Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Facultad Nacional de Agronomía Medellín
versión impresa ISSN 0304-2847
Rev. Fac. Nac. Agron. Medellín vol.69 no.1 Medellín ene./jun. 2016
https://doi.org/10.15446/rfna.v69n1.54752
DOI: http://dx.doi.org/10.15446/rfna.v69n1.54752
Thermal and thermodynamic characterization of a dye powder from liquid turmeric extracts by spray drying
Caracterización térmica y termodinámica de un colorante en polvo a partir de extractos líquidos de cúrcuma obtenidos por secado por aspersión
Aura Yazmin Coronel Delgado1, Héctor José Ciro Velásquez1 and Diego Alonso Restrepo Molina1
1 Posgrado en Ciencia y Tecnología de Alimentos - Facultad de Ciencias Agrarias - Universidad Nacional de Colombia. A.A. 1779, Medellín, Colombia. <aycoroneld@unal.edu.co>
Received: March 15, 2015; Accepted: September 22, 2015
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ABSTRACT
This study aimed to evaluate the thermodynamic properties of sorption isotherms and glass transition temperature (Tg) and the thermal properties of a dye powder obtained from turmeric extracts using spray drying. The sorption isotherms were evaluated at 15, 25 and 35 °C using the dynamic gravimetric method, wherein the isotherm data of the experiment were fit to GAB and BET models. Likewise, the Tg was measured using differential scanning calorimetry (DSC). Thermogravimetric analysis (TGA) was used to determine the mass loss, and the thermal properties (heat capacity, diffusivity and thermal conductivity) were determined using transient flow method. The results demonstrated that the GAB model best fit the adsorption data. The DSC analysis presented a glass transition temperature of 65.35 °C and a loss of volatiles at 178.07 °C. The TGA analysis indicated a considerable mass loss starting at 193 °C, resulting in degradation of the product. The thermal properties demonstrated a heat capacity of 2.45 J/g °C, a thermal conductivity of 0.164 ± 0.001 W/mK and a thermal diffusivity of 8.7x10-8 ± 0.000 m2/s.
Key words: Sorption isotherms, Turmeric powder, Glass transition temperature, TGA analysis
RESUMEN
El objetivo del trabajo fue evaluar las propiedades termodinámicas por isotermas de sorción, temperatura de transición vítrea (DSC), y propiedades térmicas del colorante en polvo obtenido a partir de extracto de cúrcuma usando secado por aspersión. Se evaluaron las isotermas de sorción a 15, 25 y 35 °C mediante el método gravimétrico dinámico donde los datos experimentales de las isotermas se ajustaron a un modelo de GAB y BET. Así mismo, la temperatura de transición vítrea mediante calorimetría diferencial de barrido (DSC), análisis termo gravimétrico (TGA) para determinar pérdida de masa y propiedades térmicas (calor especifico, difusividad y conductividad térmica) fueron evaluadas por el método transitorio. Los resultados mostraron que el modelo que mejor se ajustó a los datos experimentales de las isotermas de adsorción fue el modelo de GAB. El análisis DSC mostró una temperatura de transición vítrea de 65,35 °C y una pérdida de volátiles a 178,07 °C. El análisis TGA indicó una pérdida de masa considerable a partir de 193 °C, mostrando degradación del producto. Las propiedades térmicas del producto en polvo mostraron un valor para el calor específico de 2,45x10-3 J/kg °C, conductividad térmica de 0,164 ± 0,001 W/mK y difusividad térmica de 8,7x10-8 ± 0,000 m2/s.
Palabras claves: Isotermas de sorción, Polvo de curcumina, Temperatura de transición vítrea, Análisis TGA
Turmeric is a natural mixture of compounds, including polyphenols, with an orange-yellow color found in Curcuma longa rhizomes. It is used as a dye in the food and textile industries. It is a potential protective agent against illnesses such as cancer, HIV infection, neurological disorders and cardiovascular and skin diseases; furthermore, it possesses antioxidant, anti-carcinogenic and anti-inflammatory properties (Li et al., 2013). Some applications of turmeric have been limited by its sensitivity to alkaline conditions, thermal treatments, light, metal ions, enzymes, oxygen and ascorbic acid, demonstrating its low bioactivity. Turmeric is yellow in an acidic medium (pH 2.5-7) and red in a basic medium (pH > 7) (Paramera et al., 2011).
Food systems can present physical, chemical and biological changes, which can cause phase transitions due to resulting from temperature and pressure effects. Water is a relevant factor because it affects the physical properties of products; therefore, a relationship can be established between food preservation and the food's water content through water activity, which is equal to the relative humidity in equilibrium with the product at the same temperature (Figueira et al., 2004). The sorption isotherms relate the equilibrium water content and the equilibrium relative humidity at a fixed temperature. In accordance with the fixed relative humidity, the product can adsorb or lose moisture with the surroundings, thus the phenomena of adsorption or desorption, respectively, can be present (Chen and Chen, 2014).
In the case of powders obtained by spray drying, one important transition corresponds to first and second order phase changes, where the glass transition temperature is an important factor. A product obtained by spray drying can be considered a non-crystalline material; it can exist in the glassy state or in the super-cooled liquid state (LeBail et al., 2003).
The glass transition temperature (Tg) corresponds to a change in the plasticity of the material over a range of temperatures where the material is more brittle and glassy below the Tg and more flexible above this temperature (LeBail et al., 2003). The importance of the Tg can be seen in its relationship with the stability and shelf-life of a powdered product. Knowing the Tg is essential to ensure that the system is kept under that temperature in suitable conditions, thereby avoiding negative effects on the physical stability of the product and decreasing deterioration of the product while increasing its shelf-life. It is necessary to know the moisture sorption behavior of the material during storage because the presence of water decreases the Tg to a temperature that can be close to or below the storage temperature (Hernandez et al., 2011; Carter et al., 2012).
Little reported information exists on the thermodynamic characterization of turmeric powder, although some studies have evaluated the glass transition temperature and have performed thermogravimetric analyses (TGA) on spray-dried products (Laczkowski and Sousdaleff, 2013). Knowing these properties, characteristics and behaviors is important because they provide information on the mechanics of adsorption and the interaction between the food components and the food moisture, which are of vital importance to the storage, stability and/or shelf-life of the product (Ayala, 2011). Based on these considerations, this study aimed to evaluate the thermal and thermodynamic properties of the dye powder from liquid turmeric extracts using spray-drying technology.
MATERIALS AND METHODS
Production of the drying suspension
The drying suspension was prepared using a concentrated extract of turmeric (TECNAS, S.A.), maltodextrin (DE 10) (TECNAS, S.A.) and water. The concentrated turmeric extract was obtained in a glycerin mixture under rotoevaporation at 50 °C and 90 rpm and then stored in the dark under refrigeration at 4 °C. The curcuminoid concentration was measured in the extract using high performance liquid chromatography (HPLC).
Spray drying process
The suspension was mixed with maltodextrin (DE 10) and water and homogenized (Ultra-Turrax IKA-T25) at 7400 rpm for 2 minutes. A VIBRASEC completely automatic spray dryer was used with an evaporative capacity of 1.5 L h-1. Atomizer rotational speed, air drying temperature (inlet and outlet), air flow rate, feed flow into the dryer and feed temperature were all monitored and regulated. The drying conditions were previously optimized statistically to the following: inlet air temperature (149 °C), outlet air temperature (75 °C) and atomizer rotational speed of 28000 rpm.
Sorption isotherms
To define the sorption isotherms, the gravimetric method was used. Powder samples of 2 g were placed in triplicate in an Aqualab VSA1020 Decagon, with relative humidity varying from 10 to 90% at temperatures of 15, 25 and 35 °C. The experimental data were fitted to Brunauer, Emmett and Teller (BET) and Guggenhein, Anderson and de Boer models (GAB).The following equations were used to represent the two models (Figueira et al., 2004; Furmaniak et al., 2007; Sinija and Mishra, 2008; Ceballos et al., 2009; Perdomo et al., 2009; Ayala, 2011; Djendoubi et al., 2012):
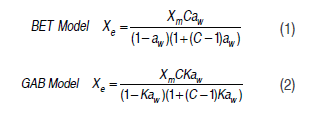
where Xe corresponds to the equilibrium water content, Xm is the monolayer water content, C corresponds to the constants of the BET and GAB models, K is a constant for the GAB model and aw is the water activity. The best fit was defined by the highest value of R2 and the lowest values of X2 and ERMS (Demir et al., 2004; Goyal et al., 2006; Ayala, 2011).
The sorption models were calculated using the following equations:
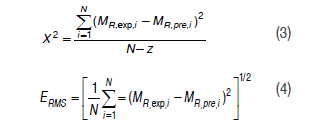
In these expressions, MR,exp,i and MR,exp,i are the calculated and experimental values for each sorption model, N corresponds to the number of experimental data and z is the number of constants for each model.
Equation (5) was used to find the thermodynamic properties (Clausius-Clapeyron equation) for the liquid vapor equilibrium at the experimental temperature and pressure (Yan et al., 2008; Bell and Labuza, 2000). With this equation, it was possible to calculate the change in enthalpy for the different moisture levels associated with the sorption processes. The water vapor partial pressure was determined by the following equations:
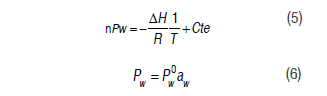
Where Pw corresponds to the water vapor partial pressure in equilibrium with the material at a determined temperature, corresponds to the water vapor pressure at a defined temperature for each product, and aw corresponds to the water activity.
The Gibbs free energy (D;G) and the differential entropy (D;S) were also calculated:
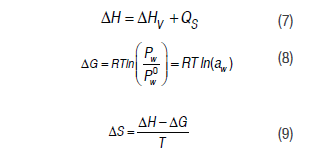
where corresponds to the differential adsorption heat (J/mol), is the latent heat of water (J/mol), Qs corresponds to the net sorption heat (J/mol), R is the universal gas constant (8.314 J/mol K), and T is the absolute temperature (K).
Differential scanning calorimetry (DSC) analysis
The glass transition temperature (Tg) was determined using a differential scanning calorimeter from Q2000-TA Instruments calibrated with indium. DSC was performed in a nitrogen atmosphere (50 mL/min flow speed) with a temperature range of 20 to 300 °C at a rate of 10 °C/min. Approximately 8 mg of each sample were hermetically sealed in an aluminum capsule, and the reference was an empty aluminum capsule. A thermogram was obtained for each sample, and the Tg was taken as the starting point of the change in the heat flow curve (Cheng et al., 2013; Enrione et al., 2010; Li et al., 2013; Sablani et al., 2007).
Thermogravimetric analysis (TGA)
The possible mass loss was quantified using a Linseis STA PT1600 thermobalance. Approximately 10 mg of powder was placed in a ceramic capsule in a nitrogen atmosphere (50 mL/min), and the sample was heated from ambient temperature to 300 °C at a heating rate of 10 °C/min (Laczkowski and Sousdaleff, 2013).
Thermal properties
The thermal conductivity (k) and the thermal diffusivity (a) were obtained using a KD2-Pro (Decagon Devices, Inc., USA) equipped with an SH-1 sensor. The heat capacity value was calculated using the classical definition (Onita and Ivan, 2005; Bergeron et al., 2012; Perusello et al., 2013), where the density (r) of the powdered product was quantified by pycnometry:
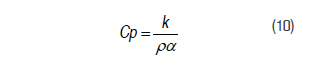
RESULTS AND DISCUSSION
Sorption isotherms
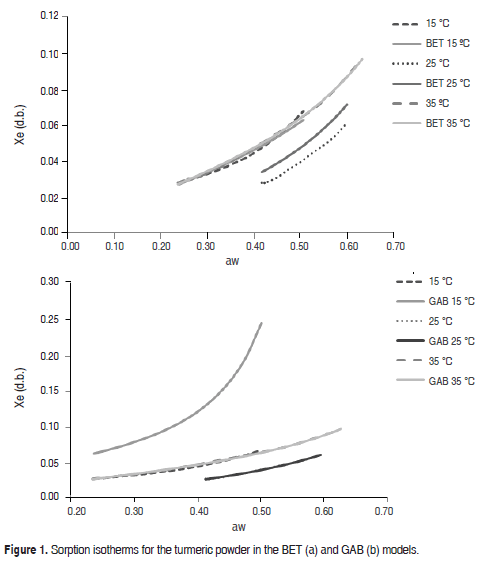
Figure 1 shows the experimental sorption isotherms fitted to the BET and GAB models. The experimental data are shown as equilibrium water content (Xe) as a function of water activity (aw), which is a characteristic relationship in systems that contain water-soluble compounds such as sugars (maltodextrin). This type of behavior has also been observed by Ceballos (2009), who studied sorption isotherms in dehydrated soursop powder, by Cano et al. (2013) in mango powders obtained by spray drying and by Gabas et al. (2007) in pineapple pulp powder.
In Figure 1, Xe of the product increased with aw, a behavior that, according to Ayala (2011), is common in many food types. The results demonstrated that for a specific aw value, the increase in temperature contributed to the decrease of Xe of the product, which was related to the hygroscopicity of the powder, which decreased at higher temperatures (Sinija and Mishra, 2008; Ayala, 2011; Cano et al., 2013).
Vega et al. (2006), Goula et al. (2008), Ayala (2011) and Martínez et al. (2014) suggested that physical and chemical changes caused by increasing temperatures could decrease the active sites in the product compounds where water interacts with the solid surface of the food. Furthermore, it is important to emphasize that the high excitation state of water molecules at high temperatures could result in decreased attraction between water and the food, which would explain why the moisture content in the powder is lower at higher temperatures (Figure 1).
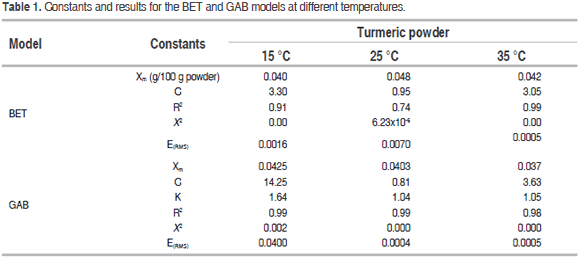
Table 1 contains the BET and GAB model parameters. The GAB model fit the experiment data better than the BET model with higher R2 values and lower X2 and ERMS values.
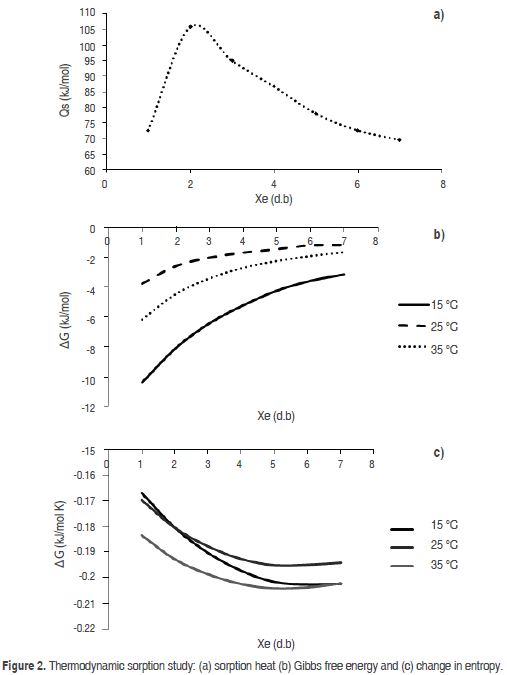
The GAB model provided information on the monolayer water content (Xm), showing that water was strongly absorbed at the active sites on the surface of the food, which relates to suitable storage conditions for low-moisture products (Cano et al., 2013). For the turmeric powder, the Xm value varied between 3.70 and 4.25 g/100 g powder, similar to the findings of Cano et al., (2013) for spray-dried mango powder and Al-Muhtaseb et al. (2004a) for starch powder. The value of the Guggenheim constant (C) indicated the relationship between the sorption heat of the monolayer molecules and temperature. (Here, this value varied indiscriminately with temperature.) The value of the constant K related the sorption heat in the multilayer molecules to the temperature increase from 15 to 25 ºC, which was very similar between 25 and 35 °C; this behavior was also reported by Sharma et al. (2009). Figure 2 shows the sorption thermodynamics of the dried product, where Figure 2(a) contains the net sorption heat value (Qs) that corresponded to the sorption isosteric heat, which described the interaction between the water and product components. There is an increase from 72,480 to 105,630 J/mol for water content between 1 and 2 g/100 g turmeric powder dry basis (d.b.). A decrease from 94,930 to 69,520 J/mol for water content between 3 and 7 g/100 g turmeric powder (d.b.) was observed. The maximum value was achieved with a moisture value close to 2 g/100 g turmeric powder (d.b.). The increase of the sorption isosteric heat in low water contents could be explained by an elevated solid-vapor interaction in the active sites of the product surface, while the sorption heat decreased with an increase in the moisture; the water occupied fewer active sites, decreasing the interaction of the existing kinetic energy between the water molecules and the powder product. A similar behavior was observed by Ayala (2011) and Martínez et al. (2014).
Figure 2(b) shows the Gibbs free energy (DG) of the turmeric powder. The change in the DG indicated that water adsorption onto the powder product is spontaneous because the obtained values were negative in the evaluated water activity range, demonstrating that the reactions that occurred in the microstructure of the product were stable in the range of temperatures evaluated. Furthermore, these values indicate that the powder product possesses a high hygroscopicity because the DG value was more negative for lower water contents. For moisture values over 7 g/100 g turmeric powder, the DG value was approximately zero, indicating that this process had a tendency to reach equilibrium. A similar behavior was observed by Cano et al. (2013) and Martínez et al. (2014).
Figure 2(c) shows the differential entropy (DS), demonstrating a strong dependency on the moisture content. Changes in entropy are related to the number of available adsorption sites at a specific energy level proportional to the change in entropy, which is related to the degree of disorder and random movement of water molecules; lower entropy is an indicator of better stability (Goneli et al., 2013). This type of behavior was observed by Goula et al. (2008) and Al-Muhtaseb et al. (2004b). The negative DS values indicated the water adsorption process by the compound molecules of turmeric powder. The highest change in entropy was obtained at a temperature of 35 °C, where the kinetic energy of the molecules is higher. Furthermore, for temperatures between 15 and 25 °C, smaller changes were observed due to the lower kinetic energy resulting from the difference between the initial and final entropy. A similar behavior was observed by Jamali et al. (2006).
Differential scanning calorimetric analysis
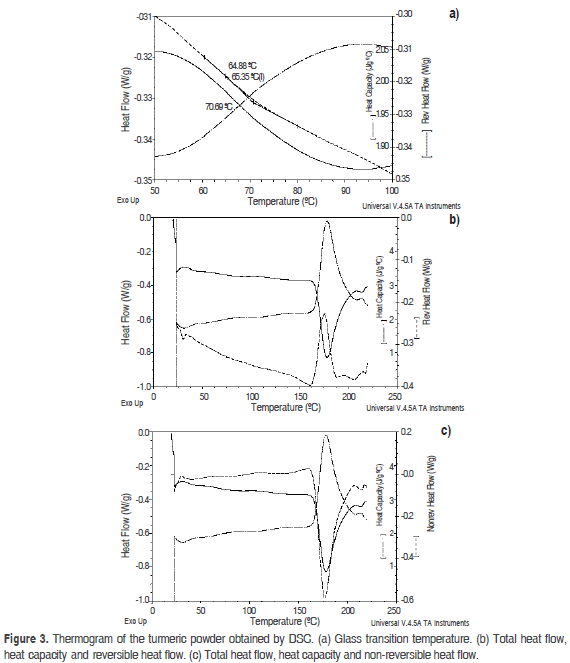
Figure 3 shows the thermogram obtained from differential scanning calorimetry (DSC). An endothermic peak was observed at 178.07 °C, which corresponded to a desolvation or loss of volatiles, which is probably why the total heat flow peak and the reversible heat flow peak did not coincide and were opposites. Likewise, fusion points were not observed, indicating the role played by maltodextrin as a protective agent, similar to the observations of Laczkowski and Sousdaleff (2013), who obtained an endothermic peak at these temperatures for turmeric encapsulated with maltodextrin. Parize et al. (2012) demonstrated endothermic peaks at similar temperatures for chitosan with turmeric obtained by spray drying. Paramera et al. (2011) found slightly higher values in endothermic peaks for turmeric encapsulated in yeast cells because, for turmeric molecules integrated within yeast cells, the transitional phases are increased. Pawar et al. (2012) found values of 182 °C for amorphous turmeric; however, this was related to a melting event because only turmeric was used.
According to the results of DSC, the glass transition temperature (Tg) was 65.35 °C. Pawar et al. (2012) observed a Tg value of 69.4 °C for amorphous turmeric, and Parize et al. (2012) found that the presence of chitosan increased the Tg of turmeric to 91.30 °C, demonstrating that the turmeric colorant is encapsulated between the polymeric chains of this compound. Wang et al. (2010) observed Tg values of 105.95-110.10 °C in turmeric contained in hyperbranched polymers, which increased the Tg. The Tg value of 65.35 °C shows that there was a phase change in the product above this temperature, so the storage temperature must be lower than this value.
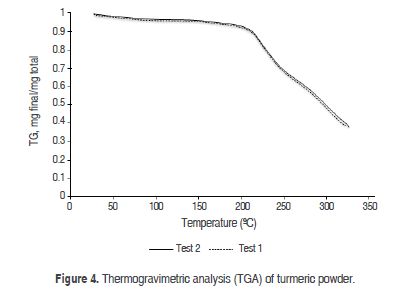
Thermogravimetric analysis (TGA).
Figure 4 shows the curve for mass loss as a function of temperature. The initial temperature of the mass loss was approximately 193 °C, resulting in degradation or decomposition of the turmeric powder; below this temperature, the decrease in the thermogram corresponded to moisture loss. Laczkowski and Sousdaleff (2013) demonstrated that the mass loss in turmeric encapsulated with maltodextrin at 228 °C was due to the interaction of the colorant with the maltodextrin protecting the compound because loose turmeric had a mass loss value starting at 201 °C. Furthermore, the drying process was performed by lyophilization; therefore, encapsulated turmeric is more stable, although it is necessary to emphasize that the profile demonstrated by the authors is very similar to those of the present study. Parize et al. (2012) reported a degradation temperature of 321 °C for turmeric as a result of the presence of chitosan and the interactions between this polymer and turmeric that contribute to the thermal stability; although in that study, the degradation temperature was higher, the mass loss profile is very similar.
Thermal properties
The thermal conductivity was 0.16 ± 0.00 W/m °C, a low value related to the moisture content of the product (Perusello et al., 2013). The thermal diffusivity value of the product was 8.7x10-8 ± 0.00 m2/s; similar values were obtained by Perusello et al. (2013) and Tiwari et al. (2011). The heat capacity value (Cp) corresponded to 2.45 J/g °C, whereas the value obtained in the DSC analysis was 1.95 J/g °C. This unexpectedly low value is probably because during the measurements with the KD2-Pro device, factors such as ambient temperature and the location of the sample sensor affected the results. The Cp value is a thermodynamic measurement of the temperature that both induces and is related to structural changes, and it is an important parameter for the characterization of the amorphous state (Pawar et al. 2012). In a study conducted by these authors on amorphous turmeric, Cp values of 530 J/mol K (1.44 J/g ºC) were found; the difference with the present study could be because, in their study, the authors did not use coadjuvant agents such as maltodextrin, whose heat capacity is 1.50 J/g ºC, because there was an increase in the Cp value of the powder turmeric (Frias et al., 2001).
CONCLUSIONS
The GAB model for the turmeric powder obtained by spray drying describes the sorption isotherms and the adsorption process well, showing the better fit modeling the experimental data. The final product is stable under storage conditions at temperatures below 65.35 °C, in the range of temperatures where the turmeric powder is in the amorphous state. Additionally, it is thermally stable, although for higher temperatures (above 200 °C), the degradation and decomposition of curcumin is high due to loss of volatiles. The values obtained for thermal properties (heat capacity, conductivity and thermal diffusivity) are low, related to the low moisture content present in the turmeric powder and as well as the properties of a mixture of maltodextrin and curcumin.
REFERENCES
Al-Muhtaseb, A., W. McMinn, T. Magee. 2004a. Water sorption isotherms of starch powders. Part 1: mathematical description of experimental data. Journal of Food Engineering 61: 297-307. doi: 10.1016/S0260-8774(03)00202-4 [ Links ]
Al-Muhtaseb, A., W. McMinn, T. Magee. 2004b. Water sorption isotherms of starch powders. Part 2: Thermodynamic characteristics. Journal of Food Engineering 62: 135-142. doi: 10.1016/S0260-8774(03)00202-4 [ Links ]
Ayala, A. 2011. Estimación de las isotermas de adsorción y del calor isostérico en Harina de yuca. Biotecnología en el Sector Agropecuario y Agroindustrial 9: 88-96. [ Links ]
Cano, D., H. Villa, J. Telis, H. Váquiro, V. Nicoletti. 2015. Influence of alternative drying aids on water sorption of spray dried mango mix powders: A thermodynamic approach. Food and Bioproducts Processing 93: 19-28. doi: 10.1016/j.fbp.2013.10.005 [ Links ]
Ceballos, A., G. Giraldo, C. Orrego. 2009. Evaluación de varios modelos de isotermas de adsorción de agua de un polvo de fruta deshidratada. Vector 4: 107-116. [ Links ]
Chen, H., C. Chen. 2014. Equilibrium relative humidity method used to determine the sorption isotherm of autoclaved aerated concrete. Building and Environment 81: 427-435. doi: 10.1016/j.buildenv.2014.07.021 [ Links ]
Cheng, Q., D. McGillivray, J. Wen, F. Zhong, S. Young. 2013. Co-encapsulation of fish oil with phytosterol esters and limonene by milk proteins. Journal of Food Engineering 117(4): 505-512. doi: 10.1016/j.jfoodeng.2013.01.011 [ Links ]
Craig, D., M. Reading. 2007. Thermal Analysis of Pharmaceuticals. CRC Press, Taylor & Francis Group, Boca Ratón, FLA. pp.15-17. doi: 10.1201/9781420014891 [ Links ]
Figueira, G., K. Park, F. Reis, S. Honório. 2004. Evaluation of desorption isotherms, drying rates and inulin concentration of chicory roots (Cichorium intybus L.) with and without enzymatic inactivation. Journal of Food Engineering 6: 273-280. doi: 10.1016/j.jfoodeng.2003.06.001 [ Links ]
Frias, J., J. Oliveira, K. Schittkowski. 2001. Modeling of maltodextrin DE12 drying process in a convection oven. Applied Mathematical Modelling 25: 449-462. doi: 10.1016/S0307-904X(00)00060-3 [ Links ]
Gabas, A., V. Telis, P. Sobral, J. Telis. 2007. Effect of maltodextrin and arabic gum in water vapor sorption thermodynamic properties of vacuum dried pineapple pulp powder. Journal of Food Engineering 82: 246-252. doi: 10.1016/j.jfoodeng.2007.02.029 [ Links ]
Goneli, A., P. Corrêa, G. Oliveira, P. Afonso. 2013. Water sorption properties of coffee fruits, pulped and green coffee. Food Science and Technology 50: 386-391. doi: 10.1016/j.lwt.2012.09.006 [ Links ]
Goula, A., T. Karapantsios, D. Achilias. K. Adamopoulos. 2008. Water sorption isotherms and glass transition temperatura of spray dried tomato pulp. Journal of Food Engineering 85: 73-83. doi: 10.1016/j.jfoodeng.2007.07.015 [ Links ]
Goyal, R., A. Kingsly, M. Manikantan, S. Ilyas. 2006. Thin-layer drying kinetics of raw mango slices. Biosystems Engineering 95: 43-49. doi: 10.1016/j.biosystemseng.2006.05.001 [ Links ]
Jamali, A., M. Kouhila, A. Mohamed, A. Idlimam, A. Lamharrar. 2006. Moisture adsorption-desorption isotherms of Citrus reticulate leaves at three temperatures. Journal of Food Engineering 77: 71-78. doi: 10.1016/j.jfoodeng.2005.06.045 [ Links ]
Laczkowski, I., M. Sousdaleff. 2013. Microencapsulação de curcumina com maltodextrina, avaliação da estabilidade e aplicação em alimentos. In Memories: VIII Encontro de Produção Cientifica e Tecnológica, Campo Mourão - PR. [ Links ]
Li, B., S. Konecke, L. Wegiel, L. Taylor, K. Edgar. 2013. Both solubility and chemical stability of curcumin are enhanced by solid dispersion in cellulose derivative matrices. Carbohydrate Polymers 98: 1108- 1116. doi: 10.1016/j.carbpol.2013.07.017 [ Links ]
Martinez, R., A. Heredia, M. Castelló, A. Andrés. 2014. Moisture sorption isotherms and isosteric heat of sorption of dry persimmon leaves. Food Bioscience 7: 88-94. doi: 10.1016/j.fbio.2014.06.002 [ Links ]
Paramera, E., S. Konteles, V. Karathanos. 2011. Stability and release properties of curcumin encapsulated in Saccharomyces cerevisiae, b-cycclodextrin and modified starch. Food Chemistry 125: 913-922. doi: 10.1016/j.foodchem.2010.09.071 [ Links ]
Parize, A., H. Stulzer, M. Marghetti, I. Da Costa, T. Rozone. 2012. Evaluation of chitosan microparticles containing curcumin and crosslinked with sodium tripolyphosphate produced by spray drying. Quimica Nova 35: 1127-1132. doi: 10.1590/S0100-40422012000600011 [ Links ]
Pawar, Y., G. Shete, D. Popar, A. Bansal. 2012. Phase behavior and oral bioavailability of amorphous curcumin. European Journal of Pharmaceutical Sciences 47: 56-64. doi: 10.1016/j.ejps.2012.05.003 [ Links ]
Perdomo, J., A. Cova, A. Sandoval, L. García, E. Laredo, A. Müller. 2009. Glass transition temperatures and water sorption isotherms of cassava starch. Carbohydrate Polymers 76: 305-313. doi: 10.1016/j.carbpol.2008.10.023 [ Links ]
Perusello, C., V. Cocco, M. Masson, F. De Castilhos. 2013. Determination of thermophysical properties of yacon (Smallanthus sonchifolius) to be used in a finite element simulation. International Journal of Heat and Mass Transfer 67: 1163-1169. doi: 10.1016/j.ijheatmasstransfer.2013.09.004 [ Links ]
Sharma, P., A. Singh, A. Patel, G. Patil. 2009. Sorption isotherms and thermodynamics of water sorption of ready-to-use Basundi mix. Food Science and Technology 42: 441-445. doi: 10.1016/j.lwt.2008.04.010 [ Links ]
Tiwari, G., S .Wang, J. Tang, S. Birla. 2011. Computer simulation model development and validation for radio frequency (RF) heating of dry food materials. Journal of Food Engineering 105: 48-55. doi: 10.1016/j.jfoodeng.2011.01.016 [ Links ]
Sablani, S., S. Kasapis, M. Rahman. 2007. Evaluating water activity and glass transitions concepts for food stability. Journal of Food Engineering 78: 266-271. doi: 10.1016/j.jfoodeng.2005.09.025 [ Links ]
Sinija, V., H. Mishra. 2008. Moisture sorption isotherms and eat of sorption of instant (soluble) green tea powder and green tea granules. Journal of Food Engineering 86: 494-500. doi: 10.1016/j.jfoodeng.2007.10.026 [ Links ]
Vega, A., E. Aravena, R. Mondaca. 2006. Isotermas de adsorción en harina de maíz (Zea mays L.). Ciência e Tecnologia de Alimentos. Campinas 26: 821-827. doi: 10.1590/S0101-20612006000400017 [ Links ]
Wang, H., Y. Wang, P. Lee, Y. Chen, C. Hang, J. Xin, K. Cheuk. 2010. Syntheses, characterization, and photophysical properties of new type of curcumin-containing hyperbranched polymer. Polymer Preprints 51: 499-500. [ Links ]
Yan, Z., M. Sousa, F. Oliveira. 2008. Sorption isotherms and moisture sorption hysteresis of intermediate moisture content banana. Journal of Food Engineering 86: 342-348. doi: 10.1016/j.jfoodeng.2007.10.009 [ Links ]