. theobromae (isolated and morphologically characterized by the Laboratorio de Sanidad Vegetal of the Universidad Nacional de Colombia, Medellín), were carried out with the methodology described by Bustillo et al. (2003), with some modifications. In Potato Dextrose Agar (PDA) medium at 50 °C, was added enough (FA) till achieve the desired concentrations (100, 500, 1000, 2000, 4000 mg L-1), and then the mixture was poured immediately into 9-cm-diameter Petri dishes. Later, a mycelial plug (6 mm in diameter) cut from the growing edge of 2-day-old culture of each funguswas transferred to the center of Petri dish plates. The cultures were incubated at room temperature and the mycelial growth diameters of the fungi were measured every 12 h for 96 h for L. theobromae and every 24 h for 168 h for C. acutatum. The measurements were taken in triplicate and each trial had the respective controls (absolute control: PDA medium devoid of FA,and solvent control: ethanol). The toxicity of the FA was expressed as the percentage of mycelial growth inhibition, which was calculated with equation (1):
Curves were constructed for the FA toxicity against the microorganisms, which was used to determine an approximate concentration that would inhibit the mycelial growth of the fungi between 80 and 90% at the midpoint of the evaluation period; this value was used as the initial concentration of the substrate in the subsequent biotransformation processes.
Preparation of the pre-inoculums of C. acutatum and L. theobromae
A Czapek-Dox medium was used (Solution A: glucose 5%, yeast extract 0.1%; Solution B: K2HPO4 0.5%, NaNO3 0.2%, MgSO4.7H2O 0.05%, FeSO4.7H2O 0.001%) in the biotransformations with an inoculum of each fungus of around 1 month of age, previously cultivated on PDA. For each liter of liquid culture medium, a Petri dish was used with the microorganism. The pathogen was inoculated in four 1.0 L Erlenmeyer flasks that contained 500 mL of the established medium. The flasks were agitated (shaker, 120 rpm; Centricol, series 0239, with an incubation chamber) under environmental conditions for 96 h. The mycelia were recovered with filtration, washed with distilled water, and used in the biotransformation at the preparative scale and in the time course experiments.
Biotransformation in the preparative scale and isolation of the metabolites
The mycelia of C. acutatum and L. theobromae, pre-incubated in 2.0 L of the medium, were transferred under sterile conditions in four, 1.0 L Erlenmeyer flasks that contained 500 mL of the corresponding culture medium and the substrate (FA at 900 mg L-1 for L. theobromae and 1400 mg L-1 for C. acutatum). The process was carried out at room temperature and under agitation (120 rpm) for 312 and 360 h for L. theobromae and C. acutatum, respectively. After the incubation period, the culture media and the mycelia were separated through filtration. The filtrate was used to isolate the major metabolic products. For the control, a biotransformation was carried out without the substrate. The resulting filtrate was saturated with NaCl and was extracted with EtOAc (3 x 400 mL). The organic phase was dried with anhydrous sodium sulfate, filtered and evaporated at a reduced pressure in a rotary evaporator. The resulting residue was fractionated with successive CC, using as stationary phase silica gel (mobile phase: systems of increasing polarity of n-hexane-EtOAc) and Sephadex® LH-20 (elusion system: mixture of n-hexane-CH2Cl2-MeOH, 2:1:1 w/v). Three major metabolic products was isolated and denominated as (II), (V) and (VII). A GC-MS analysis of the resulting fractions of the samples also revealed the presence of the compounds designated as (I), (II), (III), (IV), (V) and (VI). The metabolites were identified with spectroscopic and spectrometric methods and through a comparison of spectrums with those obtained for standard samples and/or with the data reported in the database of the NIST Mass Spectral Library, NIST'02 (version 2.0).
Time-course experiments
The material resulting from the pre-inoculation was filtered and the biomass was distributed in 12, 150.0 mL Erlenmeyer flasks with 75 mL of the Czapek-Dox culture medium and the substrate (FA) at a concentration of 900 and 1400 mg L-1 for L. theobromae and C. acutatum, respectively. The conditions of temperature, agitation and time corresponded to those cited for the preparative biotransformation. Every 24 hours, the content of one Erlenmeyer flask (culture medium containing the mycelia) was extracted with EtOAc (3 x 50 mL), following the procedure described for the isolation of metabolites. The resulting residue was re-dissolved with 5.0 mL of CHCl3, filtered with a Whatman microfilter (0.45 µm) and analyzed by TLC and GC-MS. The relative abundance of the products was determined based on the area of the peaks in CG.
RESULTS AND DISCUSSION
FA toxicity for C. acutatum and L. theobromae
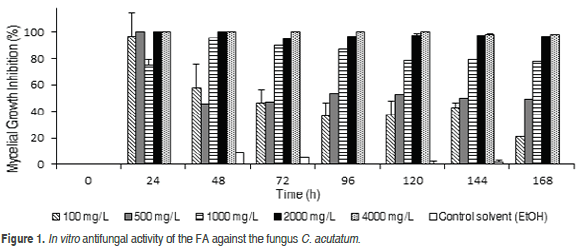
In order to determine the substrate concentration that was suitable for carrying out the biotransformation processes, the antifungal activity of the FA for both phytopathogenic fungi was evaluated. As can be seen in Figure 1, FA reduced the mycelial growth in a dose-dependent manner. C. acutatum was almost completely inhibited at the FA concentrations of 2000 and 4000 mg L-1. In the meanwhile, for the interval of 24 to 169 h, at the 100, 500, and 1000 mg L-1 levels, the inhibition of the growth varied throughout a range of 100-21%, 100-45% and 95-75%, respectively. At these concentrations, mycelial growth inhibition was decreased gradually with increasing incubation time, which could be attributed to a detoxification mechanism. In general, the results demonstrated a moderate radial growth inhibition of C. acutatum by the FA, in comparison with the values reported for other microorganisms. In particular, Sarma and Singh (2003) found that Sclerotium rolfsii was completely inhibited at a FA concentration of 1000 mg L-1, while Aspergillus niger (Baqueiro-Peña et al., 2010) and Rhizopus oryzae (Shanker et al., 2007) were inhibited at 800 and 500 mg L-1, respectively. Based on the obtained results, a FA concentration of 1400 mg L-1 was selected to carry out the biotransformation process; this value is intermediate between 2000 (fungal growth limited with inhibition over 95%) and 1000 mg L-1 (inhibition of almost 85% at the midpoint of the evaluation). At this level, enzymatic activity that results in a suitable conversion process for FA by the microorganisms is expected.
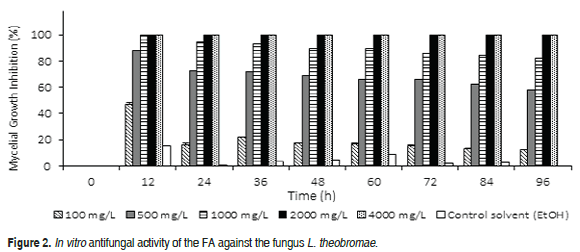
Figure 2 shows that the mycelial growth of L. theobromae presented 100% inhibition at FA concentrations of 2000 and 4000 mg L-1 during the evaluation time (96 h). In the time interval of 12 to 96 h at the 100, 500 and 1000 mg L-1 levels, the inhibition of the growth varied in ranges of 47-12%, 88-58% and 100-80%, respectively. At these concentrations, there was a decrease in the mycelial inhibition with increase in time of incubation, which could be attributed to the decrease in the FA toxicity by the microorganism, similar to the findings with C. acutatum. The FA concentration selected for the biotransformation with L. theobromae was 900 mg L-1; a slightly higher value than the concentration that inhibited the fungus at 80% at the midpoint of the evaluation period.
The moderate activity of FA agrees with different studies that have demonstrated that this organic acid has little fungitoxicity (Baqueiro-Peña et al., 2010; Shanker et al., 2007), which is why it has been suggested for possible use as material for biotransformations.
Biotransformation products of FA: isolation and identification
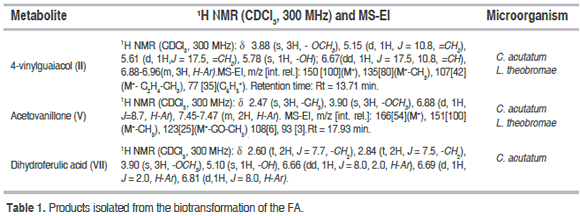
In order to isolate the metabolic products of FA, a preparative biotransformation was carried out in the Czapek-Dox medium using each of the phytopathogenic fungi. The microorganism was incubated with FA for 336 h; afterwards, the extraction of the metabolites was done with ethyl acetate and the resulting extract was fractionated with size-exclusion chromatography on Sephadex LH-20 (100 x 2 cm) with a mixture of n-hexane-CH2Cl2-MeOH (50:25:25) as eluent. In total, three metabolic compounds were isolated: 4-vinylguaiacol (II); acetovanillone (V) and dihydroferulic acid (VII), which were elucidated using1H-NMR and MS-EI techniques (Table 1).
The spectroscopic data for the compounds (II), (V), and (VII) agree with the values reported in the literature. Compound (II) presented a molecular formula C9H10O2 consistent with the molecular ion, M+ = 150 amu. The 1H-NMR spectrum of compound (II) showed the singlet characteristic of the methoxy group (d 3.88 ppm), a multiplete that corresponded to three aromatic protons (d 6.88-6.96 ppm), and the two olefinic protons (d 5.15, 5.61 and 6.67 ppm) with their respective multiplicities, which agrees with the reported by Karmakar et al. (2000). In (V), 1H-NMR spectrum showed the presence of two singlets (d 2.47 and 3.90 ppm) assigned respectively to the methyl and methoxy groups, and three aromatic protons (d 6.88 and 7.45-7.47 ppm); these characteristics, together with the molecular ion (M+ = 166 amu) and fragmentation pattern in the mass spectrum agree with the reported by Luo et al., 2008. For the compound (VII), the coupling pattern described an ABX system of three aromatic protons; furthermore, there were two up field triplets that corresponded to the methylene groups of the lateral propionic chain and a singlet (3H, d 3.90 ppm), that corresponded to the methoxy group, agreeing with the report by Saha et al.(2003).
Other biotransformation products were detected using TLC and GC-MS analyses and they were identified by comparison with the NIST 2002 mass spectral library. Both C. acutatum and L. theobromae produced the compounds 4-ethylguaiacol (I), vanillin (III), and vanillic acid(IV). Only the extract of L. theobromae had homovanillic alcohol (VI). The mass fragmentation data of the detected metabolic products corresponded to: 4-ethylguaiacol (I) (Rt = 12.07 min; m/z [int. rel.]: 152[30](M+), 137[100](M+-CH3), 122[10](M+-C2H6), 109[3], 91[3]); vanillin (III)(Rt = 15.97 min; m/z [int. rel.]: 152[90](M+), 151[100](M+-H), 137[7](M+-CH3), 123[15](M+-CO-H), 109[16](M+-CH2CO-H),93[3]; vanillic acid(IV) (Rt = 17.19 min; m/z [int. rel.]:168[77](M+), 153[100](M+-CH3), 135[7], 125[43], 110[11], 93[70](M+-CO2-H2CO-H)); and homovanillyl alcohol (VI) (Rt = 18.84 min; m/z [int. rel.]:168(M+)[32], 137[100](M+-H2CO-H), 122 [11], 107[3], 94 [7]).
Among the isolated and identified compounds produced by both fungi, those that had flavoring properties were notable. The commercial value of the compounds (I) and (II) is almost 40 times higher than that of ferulic acid (Mishra et al., 2014). The compound 4-ethylguaiacol (I) possesses a spicy to bland odor; it is responsible for the aroma and flavor of Belgian wheat and German Rauch beers (Mathew and Abraham, 2004; Priefertet al., 2001), it has also been used in soy sauce and wine and as a fragrance in the perfume industry (Krings et al., 2001; Priefert et al., 2001). Vanillin (III) is a highly appreciated aromatic compound in the world and is used as flavoring for food and drinks and is used in pharmaceutical products. Additionally, it has antioxidant, preservative, antimicrobial and antimutagenic properties (Zhao and Moghadasian, 2008; Cerrutti and Alzamora 1996; Shaughnessyet al., 2001). The vanilla flavor, that is to say vanillin, is obtained from the plant Vanilla planifolia as gluco-vanillin (Daugsch and Pastore, 2005). A large number of microorganisms has been used for the bioconversion of FA in vanillin, including Gram-negative bacteria of the Pseudomonas genus (Civolani et al., 2000; Plaggenborg et al., 2003), actinomycetes of the Amycolatopsis and Streptomyces genera (Achterholtet al., 2000), Gram-positive bacteria such as Bacillus subtilis (Plaggenborget al., 2001) and Rhodococcus sp. (Plaggenborg et al., 2006) and the basidiomycete fungus Pycnoporus cinnabarinus (Lesage-Meessen et al., 1996). For its part, acetovanillone (V) has been reported as a minor metabolite of the degradation of ferulic acid by some microorganisms (Krings et al., 2001; Priefert et al., 2001). Furthermore, acetovanillone, or apocynin as it is commonly known, is a medicament isolated from the medicinal root of Picroria kurroa (Lapperre et al., 1999); it has numerous therapeutic applications and is a potential inhibitor of the formation of NADPH and peroxynitrite, which have damaging effects on human tissue (Lafeber et al., 1999; Muijsers et al., 2000).
Time-course experiments for the biotransformation of FA with L. theobromae and C. acutatum
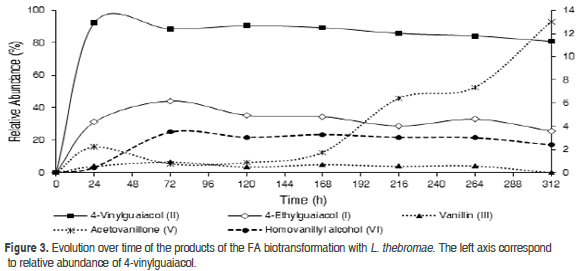
In order to evaluate the relative abundance of the substrate and the metabolic products over time, the FA was incubated with the microorganisms L. theobromae and C.acutatum for 312 and 360 h, respectively. Daily, the culture medium of each Erlenmeyer flask was removed and extracted with EtOAc. Subsequently, the extracts were analyzed with TLC and GC. Figure 3 shows how the substrate was converted by L. theobromae mainly into the metabolite (II) and other minor products [(I), (III), (V), and (VI)]. The FA was essentially transformed into (II), reaching a relative abundance of 95% in the first 24 h; afterwards, the concentration slowly decreased to 80% at 312 h. For their part, the compounds (I), (III) and (VI) increased in relative abundance after 24 h, reaching their highest concentration at 72 h. During the biotransformation (>72 h), the quantities of (III) and (VI) remained approximately constant, under 2 and 4%, respectively; while the compound (V) had a significant increase after 168 h and its relative abundance increased to 13% (312 h).
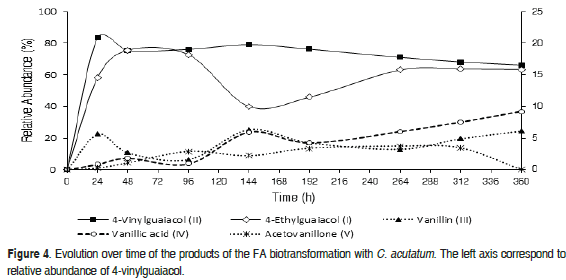
Figure 4 demonstrates how C. acutatum transformed the substrate into two major metabolites [(I) and (II)] and some minor products [(III), (IV), (V)]. Under the previously described conditions, the transformation of FA mainly produced the compound (II), reaching a relative concentration close to 90% (24 h), which subsequently decreased to 70% (360 h), while (I) reached its maximum abundance at 48 h (~20%). In addition, (III) appeared after 48 h, reaching its highest concentration at 144 h; this fact coincides with the decrease in (I) and (II). The compound (IV) had an appreciable increase after 144 h and its relative abundance increased until reaching 10% at 360 h. Meanwhile, (V) was produced at low concentrations and its concentration was always below 2%.
When comparing the progress of the biotransformation, it appeared that both microorganisms transformed the FA mainly into the compound 4-vinylguaiacol (II), with the highest abundance obtained with L. theobromae; with this microorganism, the highest proportion of acetovanillone (V) was also reached in the final period of the transformation. The elevated conversion of FA into (II) demonstrated the high efficiency of both fungi for carrying out the non-oxidative decarboxylation process. In the fermentation with C. acutatum, the content of 4-ethylguaiacol (I) was higher than that obtained with L. theobromae; while homovanillyl alcohol (VI) and vanillic acid (IV) were only detected when using L. theobromae and C. acutatum as the biocatalyizer, respectively.
Integrated metabolic pathway of FA in C. acutatum and L. theobromae
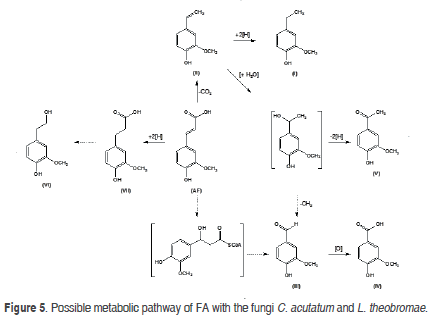
A possible metabolic pathway for the biotransformation of FA with the fungi C. acutatum and L. theobromae (Figure 5) was established with the structure of the obtained products and the experiments over time.
Both microorganisms have the ability to carry out non-oxidative decarboxylation reactions on FA in order to produce the major compound (II), via ferulic acid decarboxylase (Donaghyet al., 1999). A mechanism for the decarboxylation catalyzed by ferulic acid decarboxylase comprises the enzymatic isomerization of FA to a quinoid intermediate which is then decarboxylated spontaneously (Huang et al., 1993). The decarboxylation of FA can form part of a detoxification system in order to maintain the level of fungitoxic compounds under a threshold concentration; a similar mechanism has been observed in the secondary metabolism of phenylpropenoic acid (Seshime et al., 2005). Enzymatic studies related to the decarboxylation of FA to produce (II) have mainly been carried out with bacteria and yeasts (Mishra et al., 2014; Priefert et al., 2001). High concentrations of 4-vinylguaiacol were obtained by Karmakar et al. (2000) and Mathewet al. (2007) in cultures of Bacillus coagulans BK07 (908 mg L-1) and Debaryomyces hansenii (1470 mg L-1). The subsequent hydrogenation of (II) with the enzyme vinylphenol reductase (Godoy et al., 2008) resulted in 4-ethylguaiacol (I), which had its highest proportion when C. acutatum was used. On the other hand, minor compounds, such as vanillin (III) and vanillic acid (IV), are probably obtained through b-oxidation of FA that occurs by a mechanism analogous to the b-oxidation of fatty acids (Rosazza et al., 1995). Di Gioia et al. (2011) proposed that FA is activated by the enzyme feruloyl-CoA synthetase (EC 6.2.1.34) to produce feruloyl-CoA; subsequently, the thioester is hydrated and cleaved by the enzyme enoyl-CoA hydratase/aldolase (EC 4.2.1.101), resulting in vanillin and acetyl-CoA.Alternatively, the elimination of one acetate unit from the lateral chain (a retro-aldol elimination) to afford vanillin is one of the more common routes for the metabolism of FA by bacteria, yeasts, and fungi (Rosazza et al., 1995). Afterwards, the enzymatic action of vanillin dehydrogenase on vanillin generates vanillic acid (IV). On the other hand, Priefertet al. (2001) indicated that (III) is originated from (II) through hydration,via the intermediate 4-(1'-hydroxy) ethylguaiacol and the subsequent elimination of the methyl group. In this way, acetovanillone (V) can come from 4-(1'-hydroxy) ethylguaiacol through the oxidation. Meanwhile, dihydroferulic acid (VII) is originated from the reduction of the double bond C-C of FA. Finally, homovanillyl alcohol (VI) may result from the hydration of (II) or from reduction of (VII).
CONCLUSIONS
The results suggest that the filamentous phytopathogenic fungi C. acutatum and L. theobromae have the ability to decarboxylize the lateral chain of ferulic acid as the principal metabolic pathway. In the process, 4-vinylguaiacol is a principal product, which reaches its highest abundance in the first 48 h of the biotransformation. Other minor metabolites, such as acetovanillin, vanillin, and ethylguaiacol, were also detected. These compounds have great commercial importance in the industries of flavorings and fragrances. For this reason, C. acutatum and L. theobromae have considerable potential as biocatalyzers for the production of 4-vinylguaiacol. Nevertheless, further studies are needed to clarify the optimal conditions and the enzymes that are involved.
REFERENCES
Achterholt, S., H. Priefert and A. Steinbüchel. 2000. Identification of Amycolatopsis; sp. strain HR167 genes, involved in the bioconversion of ferulic acid to vanillin. Applied Microbiology and Biotechnology 54(6): 799-807. doi: 10.1007/s002530000431. [ Links ]
Baqueiro-Peña, I., G. Rodríguez-Serrano, E. González-Zamora, C. Augur, O. Loera and G. Saucedo-Castañeda. 2010. Biotransformation of ferulic acid to 4-vinyl guaiacol by a wild and a diploid strain of Aspergillus niger. Bioresource Technology 101(12): 4721-4724. doi: 10.1016/j.biortech.2010.01.086. [ Links ]
Bernini, R., E. Mincione, M. Barontini, G. Provenzano and L. Setti. 2007. Obtaining 4-vinylphenols by decarboxylation of natural 4-hydroxycinnamic acids under microwave irradiation. Tetrahedron 63(39): 9663-9667. doi: 10.1016/j.tet.2007.07.035. [ Links ]
Bicas, J.L., P. Fontanille, G. M. Pastore and C. Larroche. 2010. A bioprocess for the production of high concentrations of R-(+)-a-terpineol from R-(+)-limonene. Process Biochemistry 45(4): 481-486. doi: 10.1016/j.procbio.2009.11.007. [ Links ]
Bonnin, E., L. Lesage-Meessen, M. Asther and J.F. Thibault. 1999. Enhanced bioconversion of vanillic acid into vanillin by the use of natural cellobiose. Journal of the Science of Food Agriculture 79(3): 484-486. doi: 10.1002/(SICI)1097-0010(19990301)79:3<484::AID-JSFA271>3.0.CO;2-H. [ Links ]
Bustillo, A.J., C.M. García-Pajón, J. Aleu, R. Hernández-Galán and I.G. Collado. 2003. Studies on biotransformation of (±)-1-(4'-chlorophenyl)-2-phenylethanol. Tetrahedron: Asymmetry 14(23): 3755-3760. doi: 10.1016/j.tetasy.2003.08.026. [ Links ]
Cerrutti, P. and S.M. Alzamora. 1996. Inhibitory effects of vanillin on some food spoilage yeasts in laboratory media and fruit purées. International Journal of Food Microbiology29(2-3): 379-386. doi: 10.1016/0168-1605(95)00026-7. [ Links ]
Civolani, C., P. Barghini, A.R. Roncetti, M. Ruzzi and A. Schiesser. 2000. Bioconversion of ferulic acid into vanillic acid by means of a vanillate-negative mutant of Pseudomonas fluorescens Strain BF13. Applied and Environmental Microbiology 66(6): 2311-2317. doi: 10.1128/AEM.66.6.2311-2317.2000. [ Links ]
Daugsch, A. and G. Pastore. 2005. Production of vanillin: a biotechnological opportunity. Química Nova 28(4): 642-645. doi: 10.1590/S0100-40422005000400017. [ Links ]
Di Gioia, D., F. Luziatelli, A. Negroni, A.G. Ficca, F. Fava, and M. Ruzzi. 2011. Metabolic engineering of Pseudomonas fluorescens for the production of vanillin from ferulic acid. Journal of Biotechnology 156(4): 309-316. doi: 10.1016/j.jbiotec.2011.08.014. [ Links ]
Donaghy, J.A., P.F. Kelly and A. McKay. 1999. Conversion of ferulic acid to 4-vinyl guaiacol by yeasts isolated from unpasteurised apple juice. Journal of the Science of Food and Agriculture 79(3): 453-456. doi: 10.1002/(SICI)1097-0010(19990301)79:3<453:AID-JSFA284>3.0.CO;2-H. [ Links ]
Gallage, N.J. and B.L Møller. 2015. Vanillin-bioconversion and bioengineering of the most popular plant flavor and its de novo biosynthesis in the vanilla orchid. Molecular Plant 8(1): 40-57. doi: 10.1016/j.molp.2014.11.008. [ Links ]
Godoy, L., C. Martínez, N. Carrasco and M.A. Ganga. 2008. Purification and characterization of a p-coumarate decarboxylase and a vinylphenol reductase from Brettanomyces bruxellensis. International Journal of Food Microbiology 127(1-2): 6-11. doi: 10.1016/j.ijfoodmicro.2008.05.011. [ Links ]
Hu, H., L. Li and S. Ding. 2015. An organic solvent-tolerant phenolic acid decarboxylase from Bacillus licheniformis for the efficient bioconversion of hydroxycinnamic acids to vinyl phenol derivatives. Applied Microbiology and Biotechnology 99(12): 5071-5081. doi: 10.1007/s00253-014-6313-3. [ Links ]
Huang, Z., L. Dostal and J.P.N. Rosazza. 1993. Mechanisms of ferulic acid conversions to vanillic acid and guaiacol by Rhodotorula rubra. The Journal of Biological Chemistry 268(32): 23954-23958. [ Links ]
Karmakar, B., R.M. Vohra, H. Nandanwar, P. Sharma, K.G. Gupta and R.C. Sobti. 2000. Rapid degradation of ferulic acid via 4-vinylguaiacol and vanillin by a newly isolated strain of Bacillus coagulans. Journal of Biotechnology 80(3): 195-202. doi: 10.1016/S0168-1656(00)00248-0. [ Links ]
Krings, U., S. Pilawa, C. Theobald and R.G. Berger. 2001. Phenyl propenoic side chain degradation of ferulic acid by Pycnoporus cinnabarinus - elucidation of metabolic pathways using [5-2H]-ferulic acid. Journal of Biotechnology 85(3): 305-314. doi: 10.1016/S0168-1656(00)00396-5. [ Links ]
Lafeber, F.P., C.J. Beukelman, E. van den Worm, J.L. van Roy, M.E. Vianen, J. van Roon, H. van Dijk and J.W. Bijlsma. 1999. Apocynin, a plant-derived, cartilage-saving drug, might be useful in the treatment of rheumatoid arthritis. Rheumatology 38(11): 1088-1093. doi: 10.1093/rheumatology/38.11.1088. [ Links ]
Lapperre, T.S., L.A. Jiménez, F. Antonicelli, E.M. Drost, P.S. Hiemstra, J. Stolk, W. MacNee and I. Rahman. 1999. Apocynin increases glutathione synthesis and activates AP-1 in alveolar epithelial cells. FEBS Letters 443(2): 235-239. doi: 10.1016/S0014-5793(98)01723-2. [ Links ]
Lesage-Meessen, L., M. Delattre, M. Haon, J.F. Thibault, B.C. Ceccaldi, P. Brunerie and M. Asther. 1996. A two-step bioconversion process for vanillin production from ferulic acid combining Aspergillus niger and Pycnoporus cinnabarinus. Journal of Biotechnology 50(2-3): 107-113. doi: 10.1016/0168-1656(96)01552-0. [ Links ]
Luo, J.R., H.E. Jiang, Y.X. Zhao, J. Zhou and J.F. Qian. 2008. Components of the heartwood of Populus euphratica from an ancient tomb. Chemistry of Natural Compounds 44(1): 6-9. doi: 10.1007/s10600-008-0003-2. [ Links ]
Mathew, S., T.E. Abraham and S. Sudheesh. 2007. Rapid conversion of ferulic acid to 4-vinyl guaiacol and vanillin metabolites by Debaryomyces hansenii. Journal of Molecular Catalysis B: Enzymatic 44(2): 48-52. doi: 10.1016/j.molcatb.2006.09.001. [ Links ]
Mathew, S. and T.E. Abraham. 2004. Ferulic acid: an antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Critical Reviews in Biotechnology24(2-3): 59-83. doi: 10.1080/07388550490491467. [ Links ]
Mishra, S., A. Sachan, A.S. Vidyarthi and S.G. Sachan. 2014. Transformation of ferulic acid to 4-vinyl guaiacol as a major metabolite: a microbial approach. Reviews in Environmental Science and Bio/Technology 13(4): 377-385. doi: 10.1007/s11157-014-9348-0. [ Links ]
Muijsers, R.B.R., E. van den Worm, G. Folkerts, C.J. Beukelman, A.S. Koster, D.S. Postma and F.P. Nijkamp. 2000. Apocynin inhibits peroxynitrite formation by murine macrophages. British Journal of Pharmacology 130(4): 932-936. doi: 10.1038/sj.bjp.0703401. [ Links ]
Plaggenborg, R., J. Overhage, A. Steinbüchel and H. Priefert. 2003. Functional analyses of genes involved in the metabolism of ferulic acid in Pseudomonas putida KT2440. Applied Microbiology and Biotechnology 61(5-6): 528-535. doi: 10.1007/s00253-003-1260-4. [ Links ]
Plaggenborg, R., A. Steinbüchel and H. Priefert. 2001. The coenzyme A-dependent, non-beta-oxidation pathway and not direct deacetylation is the major route for ferulic acid degradation in Delftia acidovorans. Microbiology Letters 205(1): 9-16. doi: 10.1111/j.1574-6968.2001.tb10918.x [ Links ]
Plaggenborg, R., J. Overhage, A. Loos, J. Archer, P. Lessard, A. Sinskey and A. Steinbüchel. 2006. Potential of Rhodococcus; strains for biotechnological vanillin production from ferulic acid and eugenol. Applied Microbiology and Biotechnology 72(4): 745-755. doi: 10.1007/s00253-005-0302-5. [ Links ]
Priefert, H., J. Rabenhorst and A. Steinbüchel. 2001. Biotechnological production of vanillin. Applied Microbiology and Biotechnology 56(3-4): 296-314. doi: 10.1007/s002530100687. [ Links ]
Rosazza, J.P.N., Z. Huang, L. Dostal, T. Volm and B. Rousseau. 1995. Review: Biocatalytic transformations of ferulic acid: An abundant aromatic natural product. Journal of Industrial Microbiology and Biotechnology 15(6): 457-471. doi: 10.1007/BF01570016. [ Links ]
Saha, S., R.M. Smith, E. Lenz and I.D. Wilson. 2003. Analysis of a ginger extract by high-performance liquid chromatography coupled to nuclear magnetic resonance spectroscopy using superheated deuterium oxide as the mobile phase. Journal of Chromatography A 991(1): 143-150. doi: 10.1016/S0021-9673(03)00215-2. [ Links ]
Sarma, B.K. and U.P. Singh. 2003. Ferulic acid may prevent infection of Cicer arietinum by Sclerotium rolfsii. World Journal of Microbiology and Biotechnology 19(2): 123-127. doi: 10.1023/A:1023205522032. [ Links ]
Seshime, Y., P.R. Juvvadi, I. Fujii and K. Kitamoto. (2005). Genomic evidences for the existence of a phenylpropanoid metabolic pathway in Aspergillus oryzae. Biochemical and Biophysical Research Communications 337(3): 747-751. doi: 10.1016/j.bbrc.2005.08.233. [ Links ]
Shanker, K.S., K.H. Kishore, S. Kanjilal, S. Misra, U.S.N. Murthy and R.B.N. Prasad. 2007. Biotransformation of ferulic acid to acetovanillone using Rhizopus oryzae. Biocatalysis and Biotransformation 25(1): 109-112. doi: 10.1080/10242420601141721. [ Links ]
Serra, S., C. Fuganti and E. Brenna. 2005. Biocatalytic preparation of natural flavours and fragrances. Trends in Biotechnology 23(4): 193-198. doi: 10.1016/j.tibtech.2005.02.003. [ Links ]
Shaughnessy, D.T., R.W. Setzer and D.M. DeMarini. 2001. The antimutagenic effect of vanillin and cinnamaldehyde on spontaneous mutation in Salmonella TA104 is due to a reduction in mutations at GC but not AT sites. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis480-481(1): 55-69. doi: 10.1016/S0027-5107(01)00169-5. [ Links ]
Topakas, E., E. Kalogeris, D. Kekos, B.J. Macris and P. Christakopoulos. 2003. Bioconversion of ferulic acid into vanillic acid by the thermophilic fungus Sporotrichum thermophile. LWT Food Science and Technology 36(6): 561-565. doi: 10.1016/S0023-6438(03)00060-4. [ Links ]
Tsujiyama, S. and M. Ueno. 2008. Formation of 4-vinyl guaiacol as an intermediate in bioconversion of feruli acid by Schizophyllumcommune. Bioscience, Biotechnology and Biochemistry 72(1): 212-215. doi: 10.1271/bbb.60606. [ Links ]
Velasco, R., J.H Gil, C.M. García and D.L. Durango. 2010. Production of 2-Phenylethanol in the Biotransformation of Cinnamyl Alcohol by the Plant Pathogenic Fungus Colletotrichum acutatum. Vitae 17(3): 272-280. [ Links ]
Velasco, R., J.H. Gil, C.M. García and D.L. Durango. 2012. Structural modification of trans-cinnamic acid using Colletotrichum acutatum.Revista Facultad de Ingeniería Universidad de Antioquia 63(1): 20-29. [ Links ]
Velasco, R., D.L. Montenegro, J.F. Vélez, C.M. García and D.L. Durango. 2009. Biotransformación de Compuestos Aromáticos Sustituidos Mediante Hongos Filamentosos Fitopatógenos de los Géneros Botryodiplodia y Colletotrichum. Revista de la Sociedad Química de Perú 75(1): 94-111. [ Links ]
Velasco, R., I.D. Valverde, D.L. Durango and C.M. García. 2007. Biotransformación de los Compuestos 2- Feniletanol y Acetofenona Mediante el Hongo Fitopatógeno Botryodiplodia theobromae. Vitae 14(2): 43-50. [ Links ]
Xu, P., D. Hua and C. Ma. 2007. Microbial transformation of propenylbenzenes for natural flavour production. Trends in Biotechnology 25(12): 571-576. doi: 10.1016/j.tibtech.2007.08.011. [ Links ]
Zhao, Z. and M.H. Moghadasian, 2008. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chemistry109(4): 691-702. doi: 10.1016/j.foodchem.2008.02.039. [ Links ]