Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Facultad Nacional de Agronomía Medellín
versão impressa ISSN 0304-2847
Rev. Fac. Nac. Agron. Medellín vol.69 no.2 Medellín jul./dez. 2016
https://doi.org/10.15446/rfna.v69n2.59138
DOI: http://dx.doi.org/10.15446/rfna.v69n2.59138
Identification of some kefir microorganisms and optimization of their production in sugarcane juice
Identificación de algunos microorganismos del kéfir y optimización de su producción en jugo de caña
Blanca Cecilia Salazar Alzate1, Misael Cortés Rodríguez2 and Olga Montoya Campuzano1
1 Facultad de Ciencias. Universidad Nacional de Colombia. A.A. 3840, Medellín, Colombia.
2 Facultad de Ciencias Agrarias. Universidad Nacional de Colombia. A.A. 1779, Medellín, Colombia. <mcortesro@unal.edu.co>
Received: February 9, 2016; Accepted: June 2, 2016
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

ABSTRACT
Kefir grains are a consortium of bacteria and yeasts grouped in a polysaccharide called kefirano. They ferment sugar substrates to produce organic acids, CO2, vitamins and ethanol. They also have positive effects on health. This research project aimed to optimize the fermentation process of sugarcane concentrate using kefir grains. The microorganisms were first identified morphologically and biochemically, then isolated and purified in selective media. Optimization was conducted using the response surface methodology with a composite central design. The independent variables were: temperature, time and percentage of kefir grains added. As for dependent variables, we considered the following: increase in kefir grains (also measured as a percentage), acidity and microbial growth. Additionally, our study identified populations of Lactobacillus curvatus and the following yeasts: Candida famata, Can. magnoliae, Can. krusei/incospicua and Can. sphaerica in the kefir grains. The optimal conditions were 33.5 °C, 30 h and 6% w/w of added kefir grains. The increase in kefir grains reached was of 193 ± 12%. The lactobacilli, lactococci and yeast counts were 1.57x108, 8.63x107 and 2.05x107 CFU mL-1 respectively. Experimental optimization was an effective tool for the fermentation of kefir grains in sugarcane concentrate.
Key words: Functional foods, Lactic acid bacteria, Probiotics, Yeasts
RESUMEN
Los gránulos de kéfir representan un consorcio de bacterias y levaduras, agrupadas en el polisacárido kefirano, que fermentan sustratos azucarados produciendo ácidos orgánicos, CO2, vitaminas y etanol, y generan efectos positivos en la salud. El objetivo de esta investigación fue optimizar el proceso de fermentación de concentrado de caña utilizando gránulos de kefir. Los microorganismos de los gránulos, se aislaron y purificaron en medios selectivos identificándose morfológica y bioquímicamente. La optimización se realizó utilizando la metodología de superficie de respuesta con un diseño central compuesto y considerando las variables independientes: temperatura, tiempo y porcentaje de gránulo de kefir adicionados, y las variables dependientes: porcentaje de aumento del gránulo de kefir, acidez y crecimiento microbiano. Se identificaron Lactobacillus curvatus y las levaduras Candida famata, Can. magnoliae, Can. krusei/incospicua y Can. sphaerica en los gránulos de kéfir, siendo las condiciones óptimas 33,5 °C, durante 30 h y porcentaje de gránulos de kéfir adicionados 6% p/p, alcanzando un incremento de gránulos del 193 ± 12% y recuentos de lactobacilos, lactococos y levaduras de 1,57x108, 8,63x107 y 2,05x107 UFC mL-1, respectivamente. La optimización experimental fue una herramienta efectiva para el proceso de fermentación de los gránulos de kéfir en el concentrado de caña.
Palabras claves: Alimentos funcionales, Bacterias ácido lácticas, Probióticos, Levaduras
Kefir grains (KG) consist of many individual white to bone-colored, soft, gelatinous biological masses with a cauliflower-like appearance. This mass is composed of protein, lipids and a soluble polysaccharide called kefirano in which a symbiotic association of microorganisms belonging to a diverse spectrum of lactic acid bacteria, yeasts and acetic bacteria, ferment sugar substrates. They produce lactic acid, alcohol, CO2, B vitamins and other organic acids (Lopitz et al., 2006; Kabak and Dobson, 2011). Some kefir microorganisms have been isolated by different authors, namely: Lactobacillus kefiri, Lb. fermentum, Lb. acidophilus, Lb. helveticus, Lb. brevis, Lb. kefiranofaciens, Lb. kefirgranum, Lb. parakefir, Lactococcus lactis sub lactis, Lactococcus lactis sub cremoris, Leuconostoc mesenteroides, Acetobacter aceti, Acet. rasens. Yeasts such as Candida kefyr, Can. krusei, Can. lambica, Saccharomyces turicensis and Kluyveromyces marxianus, among others, have also been isolated (Witthuhn et al., 2005; Lopitz et al., 2006; Garofalo et al., 2015). Furthermore, some of them have been identified by molecular techniques (Leite et al., 2012; Leite et al., 2013; Garofalo et al., 2015). Other studies on KG, their microorganisms and kefirano explain that their consumption has positive effects on human health, these include: stimulating the immune system (Iraporda et al., 2014), producing an antitumor effect (Maalouf et al., 2011; Guzel-Seydim et al., 2011b; Diosma et al., 2014) and generating antimicrobial activity against some pathogenic microorganisms (Chifiriuc et al., 2011; Ahmed et al., 2013; Carasi et al., 2014;). Most studies on kefir have been conducted on milk (Lasik and Jan, 2012), milk whey (Teixeira et al., 2010), reconstituted skim milk (Nambou et al., 2014) and other substrates like blackcurrant juice (Luckow and Delahunty, 2004), sugarcane powder (Salazar et al., 2015) and walnut milk beverages (Cui et al., 2013). The microorganisms in the KG often reproduce in milk, but lactose-intolerant people cannot benefit from them. Thus, sugar rich substrates such as sugarcane juice, whose consumption is high in developing countries, are very interesting alternatives. A total of 20 million hectares of sugarcane are grown worldwide, and its production in Latin America is high because of the region's tropical weather. Brazil is the largest producer in the area: as of 2013, its production amounts to 11,758,663 t, and is followed by Peru and Colombia with 3,050,934 and 2,434,853 t, respectively. Furthermore, the highest increases in production rates during the last ten years have been observed in Colombia and Brazil (Faostat, 2013). Given the importance of this agribusiness for the Latin American population, these countries might be interested in exploring new alternatives for improving competitiveness and diversifying products by including new functional foods with the probiotic microorganisms from the KG. In the field of fermentation processes, some research has pointed to many factors that have an important effect on the growth of the kefir microorganisms. Some of those factors are: temperature, biomass or inoculum concentrate, pH, time and aeration (Guzel et al., 2011a; Bensmira and Jiang, 2012; Cui et al., 2013). The aim of this study was to determine the optimal conditions for the fermentation of sugarcane concentrate using KG. Such conditions should lead to a greater increase in the concentration of the grains and larger counts of lactobacilli, lactococci and yeasts.
MATERIALS AND METHODS
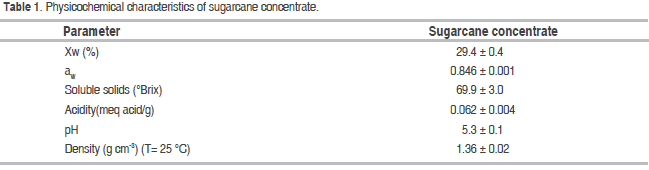
The KG were obtained from the Water and Food Microbiology Laboratory of Universidad Nacional de Colombia, Medellín. The sugarcane concentrate (69.9 ± 3.0 °Brix) was provided by a traditional company that produces jaggery in the Jamundí rural settlement in the Girardota municipality, department of Antioquia, Colombia.
The raw material and the fermented juices of each experiment were characterized three times in terms of moisture loss (Xw) when dried in an oven at 103 °C until reaching a constant weight. Other measurements were performed as follows: pH was measured with a Hanna pH-meter, and solids were measured with a refractometer. The values for the latter were expressed as °Brix at 25 °C. Acidity was measured by titration and expressed as milliequivalents of acid per milliliter (meq acid mL-1) (Horwitz, 2005), water activity (aw) with a dew point hygrometer at T = 25 °C (Decagon Aqua LAB 3TE series), density with a pycnometer.
To activate the KG, they were inoculated into sugarcane concentrate at 30 °Brix, 25 °C for 24 h. They were then separated with a clothperla® 500312 filter and washed with distilled water. This procedure was repeated for three days. The KG were homogenized in 0.1% (w/v) peptone water, and aliquots of 0.1 mL were placed into the following selective culture media through surface depletion: MRS, M17 and YGC agar (Aquilanti et al., 2012; Chen et al., 2008). Additionally, each colony was isolated and purified on the basis of its morphological features and the microscopic characteristics of its microorganisms. In order to identify the microbial genus, the colonies were characterized biochemically through bioMérieux's API® galleries: 50CHL for lactobacilli and 20CAUX for yeasts, as stated in the manufacturer's instructions. In addition, they were identified using the API WEB software.
Fermentation processes took place in Erlenmeyer flasks made of glass with a total volume of 200 mL of sugarcane juice in accordance with the proposed experiment design (Table 2). Counts of viable microorganisms (CFU mL-1) in each fermentation were conducted with the serial dilution method in the previously mentioned selective media. Microorganisms were incubated at 37 °C / 5 d anaerobically for lactobacilli and cocci, and at 37 °C / 24 to 48 h for yeasts. The percentage of increase in KG was calculated as follows:
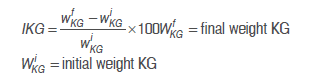
The optimization of the fermentation process was performed using the response surface methodology based on a central composite experiment design (15 experiments). In addition, three factors or independent variables were considered: added KG (2.4-9.6%) (Factor A), fermentation temperature (29-38 °C) (Factor B) and fermentation time (12-48 h) (Factor C). The response or dependent variables were: Increase in Kefir grains (IKG) (%) (to be maximized), acidity (meq acid mL-1) (to be minimized) and lactobacilli, lactococci and yeast counts (to be maximized). The statistical analysis of the results was conducted with the Design Expert 8.0, software, State-Ease, Inc., additionally, an analysis of variance (ANOVA) with a level of significance of 5% was conducted. The regression analysis and the relationship between the independent and dependent variables were calculated using a second order polynomial model.
RESULTS AND DISCUSSION
Table 1 shows the mean values and standard deviations of the sugarcane concentrate used in the fermentation process. The sugarcane concentrate was obtained as an intermediate product of the jaggery manufacturing process during the final stage of juice concentration and prior to the mixing and molding of the product (Osorio, 2007). This concentrate has an important content of soluble solids provided mainly by sucrose, glucose and fructose (Wisuthiphaet and Napathorn, 2016). Moreover, its Xw and aw identified this substrate as susceptible to microbial activity, which is favored by its acidity and pH conditions. Information on sugarcane concentrate is scarce in the reviewed literature, but some studies stand out: Largo et al. (2015), reported findings on sugarcane concentrate at 48.6 °Brix, an Xw: 41.0; aw: 0.962; pH: 5.7; acidity: 0.026%; Naranjo (2008) obtained similar values for density (1.35 g cm-3) and Xw (30%) with sugarcane concentrate at 70 °Brix.
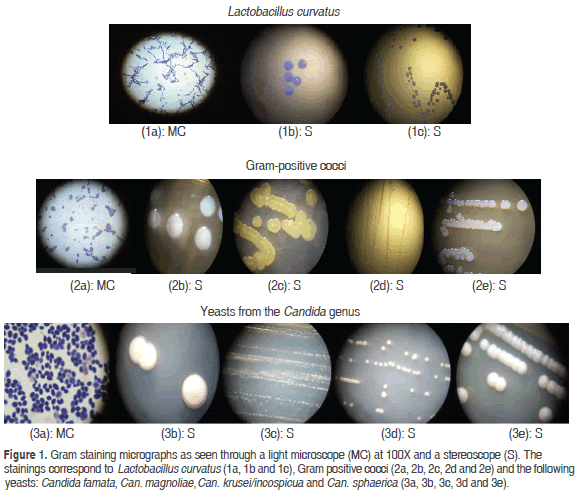
The microorganism isolation process identified six bacterial colonies in the KG: two colonies of Lactobacillus curvatus with a degree of confidence of 99.9% that were white, bright, umbonate and round with even borders, and four colonies of gram-positive, catalase and oxidase negative cocci that grew properly in the selective culture medium for cocci (M17) and showed a tendency to cluster in strings. Microscopic observations confirmed that these colonies were transparent, bright, punctuate and flat, but their genus and species need to be confirmed via molecular techniques. Other studies reported that the composition of microorganisms in the KG is variable and depends on their source, culture conditions and substrate. However, the Lactobacillus genus is predominant (Leite et al., 2013; Garofalo et al., 2015).
Lactobacillus curvatus is a sucrose-fermenting and facultative heterofermentative microorganism that lowers the pH of the substrate as it produces lactic acid (De Vos et al., 2009). It has been isolated from KG, fermented vegetables, meat and dairy products. Witthuhn et al. (2004) isolated it from South African KG; Ahmadova et al. (2013) isolated it from homemade cheese in Azerbaijan and observed antimicrobial effects against Listeria monocytogenes and Bacillus cereus as well as antifungal activity against Cladosporium and Fusarium ssp caused by a bacteriocin called curvacin. In addition, it also showed resistance to physiological concentrations of bile salts and no multiresistance to antibiotics. Similarly, Cocolin et al. (2009) reported that Lactobacillus curvatus and Lb. sakei were the most frequent bacteria in three types of salami. These results are consistent with those obtained by a study with spontaneously matured European style sausages (Amor and Mayo, 2007, cited by Rivera and Gallardo, 2010). Likewise, Hebert et al. (2012) sequenced the genome of this lactobacillus after isolating it from artisanal sausages from Argentina that produced this bacteriocin.
Strains of Lactobacillus curvatus have been isolated from vegetables such as kimchi with good results in terms of acidification properties and excellent results when used as starter culture for fermentation in sausages. Equal results have been obtained with Lb. casei, Lb. pentosus, Lb. plantarum, Lb. sakei, Pediococcus acidilactici and Ped. Pentosaceus (Lee et al., 2006). Rühmkorf et al. (2013) found that the exopolysaccharide produced by this lactobacillus together with that of Lb. reuteri and Lb. animalis, improved the quality of gluten in bread making. This shows the potential uses of Lb. curvatus in the food industry as well as its behavior as a probiotic.
Figure 1 shows gram staining micrographs for Lactobacillus curvatus, gram positive cocci and the Candida famata, Can. magnoliae, Can. krusei/incospicua and Can. sphaerica yeasts. These micrographs were taken with an optical microscope and a stereoscope.
The gram positive cocci and Lactococci have also been isolated from KG (Hsieh, et al 2012; Witthuhn et al., 2005; Leite et al., 2015) and dairy beverages prepared with them (Altay et al., 2013). Monteagudo et al. (2012) found that samples of Lactoccus lactis, isolated from cow and sheep milk had high adherence to Caco-2 cells, low pH tolerance, and tolerance to pancreatin and bile salts. In addition, they also showed antimicrobial activity against Bacillus cereus, Listeria innocua, L. monocytogenes, Staphylococcus aureus and Pseudomonas fluorescens. These results show the potential uses of Lactococcus lactis as a probiotic microorganism.
As for the yeasts found in the KG in our study, they were more diverse for species from the Candida genus: Can. magnoliae, Can. sphaerica. Can. famata, Can. krusei/incospicua, with confidence values of 81.0%, 67.2%, 93.9% and 99.9% respectively. The latter two having been identified on the basis of the cycle in which they were found: Candida krusei (anamorphic) or Issatchenkia orientalis (telomorphic); Candida famata (anamorphic) or Debaromyces hansenii (telomorphic) (Lopitz et al., 2006). The following morphological characteristics were observed in the colonies: they were bright, white, creamy, umbonate and round with even borders. Candida krusei, Debaromyces hansenii (Candida famata) and Candida inconspicua have been isolated directly from the KG (Witthuhn et al., 2005; Loretan, et al., 2003); moreover, Candida inconspicua has been also isolated from dairy beverages (Altay et al., 2013). In studies conducted with eight strains of yeasts isolated from cheese and kefir, Debaromyces hansenii (Candida famata) showed good adhesion to cultures of human Caco-2 cells as well as resistance to acidic conditions. However, the strain of Kluyveromyces lactis exhibited the best probiotic characteristics (Kumura et al., 2004).
In general, we can say that the study using South African KG shares similarities with ours regarding the microorganisms contained in the kefir. This is due mainly to the presence of Lactobacillus curvatus, Candida krusei and Debaromyces hansenii (Candida famata) (Witthum et al. 2004).
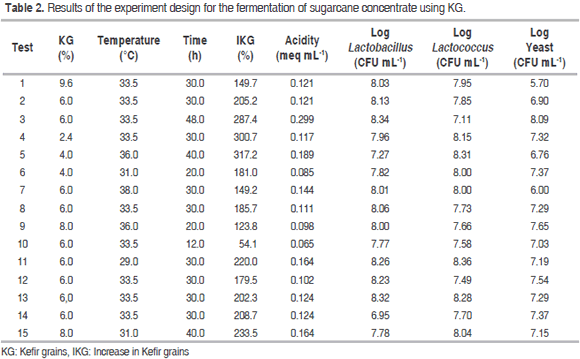
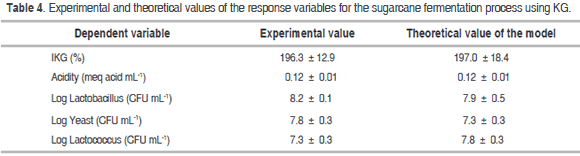
Tables 2 and 3 show the results of the experiment design for the fermentation process, as well as the ANOVA for the dependent variables labeled IKG (%), acidity (meq mL-1) and Log yeast (CFU mL-1) in relation to factors A (KG), B (temperature) and C (time) respectively.
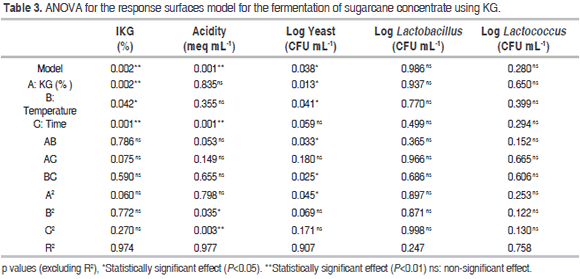
There were statistically significant differences (P<0.05) for IKG in relation to factors A, B and C; in contrast, there were no significant differences (P>0.05) for their interactions or for quadratic interactions. The entire fermentation temperature range showed a higher IKG with low percentages of KG. For instance, 2.4% of KG led to values of approximately 350 and 250% at 29 and 38 °C respectively, while 9.6% of KG led to an IKG ranging from 150 to 170% IKG. The interaction of factors A and B on IKG had a negative effect, as there is less competition for space and nutrients among the microorganisms as well as lower production of inhibitory metabolites. However, since this is a microbial succession, some of the generated metabolites become the substrate for the growth of others. Furthermore, these microorganisms are considered mesophilic (optimal temperature: 30-40 °C) and their growth is favored in the lower limit of this range because of the balance in their metabolic processes. In the entire range of studied KG concentrations, the IKG showed little variation with low fermentation times, but became maximized once time was increased; this happened mainly when adding 2.4 to 6.0% of KG. The long time required for higher IKG and log yeast values could be due to the fact that the growth of lactobacilli and lactococci starts when the pH is close to neutrality (the initial stage of fermentation) and decreases when acids are produced from glucose. Moreover, high acidity stimulates yeast growth (long fermentation times) (De Vos et al., 2009).
Some researchers have found that the IKG is favored when Lactobacillus kefiranofaciens and Saccharomyces cerevisiae are present in the fermentation process (Cheirslip et al., 2003). This confirms the symbiosis between bacteria and yeasts occurring in the KG. Gao et al. (2012) showed a lower IKG in a dairy substrate at 30.05 °C with 20 hours of fermentation. These conditions caused an increase of only 39.4%. Furthermore, Guzel et al. (2011a) obtained an IKG of around 392 and 223% in dairy substrates enriched with whey proteins; however, this required higher times (30 days). This study, on the other hand, reached similar IKG values with shorter times and no dairy or enriched substrates.
Acidity showed statistically significant differences (P<0.05) when compared to factor C. This was also true for the B2 and C2 quadratic interactions. In contrast, no significant differences (P>0.05) were found with factors A and B, interactions between factors or in the A2 quadratic interaction. Low acidity was observed throughout the entire fermentation temperature range at short fermentation times (values ranged from 0.06 to 0.1 meq acid mL-1). Additionally, increasing the fermentation time resulted in higher acidity, with values approaching 0.3 meq acid mL-1. Cui et al. (2013) found similar acidity values (meq acid 0.16 mL-1) using a similar fermentation temperature, a shorter time and fewer KG in a different substrate.
The log yeast variable showed statistically significant differences (P<0.05) with factors A and B, the AB and BC interactions and the A2 quadratic interaction. No difference was observed regarding the C factor, the AC interaction or the B2 and C2 quadratic interactions. Little variation was observed in the yeast growth at high fermentation temperatures. However, there was a tendency to become maximized once the temperature and KG decreased. The AB and BC interactions on acidity had a positive effect.
The optimization of the fermentation process using the Design Expert 8.0 software was performed by maximizing both the IKG and the microbial counts while keeping the acidity levels within the experimental range, thus generating the following optimal conditions for fermentation: KG = 6.0% (w/w), temperature = 33.5 °C and time = 30 hours. Table 4 shows the values obtained experimentally using the optimal process conditions and the theoretical values from the models for each variable. The ANOVA (Table 3) showed that the factorial regression model was statistically significant (P<0.05) for the IKG, acidity (meq mL-1) and log yeast (CFU mL-1) response variables. The regression coefficients for the quadratic model were 97.4%; 97.7% and 90.4% respectively. In contrast, no statistically significant differences (P>0.05) were observed for the other dependent variables, factors or interactions within the studied range.
Additional research conducted on fermentation processes using 3% of KG in nut milk supplemented with 8% of sucrose for 12 h at 30 °C showed yeast counts of approximately 6 logs (1 log unit less than in our study), probably due to the lower fermentation times; on the other hand, the lactobacilli and lactococci counts were similar (Cui et al., 2013). Furthermore, the counts found by Leite et al. (2012) upon fermenting UHT skim milk with 3% of KG at 25 °C for 24 h were similar in terms of lactobacilli and yeasts, but higher for lactococci.
A number of studies have shown that the use of KG produces foods with better quality and greater acceptance than those obtained from starter cultures that have been directly isolated from the granule (Assadi et al., 2000). Therefore, it is more effective to use grains given the symbiosis existing between the organisms that compose them because they produce a wider variety of metabolites that provide the food with better sensory and nutritional characteristics.
CONCLUSIONS
Sugarcane concentrate is an effective alternative for the development of non-dairy foods with probiotics using KG as the starter culture for the fermentation process. Consumption of this food might prevent gastrointestinal diseases and strengthen the immune system.
Six bacterial and four yeast strains were isolated from KG activated in sugarcane concentrate; their microorganism composition showed greater similarity with that of KG from South Africa. The yeasts found showed greater variety (Candida famata, Can. magnoliae, Can. krusei/incospicua and Can. sphaerica) than those from the lactobacilli genus (Lactobacillus curvatus).
Results made it possible to establish a technological, effective, simple and practical fermentation process that is likely to be replicable at a large scale under the following conditions: a temperature of 33.5 °C, a time of 30 hours and with 6% w/w of added KG. The increase in KG reached was of 193 ± 12% and the lactobacilli, lactococci and yeast counts were 1.57x108, 8.63x107 and 2.05x107 CFU mL-1 respectively. In addition, they showed many advantages over those found by other studies using milk-based substrates with additional nutrients.
REFERENCES
Ahmadova A, Todorov SD, Hadji I, Choiset Y, Rabesona H, Messaoudi S, Kuliyev A, Gombossy BJ, Chobert JM and Haertlé T. 2013. Antimicrobial and antifungal activities of Lactobacillus curvatus strain isolated from homemade Azerbaijani cheese. Anaerobe 20: 42-49. doi: 10.1016/j.anaerobe.2013.01.003 [ Links ]
Ahmed Z, Wang Y, Ahmad A, Khan ST, Nisa M, Ahmad H and Afreen A. 2013. Kefir and health: A contemporary perspective. Critical Reviews in Food Science and Nutrition 53: 422-434. doi: 10.1080/10408398.2010.540360 [ Links ]
Altay F, Karbancıoglu-Güler F, Daskaya-Dikmen C and Heperkan D. 2013. A review on traditional Turkish fermented non-alcoholic beverages: Microbiota, fermentation process and quality characteristics. International Journal of Food Microbiology 167: 44-56. doi: 10.1016/j.ijfoodmicro.2013.06.016 [ Links ]
Aquilanti L, OzgeK, Zannini E, Osimani A, Silvestri G, Ciarrocchi F, Garofalo C, Tekin E and Clementi F. 2012. Response of lactic acid bacteria to milk fortification with dietary zinc salts. International Dairy Journal 25: 52-59. doi: 10.1016/j.idairyj.2011.12.006 [ Links ]
Assadi MM, Pourahmad R and Moazami N. 2000. Use of isolated kefir starter cultures in kefir production. World Journal of Microbiology and Biotechnology 16: 541-543. doi 10.1023/A:1008939132685 [ Links ]
Bensmira, and Jiang B. 2012. Effect of some operating variables on the microstructure and physical properties of a novel Kefir formulation. Journal of Food Engineering 108: 579-584. doi: 10.1016/j.jfoodeng.2011.07.025 [ Links ]
Carasi P, Jacquot C, Romanin DE, Elie AM, De Antoni GL, Urdaci MC and Serradell MA. 2014. Safety and potential beneficial properties of Enterococcus strains isolated from kefir. International Dairy Journal 39: 193-200. doi: 10.1016/j.idairyj.2014.06.009 [ Links ]
Cheirslip B, Shimizu H and Shioya S. 2003. Enhanced kefirano production by mixed culture Lactobacillus kefiranofaciens and Saccharomyces cereviciae. Journal of Biotechnology 100: 43-53. doi: 10.1016/S0168-1656(02)00228-6 [ Links ]
Cocolin L, Dolci P, Rantsiou K, Urso R, Cantoni C and Comi G. 2009. Lactic acid bacteria ecology of three traditional fermented sausages produced in the North of Italy as determined by molecular methods. Meat Science 82: 125-132. doi: 10.1016/j.meatsci.2009.01.004 [ Links ]
Cui XH, Chen SJ, Wang Y and Jan JR. 2013. Fermentation conditions of walnut milk beverage inoculated with kefir grains. LWT - Food Science and Technology 50: 349-352. doi: 10.1016/j.lwt.2012.07.043 [ Links ]
Chen HC, Wang SY and Chen MJ. 2008. Microbiological study of lactic acid bacteria in kefir grains by culture-dependent and culture-independent methods. Food Microbiology 25: 492-501. doi: 10.1016/j.fm.2008.01.003 [ Links ]
Chifiriuc MC, Cioaca AB and Lazar V. 2011. In vitro assay of the antimicrobial activity of kephir against bacterial and fungal strains. Anaerobe 17: 433-435. doi: 10.1016/j.anaerobe.2011.04.020 [ Links ]
De Vos P, Garrity GM, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer KH and WhitmanWB. 2009. Bergey's Manual of systematic bacteriology. Second edition. Vol. 3. Springer, New York. 1450 p. [ Links ]
Diosma G, Romanin DE, Rey-Burusco MF, Londero A, Garrote GL. 2014. Yeasts from kefir grains: isolation, identification, and probiotic characterization. World Journal of Microbiology and Biotechnology 30: 43-53. doi: 10.1007/s11274-013-1419-9 [ Links ]
Faostat. 2013. United Nations Food and Agriculture Organization. < Disponible en: http://faostat3.fao.org/browse/Q/QC/E>; accessed: October 2014. [ Links ]
Gao J, Gu F, Ruan H, Chen Q, He J and He G. 2012. Culture conditions optimization of tibetan kefir grains by response surface methodology. Procedia Engineering 37: 132-136. doi: 10.1016/j.proe ng.2012.04.215 [ Links ]
Garofalo C, Osimani A, Milanovi V, Aquilanti L, De Filippis F, Stellato G, Di Mauro S, Turchetti B, Buzzini P, Ercolini D and Clementi F. 2015. Bacteria and yeast microbiota in milk kefir grains from different Italian regions. Food Microbiology 49: 123-133. doi: 10.1016/j.fm. 2015.01.017 [ Links ]
Guzel-Seydim Z, Kok-Tas T, Ertekin-Filiz B and Seydim AC. 2011a. Effect of different growth conditions on biomass increase in kefir grains. Journal of Dairy Science 94: 1239-1242. doi: 10.3168/jds. 2010-3349 [ Links ]
Guzel-Seydim Z, Tugba KT, Annel KG and Seydim AC. 2011b. Review: Functional properties of kefir. Critical Reviews in Food Science and Nutrition 51: 261-268. doi: 10.1080/10408390903579029 [ Links ]
Hebert EM, Saavedra L, Taranto MP, Mozzi F, Magni Ch, Nader ME, Font G, Sesma F, Vignolo G and Raya R. 2012. Genome sequence of the bacteriocin-producing Lactobacillus curvatus strain CRL705. Journal of Bacteriology 194: 538-539. doi: 10.1128/JB.06416-11 [ Links ]
Horwitz W. 2005. Official Methods of Analysis Association of Official Analytical Chemists. 17th edition. AOAC International, Virginia. 1115 p. [ Links ]
Hsieh H, Wang SY, Chen TL, Huang YL and Chen MJ. 2012. Effects of cow's and goat's milk as fermentation media on the microbial ecology of sugary kefir grains. International Journal of Food Microbiology 157: 73-81. doi: 10.1016/j.ijfoodmicro.2012.04.014 [ Links ]
Iraporda C, Romanin DE, Rumbo M, Garrote GL and Abraham AG. 2014. The role of lactate on the immunomodulatory properties of the nonbacterial fraction of kefir. Food Research International 62: 247-253. doi: 10.1016/j.foodres.2014.03.003 [ Links ]
Kabak B and Dobson ADW. 2011. An introduction to the traditional fermented foods and beverages of Turkey. Critical Reviews in Food Science and Nutrition 51: 248-260. doi: 10.1080/10408390903569640 [ Links ]
Kumura H, Tanoue Y, Tsukahara M, Tanaka T and Shimazaki K. 2004. Screening of dairy yeast strains for probiotic applications. Journal Dairy Science 87(12): 4050-4056. doi: 10.3168/jds.S0022-0302(04)73546-8 [ Links ]
Largo-Ávila E, Cortés M, Ciro-Velásquez HJ. 2015. Influence of Maltodextrin and Spray Drying Process Conditions on Sugarcane Juice Powder Quality. Revista Facultad Nacional de Agronomía 68(1): 7509-7520. doi: 10.15446/rfnam.v68n1.47839 [ Links ]
Lasik A and Jan P. 2012. Production of fermented goat beverage using a mixed starter culture of lactic acid bacteria and yeasts. Engineering in Life Sciences 4: 486-493. doi: 10.1002/elsc.201100126 [ Links ]
Lee JY, Kim ChJ and Kunz B. 2006. Identification of lactic acid bacteria isolated from kimchi and studies on their suitability for application as starter culture in the production of fermented sausages. Meat Science 72: 437-445. doi: 10.1016/j.meatsci.2005.08.013 [ Links ]
Leite AMO, Mayo B, Rachid CTCC, Peixoto RS, Silva JT, Paschoalin VMF and Delgado S. 2012. Assessment of the microbial diversity of Brazilian kefir grains by PCR-DGGE and pyrosequencing analysis. Food Microbiology 31(2): 215-221. doi: 10.1016/j.fm.2012.03.011 [ Links ]
Leite AM, Leite DC, Del Aguila EM, Alvares TS, Peixoto RS, Miguel MA, Silva JT and Paschoalin VM. 2013. Microbiological and chemical characteristics of Brazilian kefir during fermentation and storage processes. Journal of Dairy Science 96: 4149-4159. doi: 10.3168/jds.2012-6263 [ Links ]
Leite AMO, Miguel MAL, Peixoto RS, Ruas-Madiedo P, Paschoalin VMF, Mayo B and Delgado S. 2015. Probiotic potential of selected lactic acid bacteria strains isolated from Brazilian kefir grains. Journal of Dairy Science 98(6): 3622-3632. doi: 10.3168/jds.2014-9265 [ Links ]
Lopitz O, Rementeria FA, Elguezabal N, Garaizar J. 2006. Kefir: A symbiotic yeasts-bacteria community with alleged healthy capabilities. Revista Iberoamericana de Micología 23: 67-74. [ Links ]
Loretan T, Mostert JF and Viljoen BC. 2003. Microbial flora associated with South African household kefir. South African Journal Science 99(1): 92-94. [ Links ]
Luckow T and Delahunty C. 2004. Which juice is healthier'? A consumer study of probiotic non-dairy juice drinks. Food Quality and Preference 15: 751-759. doi: 10.1016/j.foodqual.2003.12.007 [ Links ]
Maalouf K, Baydoun E and Rizk S. 2011. Kefir induces cell-cycle arrest and apoptosis in HTLV1-negative malignant T-lymphocytes. Cancer Management and Research 3: 39-47. doi: 10.2147/CMR.S15109 [ Links ]
Monteagudo A, Rodríguez L, Rúa J, Martínez H, Navasa N, García MR and Ferrero MA. 2012. In vitro evaluation of physiological probiotic properties of different lactic acid bacteria strains of dairy and human origin. Journal of Functional Foods 4(2): 531-541. doi: 10.1016/j.jff.2012.02.014 [ Links ]
Nambou K, Gao C, Zhou F, Guo B, Ai L and Wu ZJ. 2014. A novel approach of direct formulation of defined starter cultures for different kefir-like beverage production. International Dairy Journal 34: 237-246. doi: 10.1016/j.idairyj.2013.03.012 [ Links ]
Naranjo, WD 2008. Caracterización térmica y reológica de miel de dos variedades de caña. Trabajo de grado Ingeniería en Alimentos. Facultad de Ciencia e Ingeniería en Alimentos. Universidad Técnica de Ambato. Ambato, Ecuador. 110 p. [ Links ]
Osorio G. 2007. Buenas Prácticas Agrícolas -BPA- y Buenas Prácticas de Manufactura -BPM- en la producción de caña y panela. Corpoica-Mana-FAO. p. 101-126. [ Links ]
Rivera Y and Gallardo Y. 2010. Non-dairy probiotic products. Food Microbiology 27: 1-11. doi: 10.1016/j.fm.2008.06.008 [ Links ]
Rühmkorf Ch, Bork Ch, Mischnick P, Rübsam H, Becker T and Vogel R. 2013. Identification of Lactobacillus curvatus TMW 1.624 dextransucrase and comparative characterization with Lactobacillus reuteri TMW 1.106 and Lactobacillus animalis TMW 1.971 dextransucrases. Food Microbiology 34: 52-61. doi: 10.1016/j.fm.2012.11.002 [ Links ]
Salazar BC, Cortés M and Montoya OI. 2015. The impact of storage conditions on the stability of sugarcane powder biofortified with kefir grains. Revista Facultad Nacional de Agronomia 68(2): 7703-7712. doi: 10.15446/rfnam.v68n2.50987 [ Links ]
Teixeira M, Pereira MA, Giuliano AN, Domingues L, Teixeira JA, De Almeida JB and Schwan RF. 2010. Production of fermented cheese whey-based beverage using kefir grains as starter culture: Evaluation of morphological and microbial variations. Bioresource Technology 101: 8843-8850. doi: 10.1016/j.biortech.2010.06.083 [ Links ]
Wisuthiphaet N and Napathorn SC. 2016. Optimisation of the use of products from the cane sugar industry for poly (3-hydroxybutyrate) production by Azohydromonas lata DSM 1123 in fed-batch cultivation. Process Biochemistry 51(3): 352-361. doi: 10.1016/j.procbio.2015.12.009 [ Links ]
Witthuhn R, Schoeman T and Britz TJ. 2004. Isolation and characterization of the microbial population of different South African kefir grains. International Journal of Dairy Technology 57(1): 33-37. doi: 10.1111/j.1471-0307.2004.00126.x [ Links ]
Witthuhn R, Schoeman T and Britz TJ. 2005. Characterization of the microbial population at different stages of kefir production and kefir grains mass cultivation. International Dairy Journal 15(4): 383-389. doi: 10.1016/j.idairyj.2004.07.016 [ Links ]