Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Facultad Nacional de Agronomía Medellín
versão impressa ISSN 0304-2847
Rev. Fac. Nac. Agron. Medellín vol.69 no.2 Medellín jul./dez. 2016
https://doi.org/10.15446/rfna.v69n2.59140
DOI: http://dx.doi.org/10.15446/rfna.v69n2.59140
The effect of storage temperature and time on total phenolics and enzymatic activity of sapodilla (Achras sapota L.)
Efecto de la temperatura y el tiempo de almacenamiento en los fenoles totales y la actividad enzimática del níspero (Achras sapota L.).
Jairo Mercado Camargo1, Arnulfo Taron Dunoyer1 and Luis A. García-Zapateiro2
1 Facultad de Ciencias Farmacéutica. Universidad de Cartagena. Campus de Ciencias de la Salud, Cartagena, Colombia. <jmercadoc@unicartagena.edu.co>
2 Facultad de Ingeniería. Universidad de Cartagena. Campus de Piedra de Bolívar, Cartagena, Colombia.
Received: March 31, 2016; Accepted: June 13, 2016
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

ABSTRACT
The tropical fruits are sensitive to low storage temperatures, so optimal parameters have been searched for storage and transport for the purpose of maintaining its overall quality as long as possible to the consumer. The effect of different storage temperatures (6, 10, 15, 21 and 27 °C) and storage durations (0 to 20 d) on total phenolics and enzymatic activity of peroxidase (POD), catalase (CAT), and polyphenol oxidase (PPO) on sapodilla (Achras sapota L.) fruit was investigated. The extraction and quantitation of protein and phenols from fruit was performed, then the enzymatic activity of PPO, POD and CAT was determined. The concentration of total phenolics decreased in the control fruit. POD activity was 3268.7 ± 1.4 U g-1 in ripening and senescence of sapodilla stored at 27 °C. CAT activity reached a peak of 34.0 ± 0.25 U g-1 in senescence in control fruit. PPO activity remained unchanged in the ripening stage and until consumption. The best storage temperatures to prolong the post-harvest life of the sapodilla fruit were 6 °C and 10 °C when storage was at low temperatures. POD activity was inactivated during sapodilla storage at low temperatures (6 and 10 °C) and after being transferred to 27 °C the activity was reactivated. Likewise of fruits stored at 21 °C after being transferred to 27 °C the POD activity was reactive with a maximum value of 46.3 ± 0.012 U g-1. Enzyme activity decreased at low temperatures, which contributed to the preservation of the fruit, showing that the cold retards the maturation processes.
Key words: Total phenolics, Catalase, Peroxidase, Polyphenol oxidase, Low temperature storage
RESUMEN
Las frutas tropicales son sensibles a bajas temperaturas de almacenamiento, debido a esto los parámetros de almacenamiento y transporte deben ser los óptimos con el fin de mantener su calidad el mayor tiempo posible para el consumidor. En esta investigación se estudió el efecto de las temperaturas (6, 10, 15, 21 y 27 °C) y tiempos (0 a 20 d) de almacenamiento en el contenido de fenoles totales y actividad enzimática (peroxidasa (POD), Catalasa (CAT) y Polifenol Oxidasa (PPO)), además extracción y cuantificación de proteínas del níspero (Achras sapota L.) y un fruto control. La actividad POD fue 3268,7 ± 1,4 U g-1 en la maduración y senescencia del níspero almacenado a 27 °C, la actividad CAT alcanzó un pico de 34,0 ± 0,25 U g-1 en la senescencia del fruto control. La actividad PPO permaneció sin cambios en el estado de madurez hasta su consumo. Las temperaturas de almacenamiento de 6 °C y 10 °C fueron las mejores para prolongar la vida poscosecha del fruto. La actividad POD se inactivó durante el almacenamiento del níspero a bajas temperaturas (6 y 10 °C) pero esta se reactivó con la transferencia del fruto a 27 °C. La actividad PPO de los frutos almacenados a 21 °C se incrementó después de transferirse a una temperatura de 27 °C, presentando un valor máximo de 46,3 ± 0,012 U g -1. La actividad enzimática disminuye a bajas temperaturas lo que contribuye a la preservación de la fruta, demostrando que el frío retrasa los procesos de maduración.
Palabras claves: Fenoles totales, Catalasa, Peroxidasa, Polifenoloxidasa, Almacenamiento a baja temperatura
Fruits and vegetables are an indispensible part of the human diet. They provide a diversified flavoured, colourful, tasty, low caloric and protective, micronutrient rich diet (Sachdeva et al., 2013). The intake of fruits reduces the risk of chronic degenerative diseases such as cancer, diabetes heart disease and obesity (OMS and FAO, 2005).
Maturity is the most important and complex stage in fruit development, which can be divided into two phases: physiological maturity and ripening period. Physiological maturity begins before the growth of cells ends, and finish when the fruit has seeds in a position to produce new plants; the evolution of this phase is completed when the fruit is in the plant. The ripening period is the process when physiologically mature tissue becomes a visual and olfactory attractive tissue; this is the result of complex transformations including seed maturation, colour changes, organic acid modifications, changes in the composition of peptic substances, production of volatile substances and development of wax in the skin (Lara et al., 2007).
Low temperatures can extend the storage period, but this does not completely inhibit the decline in the levels of organic acids or the loss of moisture during a prolonged time (Chachin et al., 1990). In addition, tropical and subtropical fruits are particularly sensitive to storage at low temperatures; therefore, optimal parameters of conservation and transport have been investigated in order to maintain the quality as much as possible for the consumer (Pérez et al., 1999). Darkening and chilling injury in fruits has been associated with the metabolism of phenolics (Salveit and Morris, 1990), It is leading to an increase in enzyme activity of polyphenol oxidase (EC. 1.14.18.1, PPO) and peroxidase (EC: 1.11.1.7, POD) are relevant in the oxidative degradation of phenolic compounds in terms of quality because they produce darkening (Tomas and Espin, 2001). The chilling injury is also considered as oxidative stress, and it is related to a decrease of the enzyme that removes reactive oxygen species, such as catalase (EC. 1.11.1.6, CAT) (Sala, 1998).
The balance between the reactive oxygen species (ROS), i.e. superoxide radicals (O-2), singlet oxygen (1O2), hydroxyl radicals (OH) and hydrogen peroxide (H2O2), and the antioxidant system play an important role in the loss of quality in fruits (Duarte-Baquero et al., 2005). Although the production of ROS is a normal process, this can be increased for the ripening and senescence, and also for the agents that cause stress, such as heavy metals, radiations, changes in the atmosphere, extreme temperatures and microorganism attacks (Kaur and Kapoor, 2008).
Sapodilla (Achras sapota L.) belongs to the Sapotaceae family, and is one of the major fruit crops in India, Mexico, Guatemala and Venezuela (Siddappa and Bhatia, 1954), where it is grown commercially, with large economic value. Sapodilla is a fleshy berry, generally globose, conical or oval with one or more seeds. The fruit generally weighs about 75-200 g, ranging from 5 to 9 cm in diameter. The fruit has a thin rusty brown scurfy skin and a yellowish brown or red pulp with a pleasant, mild aroma and an excellent taste (Mickerbart, 1996; Thompson, 2003). The fruit contains sugars, ascorbic acid, proteins, phenols, carotenoids and minerals like potassium, calcium and iron (Kulkarini et al., 2007), establishing that sapodilla (A. sapota L.) has average nutritional elements make it a fruit with excellent features for agro-processing.
Raw sapodilla (A.sapota L.) fruits are astringent, while ripe fruits are sweet. Mature fruits are used for making mixed fruit jams and provide a valuable source of raw material for the manufacture of industrial glucose, pectin, and natural fruit jellies. They also are canned as slices. Fully ripened sapodilla (A. sapota L.) fruit pulp is eaten as a dessert fruit, but sometimes it is served as candy, dehydrated slices, jelly and juices (Ganjyal et al., 2003).
Sapodilla (A. sapota L.) is a non-climateric and subtropical fruit, which should be harvested when it reaches full maturity; if harvested before its flavor it is regular (Razeto, 1998), there is a need to find methods to achieve mass productions that permit export. In Colombia there is production that covers the domestic market, which is mainly represented by the consumption of fresh fruit (pulp) and to a lesser extent, used for prepared juices, sorbets, ice creams and jams. This represents a production of 0.002% in the domestic market (Guia Ambiental de Hortifruticola de Colombia, 2009). Therefore, it is necessary to know the feasibility and storages techniques to prolong the life of this fruit-what is perishable and sensitive to browning if not handled with care, and trying to keep longer post-harvest life of this fruit. For this, the aim of this work is to determinate the chilling injury in sapodilla (A. sapota L.) from the quantification of total phenols and enzymatic activity, by different conditions of conservation that ensure a better condition for the time when it reaches the consumer.
MATERIALS AND METHODS
Plant material and storage process
Sapodilla (A. sapota L.) fruit was harvested in the Clemencia-Bolivar department and transported to the laboratory of food science at Cartagena University (Cartagena de Indias- Colombia). Fruits were selected according to uniformity of size and colour; blemished and damaged fruits were discarded.
One hundred eighty fruits were selected, which were conditioned in the laboratory at room temperature (27 °C) for 5 h. Sapodilla fruits were distributed into five groups (36 fruits in each group) to be stored at different temperatures for experimentation. The storage temperatures were 6, 10, 15, and 21 °C for 10 d for each group. After that time, each group of fruit was stored at room temperature (27 °C) to complete 20 d of storage. The fifth group (control sample) was stored at 27 °C for 10 d, after this period, the fruits lost texture and there was a considerable decrease in the concentration of total phenolics compounds. Three fruits were taken at random from each group every two days in order to determine changes that occurred during storage.
Extraction and quantification of proteins
The samples of sapodilla fruits were homogenized with acetone powders at 4 °C; the white sediment was homogenized for 24 h at 2 °C with 200 mM phosphate buffer solution adjusting to pH 6.8. The suspension was centrifuged at 12,000 G for 30 min at 2 °C. Then, 2 mL of supernatant were taken for the enzymatic activity. These analyses were done using fresh extracts since low temperatures (refrigeration or freezing) could affect the activity (Tejacal et al., 2005). For quantification of soluble proteins, the Bradford (1976) method modified by Zor and Sellinger (1995) was used and the absorbance readings were at 590 nm using bovine serum albumin (BSA) as standard curve with concentrations between 0.5 to 2 mg L-1. Three replicates were conducted for each sample.
Extraction and quantification of total phenols
Ten grams of pulp was homogenized, adding 20 mL of acetone at 6 °C, divided into four parts of 5 mL for the successive extraction. Extracts were centrifuged for 5 min, supernatant samples approximately 2 mL and 0.7 mL of distilled water were added to aliquots of 0.1 mL of extracts and 0.5 mL of commercial reagent of Folín-Ciocalteu (Sapan et al., 1999), and 1.5 mL of sodium carbonate (NaCO3) at 20%. Finally, the volume was completed with 10 mL of distillated water. Absorbance measuring was done 2 h later at 760 nm for quantification of phenols, using a standard curve of calibration of tannic acid in concentrations between 3 mg L-1 to 50 mg L-1. The spectrophotometer UV-VIS (Shimadzu UV-1700, Pharmaspec, Japan) was used for the analysis. Three replicates were conducted for each sample (Ayala-Zavala et al., 2004).
Determination of enzymatic activity of polyphenol oxidase
For determination the activity of PPO were used 3 mL of 60 mM catechol dissolved in 200 mM phosphate buffer pH 6.8 were mixed in a quartz cell with 200 µL of extracts. The absorbance was evaluated at a wavelength of 420 nm for 1 min. The enzymatic activity was reported as U g-1 of fresh weight, where one unit of enzymatic activity is equal to 1 mol formation of o-benzoquinone min-1 (Tejacal et al., 2005). Three replicates were conducted for each sample.
Determination of enzymatic activity of peroxidase
For EC: 1.11.1.7-POD, 2.6 mL of 200 mM phosphate buffer, pH 6.8, 0.25 mL of guaiacol 0.1 M, 0.1 mL of hydrogen peroxide (H2O2) 0.25 % and 100 µL of the extract were mixed in a quartz cell. The change in absorbance at 470 nm was determined for 3 min. The enzymatic activity was reported as U g-1 of fresh weight, where one unit of enzymatic activity equals forming 1 µmol of tetraguaicol min-1 (Tejacal et al., 2005) was used for the analysis. Three replicates were conducted for each sample.
Determination of enzymatic activity of catalase
For EC. 1.11.1.6-CAT, 3 mL of 10 mM Tris-HCl buffer, pH 8.5, were mixed to 0.1 mL of hydrogen peroxide (H2O2), 0.88% in 100 mL of Tris-HCl mM (pH 8.5) and 100 µL of extract were mixed in a quartz cell. The change in absorbance at 240 nm was determined for 1 min and enzyme activity was reported as U g-1 of fresh weight, where one unit of enzymatic activity equals to decomposition of 1 µmol min-1 of H2O2. Three replicates were conducted for each sample (Dueñas-Gómez et al., 2008).
Statistical analysis
In order to evaluate the effects of the quantification of proteins, of total phenols and the enzymatic activity on the temperatures and the time of storage of sapodilla (A. Sapota L.) fruits, three replications were performed. Significant differences between treatments were determined by performing ANOVA and a multiple comparison of means test (P<0.05). Data were expressed as the mean ± standard deviation. The analyses were carried out using STATGRAPHICS Centurion XV.
RESULTS AND DISCUSSION
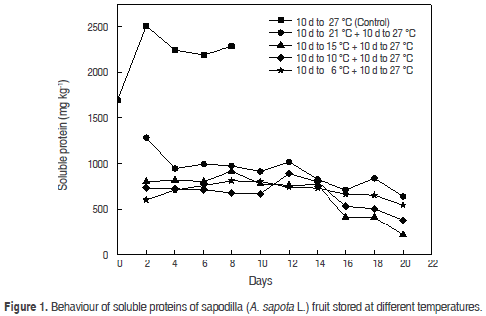
Quantification of soluble proteins
The concentration of soluble proteins has shown a significant increase in the stored time (Figure 1). The control fruit presented a value of 1699.9 ± 0.032 mg kg-1, reaching a peak on the fourth day (2506.1 ± 0.029 mg kg-1) in physiological maturity stage. In senescent fruit, the protein concentration was 2189.6 ± 0.084 mg kg-1. This behavior is due to changes in metabolic pathways of maturation that involve synthesis of protein; hydrolytic enzymes-e.g. α-amylase, β-amylase, starch phosphorylase accumulate inverted sugars and polygalacturonase involved in softening tissue, and promote and accelerate the change in ripening. Ripening has been rationed with the accumulation of organic compounds, fruit abscission and senescence or aging tissue. Tejacal et al. (2005) have reported a value of 82 mg kg-1 in physiological maturity and 950 mg kg-1 in ripening and 299.1 mg kg-1 in senescence for zapote mamey (Pouteria sapota Jacq.), showing an increase in soluble protein concentration. Storage temperature did not significantly show an effect in soluble protein concentration of fruits stored at 6, 10, 15 and 21 °C, but a decrease was observed when the produce was moved to room temperature (27 °C); this is probably due to several changes and metabolic pathways involving protein synthesis, which may be inhibited due to the low temperature to which the fruit was exposed (Dueñas-Gómez et.al., 2008; Tejacal et al., 2005). Statistically significant differences were not detected in soluble protein concentration of fruit stored at different temperatures (P<0.05) until day 14; at day 15 a decrease was observed as a consequence of ripening and senescence of fruit. The temperature and time showed an effect on the concentration of protein (P<0.05) on fruit, showing a decreased concentration thereof, to elapse the ageing time at different temperatures.
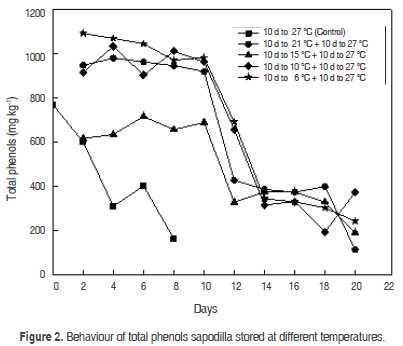
Total phenols
The maturity stage is an important factor that influences the compositional quality of fruit and vegetables, and during fruit ripening, several biochemical, physiological and structural modifications occur; these changes determine the fruit quality attributes. Harvesting at the proper maturity stage is essential for optimum quality and often for the maintenance of this quality after harvest and storage. In fact, storage can influence the quality indices and nutritional content of fresh fruit (Ayala-Zavala et al., 2004).
Phenolic compounds are responsible for colour, astringency and flavour (taste and aroma) of fruits and vegetables. Total phenols in control fruits showed a decrease in physiological maturity from 768.1 ± 10.2 mg kg-1 to 308.9 ± 7.9 mg kg-1, and senescence to 161.7 ± 2.4 mg kg-1 (Figure 2). These results are similar to those reported for mamey sapote (Pouteria sapota Jacq.) where the total phenol content was 1653 mg kg-1, in physiological maturity and 664 mg kg-1 in consumption maturity, and then decreased to 66 mg kg-1 in senescent fruit (Tejacal et al., 2005); similar results are associated with a decrease in the astringency of mango (Mangifera indica L.) (Seymour et al., 1993).
The amount of total phenols decreases in the ripening process of Manilkara sapota, which is due to the oxidation of monohydric or dihydric phenols that occurs, and with this, the reduction of active phenols (Reyes et al., 2005). The decrease in the concentration of total phenolic (3619.8 mg kg-1 fresh weight) during post-harvest storage could be attributed to phenolic degradation as a result of enzymatic activities occurring in the fruit, as previously reported in other fruit such as rowanberries (Baltacioğlu et al., 2011).
Temperature management is the most important tool to extend shelf-life and maintain quality of fresh fruit and vegetables (Lee and Kader, 2000). The temperature upon storage is the most important factor in the post-harvest life of fresh produce because of its dramatic effect on rates of biological reactions (Li and Kader, 1989). The interaction between harvest temperature and storage time has an influence on the phenol content of Sapodilla (A. sapota L.) fruit. The behaviour of total phenols concentration during storage are shown in figure 2; the values of concentrations at 6, 10, 15 and 21 °C were 981.4 ± 7.9 mg kg-1, 961.3 ± 7.8 mg kg-1, 687.0 ± 7.3 mg kg-1 and 918.9 ± 7.7 mg kg-1, respectively. A significant drop was observed in these fruits at the second storage at 27 °C. A significant rise was observed in the fruits stored at 10 and 21 °C, but at the end there was a decrease in total phenols content. The fruits stored at 15 °C presented behaviour similar to control fruits after six days of storage, showing a decrease in phenols concentration.
Cold storage conditions significantly increased total phenolics, which can be attributed to changes occurring in phenol metabolism during storage. The analysis of variance detected significant differences in the concentration of total phenols in fruits stored at different temperatures, compared to control fruits (P<0.05). Statistically significant differences were in fruits taken at room temperature (P>0.05). The relationship between temperature and days of refrigeration presented an influence on total phenolics during the maturation process of sapodilla fruit (P<0.05).
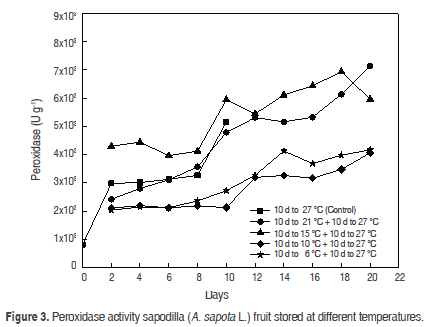
Enzymatic activity
Peroxidase (POD). The POD remained increased until maturity (3126.5 ± 0.014 U g-1) and at the end was 5151.3 ± 0.023 U g-1 in the case of control fruits; a similar behaviour was observed in apple (Malus domestica) (Ingham et al., 1998). In all treatments, peroxidase activity decreased during storage and could not be reactivated after the fruit was transferred to 27 °C, as shown in figure 3.
Previous studies (Ortiz et al., 2007) have shown that the peak activity of POD is reached at temperatures around 25 °C. The same study shows that the POD activity is considerably reduced when it is subjected to low temperatures (20 °C below the optimum temperature), which infers that the POD is an enzyme highly susceptible to temperature changes. This explains the behaviour of the fruit stored at 6 °C and 10 °C, but does not really explain the behaviour of the fruit stored at 21 °C. The variance analysis detected statistically significant differences in the activity of POD in the cooler days for fruits compared with controls (P<0.05).
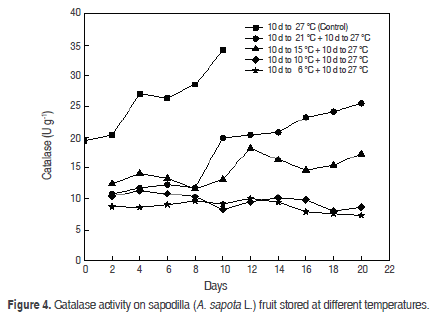
Catalase (CAT). The increase of CAT from physiological maturity (19.4 ± 0.08 U g-1) and ripening (26.3 ± 0.0003 U g-1) to senescence (Figure 4) in control fruits. A continuous decrease was observed in relation to the temperature of storage, and at the lowest temperatures the catalase activity declines, showing oscillations that trends to decreased activity. After the transfer to 27 °C these oscillations dwindled; this happened after 2 d at 27 °C.
The behaviour of control fruits is different from reports previously investigated. Compared to the activity of POD and PPO, the CAT activity is higher in the same period as the other enzymes, and it is known that between physiological maturity and sensory maturity must be a high oxidative metabolism with high consumption of O2, favouring the appearance of O2- due to superoxide dismutase (SOD) to H2O2 when catalase plays an important role in its degradation. CAT is high in a temperature range between 37 °C to 40 °C with a maximum at 37 °C (Duarte-Baquero et al., 2005). This would support the respective values to 17 °C of CAT is not as diminished, according to studies carried out previously, which conclude that between CAT, POD and PPO, the CAT is the least susceptible to temperature changes (Duarte-Baquero et al., 2005).
The CAT begins to affect as the fruit begins its maturation (P<0.05), and finally, when the two factors (temperature and time) are compared show that catalase activity is power significantly (P<0.05).
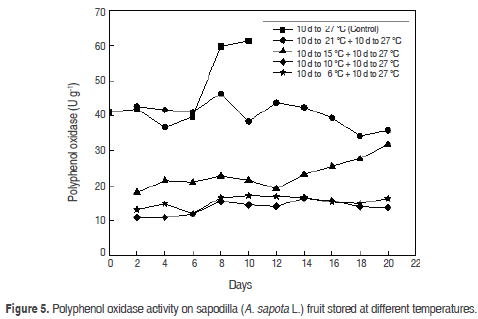
Polyphenol oxidase (PPO). PO showed (Figure 5) an increase in values of 41.09 ± 0.11 U g-1 in physiological maturity, 39.7 ± 0.45 U g-1 in ripening and 61.4 ± 0.7 U g-1 in senescence. Sapodilla fruits presented a gradual darkening during maturation because PPO are responsible for the enzymatic browning reaction, which is triggered during the handling, storage and processing of fruits and vegetables (Steffens et al., 1994). This enzyme catalyses the oxidation of polyphenol substances naturally present in raw materials, producing substances responsible for changing in the colour, sensory and nutritional characteristics (Rosenthal et al., 2002).
Several reports suggest that the enzyme is localised exclusively in plastids and is released to the cytosol after senescence damage or deterioration of the organ (Steffens et al., 1994).
Fruits stored at 6 °C and 10 °C presented an inactivity of PPO until day 10 without being could reactivated after the transfer at room temperature of 27 °C. At 15 oC PPO activity showed a significant increase on day 10 and a maximum at 20 days stored at this temperature. At the end of storage, the activity of the PPO of fruits stored at 21 °C was increased in the transfer at room temperature of 27 °C with a maximum value of 46.3 ± 0.12 U g-1, This is seen a continuous decline in the curve PPO activity (Figure 5).
PPO increases during the ripening process; similar behaviour has been reported with the Zapote mamey (Selles, 2007). The temperature decreases in PPO activity were statistically different (P<0.05) at 6, 10 and 15 °C with respect to 21 °C and 27 °C. When comparing the time and temperature, there is a marked influence of the temperature over time on the PPO activity in the time (P<0.05), so that the two factors together reduce the observed PPO activity.
CONCLUSIONS
The ripening process of sapodilla (A. sapota L.) at room temperature (27 °C) was characterized by an increase in the concentration of phenols and the activity of catalase along with an increased concentration of soluble proteins and the activities of the polyphenol oxidase and peroxidase. The ripening period and senescence of sapodilla fruits was 10 days at 27 °C, while for the fruit stored in cold temperature conditions (6, 10, 15 and 21 °C) it was 20 days, showing that the cold retards the maturation processes like phenols and proteins concentration, and catalytic activity of enzymes such as CAT, PPO and POD.
ACKNOWLEDGEMENTS
This work is part of the research project of the University of Cartagena. The authors gratefully acknowledge its financial support.
REFERENCES
Ayala-Zavala JF, Wang SY and Gonzalez-Aguilar GA. 2004. Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit. LWT - Food Science and Technology 37(7): 687-695. doi: 10.1016/j.lwt.2004.03.002 [ Links ]
Baltacioğlu C, Velioğlu S and Karacabey E. 2011. Changes in total phenolic and flavonoid contents of rowanberry fruit during postharvest storage. Journal of Food Quality 34(4): 278-283. doi: 10.1111/j.1745-4557.2011.00389.x [ Links ]
Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72(1-2): 248-254. [ Links ]
Chachin K, Hamauzu Y, Kurooka H and Iwata T. 1990. Physiological changes of loquat fruit after harvest. In: Proceedings of XXIII International Horticultural Congress. International Society for Horticultural Science, Firenze, Italy. 1.350 p. [ Links ]
Duarte-Baquero LE, Castro JA and Narvaez CE. 2005. Catalasa, peroxidasa y polifenoloxidasa en pitaya amarilla (Acanthocereus pitajaya): maduración y senescencia. Acta Biologica Colombiana 10(2): 49-60. doi: 319028577001 [ Links ]
Dueñas-Gómez YM, Narváez CE and Restrepo E. 2008. Inhibición de lesiones por frío de pitaya amarilla (Acanthocereus pitajaya) a través del choque térmico: catalasa, peroxidasa y polifenoloxidasa. Acta Biologica Colombiana 13(1): 95-106. [ Links ]
Ganjyal GM, Hanna MA and Devadattam DSK. 2003. Processing of sapota (Sapodilla): Drying. Journal of Food Science 68(2): 517-520. doi: 10.1111/j.1365-2621.2003.tb05704.x [ Links ]
Guia Ambiental Hortifruticola de Colombia. 2009. Ministerio de Ambiente y Vivienda y Desarrollo Territorial. Asociación Hortifrutícola de Colombia. 92 p. [ Links ]
Ingham LM, Parker ML and Waldron KW. 1998. Peroxidase: Changes in soluble and bound forms during maturation and ripening of apples. Physiologia Plantarum 102(1): 93-100. doi: 10.1034/j.1399-3054.1998.1020113 [ Links ]
Kaur C and Kapoor HC. 2008. Antioxidants in fruits and vegetables -the millennium's health. International Journal of Food Science and Technology 36(7): 703-725. doi: 10.1111/j.1365-26 21.2001.00513.x [ Links ]
Kulkarini AP, Policegouda RS and Aradhya SM. 2007. Chemical composition and antioxidant actvity of sapota (Achras sapota Linn.) fruit. Journal of Food Biochemistry 31: 399-414. doi: 10.1111/j.1745-4514.2007.00122.x [ Links ]
Lara C, Neiro LS and Oviedo LE. 2007. Evaluación fisicoquímica y bromatológica de la guayaba agria (Psidium araca) en dos estados de maduración. RevistaTemas Agrarios 12(1): 13-21. [ Links ]
Lee SK and Kader AA. 2000. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biology and Technology 20(3): 207-220. doi: 10.1016/S0925-5214 (00)00133-2 [ Links ]
Li C and Kader AA. 1989. Residual effects of controlled atmopsheres on post-harvest physiology and quality of strawberries. Journal of the American Society for Horticultural Science 114(4): 629-634. [ Links ]
Mickerbart MV. 1996. Sapodilla: A potential crop for subtropical climates. pp. 439-446. In: Janick J. (ed.). Progress in new crops. ASHS Press, Alexandria, VA. [ Links ]
Ortiz-Ortiz H, Benavides-Mendoza A, Mendoza-Villarreal R, Ramírez-Rodríguez H and De Alba-Romerus K. 2007. Enzymatic activity in tomato fruits as a response to chemical elicitors. Journal of the Mexican Chemical Society 51(3): 141-144. [ Links ]
Pérez-Tello GO, Diaz-Perez JC, Arispuro-Vargas I, Briceño TO and Martinez-Tellez MA. 1999. Actividad de polifenoloxidasa y peroxidasa en frutos de mamey sapote (Pouteria sapota). Revista Iberoamericana de Tecnologia Poscosecha 1: 120-125. doi: 10.51 54/r.rchsh.2015.01.003 [ Links ]
Razeto B. 1998. El nispero. pp. 58-72. In: Sudzuki F. and Gomez A (eds.). Frutales no tradicionales. Universidad de Chile, Santiago, Chile. [ Links ]
Reyes BB, Arevalo ML, Saucedo C and Martínez MT. 2005. Proceso de maduración de frutos de chicozapote (Manilkara sapota L.) P. Royen tipo fino. Revista Chapingo serie Horticultura 11(2): 387-391. [ Links ]
Rosenthal A, Ledward D, Defaye A, Gilmour S and Trinca L. 2002. Effect of pressure, temperature, time and storage on peroxidase and polyphenoloxidase from pineapple. Trends in High Pressure Bioscience and Biotechnology, Proceeding First International Conference on High Pressure Bioscience and Biotechnology. Elsevier BV, pp. 525-532. [ Links ]
Sachdeva S, Sachdevand TR and Sachdeva R. 2013. Increasing fruit and vegetable consumption: Challenges and opportunities. Indian Journal of Community Medicine: Official publication of Indian Association of Preventive and Social Medicine 38(4): 192-197. doi: 10.4103/0970-0218.120146 [ Links ]
Sala JM. 1998. Involvement of oxidative stress in chilling injury in cold-stored mandarin fruits. Postharvest Biology and Technology 13(3): 255-261. doi: 10.1016/S0925-5214(98)00011-8 [ Links ]
Salveit M and Morris L. 1990. Overview on chilling injury of horticultural crops. pp. 3-14. In: Wang CY (ed.). Chilling Injury of Horticultural Crops. CRC Press, Boca Raton, FL. [ Links ]
Sapan CV, Lundblad RL and Price NC. 1999. Colorimetric protein assay techniques. Biotechnology and Applied Biochemistry 2(2): 99-108. doi: 10.1111/j.1470-8744.1999.tb00538.x [ Links ]
Selles S. 2007. Pardeamiento enzimático del fruto del níspero (Eriobotrya japonica cv. Algerie): Enzimología y fisiología de las polifenol oxidasas. Tesis Doctoral en Química. Facultad de Ciencias. Universidad de Alicante. Alicante, España. 149 p. [ Links ]
Seymour GB, Taylor JE and Tucker GA. 1993. Biochemistry of fruit ripening. Springer, Dordrecht, Netherlands. 442 p. [ Links ]
Siddappa GS and Bhatia BS. 1954. The identification of sugars in fruits by paper chromatography. Indian Journal of Horticulture 11(1): 19-23. [ Links ]
Steffens JC, Hareland E and Hunt MD. 1994. Polyphenol oxidase. pp. 275-312. In: Ellis BE, Kuroki GW and Stafford HA (eds.). Genetic engineering of plant secondary metabolism. Plenum Press, New York, USA. [ Links ]
Tejacal IA, Colinas MT, Martínez MT and Soto RM. 2005. Daños por frío en zapote mamey (Pouteria sapota (Jacq.) H.E. Moore and Stearn). II. Cambios en fenoles totales y actividad enzimática. Revista Fitotecnia Mexicana 28(1): 25-32. [ Links ]
Thompson AK. 2003. Fruit and vegetable harvesting, handling and storage. Blackwell Publ., Oxford, UK. 460 p. [ Links ]
Tomas FA and Espin JC. 2001. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. Journal of the Science of Food and Agriculture 81(9): 853-876. doi: 10.1002/jsfa.885 [ Links ]
Zor T and Selinger Z. 1995. Linearization of the bradford protein assay increases its sensitivity: theoretical and experimental studies. Analytical Biochemistry 236: 302-308. doi: 10.1006/abio.1996.0171 [ Links ]