The common bean (P. vulgaris L.) is the most important edible legume on the planet. It is an important source of food for at least 300 million people who live mostly in developing countries. Common beans are especially important sources of nutrition for women and children, and the crop also generates income for millions of smallholders (Velásquez and Giraldo, 2005, cited by Torres et al., 2013). For this reason, common bean is in the eighth position among the pulse crops cultivated in the world (Torres et al., 2013). According to statistical data from the Food and Agriculture Organization of the United Nations (FAO), dry bean production in the world during 2012 reached 23.1 million tons in a cultivated area of 29.2 million hectares.
Common Bean production is affected by some diseases that are widespread in production areas worldwide (Ddamulira et al., 2014). Angular Leaf Spot (ALS), caused by the hemibiotrophic fungus Pseudocercospora griseola (Sacc.) Crous and Braun, is one of the most devastating diseases, causing yield losses of up to 80% (Singh and Schwartz, 2010). Genetic resistance is an effective and environmentally friendly strategy for disease management. However, the diversity and high virulence of P. griseola and the emergence of new races of this pathogen are a challenge for the development of cultivars with a long-lasting resistance (Sartorato and Alzate-Marin, 2004; Abadío et al., 2012).
According to Hernández-López (2013), there were two centers of domestication of common bean: one primary (Mesoamerica) and one secondary (Andean). The isolates of P. griseola have also been divided into Andean and Mesoamerican groups that correspond to the two groups of the common bean origin (Pastor-Corrales and Jara, 1995; -Pastor Corrales et al., 1998). The co-evolution of P. griseola and common bean offers the possibility of combining resistance genes from both gene pools (Andean and Mesoamerican) to achieve a long-lasting resistance.
P. griseola virulence is assessed using a system proposed by the International Center for Tropical Agriculture (CIAT) that is based on the reaction of the ALS isolated pathogen with a standard differential set of six Mesoamerican and six Andean common bean cultivars of diverse origin (Pastor-Corrales and Jara, 1995). Screening the standard differential set of genotypes with endemic isolates of the ALS pathogen provides information about the pathogenic variability of P. griseola. These findings are useful to determine the distribution and frequency of pathotypes (races) and to select the best sources of resistance for different geographic regions.
According to Souza et al. (2016), six ALS resistance genes have been identified: Phg-1, Phg-2, Phg-2 2, Phg-3, Phg-4, and Phg-5. The Phg-1, Phg-4, and Phg-5 genes are from an Andean origin, and the Phg-2 and Phg-3 genes are from a Mesoamerican origin. Continuing identification and evaluation of additional genotypes with broad resistance should increase the genetic diversity of the sources of resistance to this disease. Therefore, the objective of this study was to identify the most resistant genotypes from a group of 181 bean genotypes of diverse geographic origins when inoculated with two highly virulent races of P. griseola.
MATERIALS AND METHODS
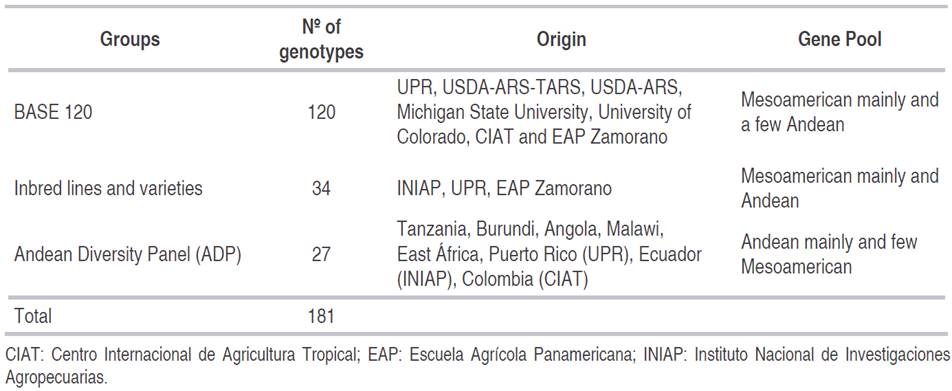
The experiment was carried out in two greenhouses of the University of Puerto Rico (UPR). Two isolates of P. griseola collected in Puerto Rico, coded as ALS 9029JD2 (collected in the Juana Díaz locality) and ALS 1146C (collected in the Isabela locality), were used for this study. Based on their reaction to Angular Leaf Spot differentials (Pastor-Corrales and Jara, 1995), these isolates were characterized as races 61:11 and 63:51, respectively (Estevez de Jensen et al., 2015). Three groups of common bean genotypes were inoculated with these P. griseola isolates. The first group was the BASE 120 (Bean Abiotic Stress Evaluation) trial consisting of 118 lines of common bean and two of tepary bean (Phaseolus acutifolius L.) lines (Table 1). This group is composed mainly of Mesoamerican genotypes and a few of Andean origin from breeding programs in Puerto Rico, Honduras, Colombia, and the United States. The second group of common bean genotypes included 34 lines and varieties of Andean and Mesoamerican origin from Honduras, Ecuador, and Puerto Rico that were previously selected for resistance to P. griseola (Table 1). The third group of common bean lines included 27 lines and varieties from the Andean Diversity Panel (ADP) (Table 1). The ADP consists of 396 bean accessions and includes important improved lines and local varieties that originated mainly from Africa, the Caribbean, and North and South America (Cichy et al., 2015). The 27 genotypes of the ADP used in this study were selected because they presented resistance to ALS in field evaluations in Cedara, South Africa (Cichy et al., 2015).
The methodology of sowing, inoculation, and evaluation was the same for the three groups of bean genotypes. Each genotype was planted in a 9 cm diameter plastic bag containing commercial substrate (Sunshine mix #1). Five seeds of each genotype were planted and thinned to three seedlings in a pot after germination. Each pot was considered as an experimental unit. The inoculum consisted of the two P. griseola races increased in V8 culture medium (200 ml V-8 juice, 3 g CaCO3, 18 g Bacto agar and 800 mL sterile distilled water) following the methodology of Castellanos et al. (2011). The isolates were grown in a V8 agar media and incubated at about 24 °C for 15 d. The inoculations were carried out 15 d after sowing using the first trifoliate leaf by spraying the inoculum on the underside of the leaf. The inoculum concentration was 1×104 conidia mL-1 adjusted using a hemocytometer (1/400 square mm, Hausser Scientific). The inoculated plants were exposed to a relative humidity of 90-100%, using a humidifier for 72 h after the inoculation. Afterward, they were placed under benches in the greenhouse and submitted to constant humidity (80-90%) every night until evaluation.
The evaluation of ALS severity was scored after 15 d of inoculation according to the 1-9 CIAT scale, where 1= plants have no symptoms; 3= plants with 2% of the leaf surface with lesions; 5= plants with 5% of the leaf area with lesions and sporulation; 7= plants with up to 10% of the leaf surface with lesions and sporulation associated with chlorosis and necrotic tissues; 9= 25% of the leaf area with lesions, frequently associated with early leaf fall and plant death (Schoonhoven and Pastor-Corrales, 1987).
The severity observed in the first three groups of genotypes were classified as follows: plants with mean values from 1 to 3 were considered resistant; plants with values from >3 to ≤6 were considered to have intermediate resistance; plants with values from >6 to 9 were considered susceptible (Schoonhoven and Pastor-Corrales, 1987). At the time of the evaluation, the plants that presented leaves with lesions without the development of sinemas were exposed to humidity >80% for 24 h with a humidifier, and leaves from plants that did not develop sinemas after that treatment were considered resistant (Sartorato, 2002). Temperature and humidity were recorded using an iButton® sensor (Maxim Integrated TM, USA) every 15 min.
The data analysis was performed using the statistical software Infostat (version 2008). The experimental design was a randomized complete block (RCBD) with two replications. Analyses of Variance (ANOVA) was completed using the General Linear Model with a significant level of P<0.05. Means were compared using Fisher’s least significant difference with a significant level of P<0.05.
RESULTS AND DISCUSSION
The temperature ranged from 20 to 36 °C with an average of 26±3 °C, and the relative humidity ranged (during three days of continuous humidification after inoculation) from 60-79% during the day, and 80-90% during the night. Allorent and Savary (2005) presented different limits in temperature ranges for each stage of the disease development. Spore germination, disease development, and sporulation can occur between 12 and 30 °C. In this research, the inoculations were conducted in the afternoon (after 5 PM) to favor the initiation of infection process because temperatures were <30 °C during this time. However, the temperature increased up to 36 °C during short periods, mainly at midday, which exceeded by 3 °C the maximum temperature indicated for the development of this disease. During the evaluation, susceptible checks showed symptoms with abundant sporulation; thus temperatures higher than 33 °C during short periods did not affect the development of the disease. The provision of high humidity (using humidifiers) during the evaluations was critical for the development of the disease.
There were significant differences between lines of reaction to both isolates in all inoculated trials (Table 2) because of the lines in the three groups of genotypes presented different levels of disease severity for each isolate. There were a small number of lines (<20) with resistance to both isolates.
Table 2 Summary of ANOVA for the severity of two isolates of angular leaf spot in three bean lines groups.
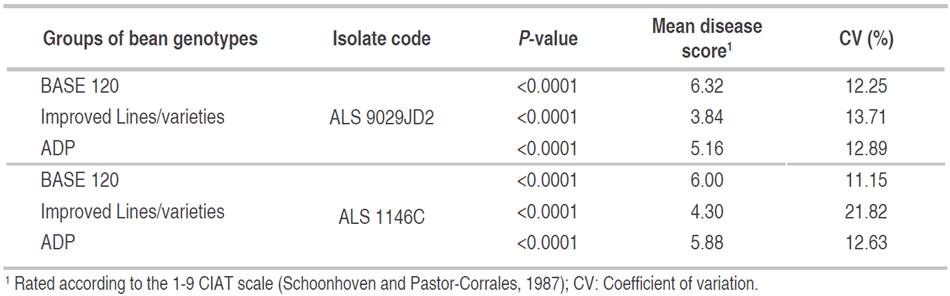
In the first group of genotypes (BASE 120) evaluated with the isolate ALS 9029JD2 (race 61:11) 60 lines were susceptible (>6.0), four lines had resistant scores (≤3.0), and 42 lines showed intermediate resistance (>3 and ≤6). With the isolate ALS 1146C (race 63:51), 55 lines were rated as susceptible, four lines were resistant, and 48 lines had intermediate resistance. Only the lines G21212 and SER125 were resistant to both isolates.
In the second group of genotypes (the bean lines and varieties developed by various breeding programs for resistance to angular leaf spot), 15 lines were found to be resistant to the ALS 9029JD2 isolate, 11 lines had intermediate resistance, and eight lines were susceptible. With the isolate ALS 1146C, 15 lines were found to be resistant, 13 lines had intermediate resistance, and six lines were found to be susceptible. 12 lines with resistance to both angular leaf spot isolates were identified: Ouro Negro, INIAP 484 Centenario, INIAP 483 Intag, AND 277, PR 0637-6, PR 1530-57, ALS 0546-78, ALS 0532-6, ALS 0531-41, ALS 0531-97, ALS 0546-60, and ALS NIL 604-29.
In the evaluation of the lines from the Andean Diversity Panel (ADP) inoculated with the ALS 9029JD2 isolate, 10 lines were resistant, five lines showed intermediate resistance and 11 lines were identified as susceptible. Inoculations with the ALS 1146C isolate identified four resistant lines, seven lines with intermediate resistance and 12 susceptible lines. Only two lines, CAL 143 and AFR 612, were resistant to the two angular leaf spot isolates.
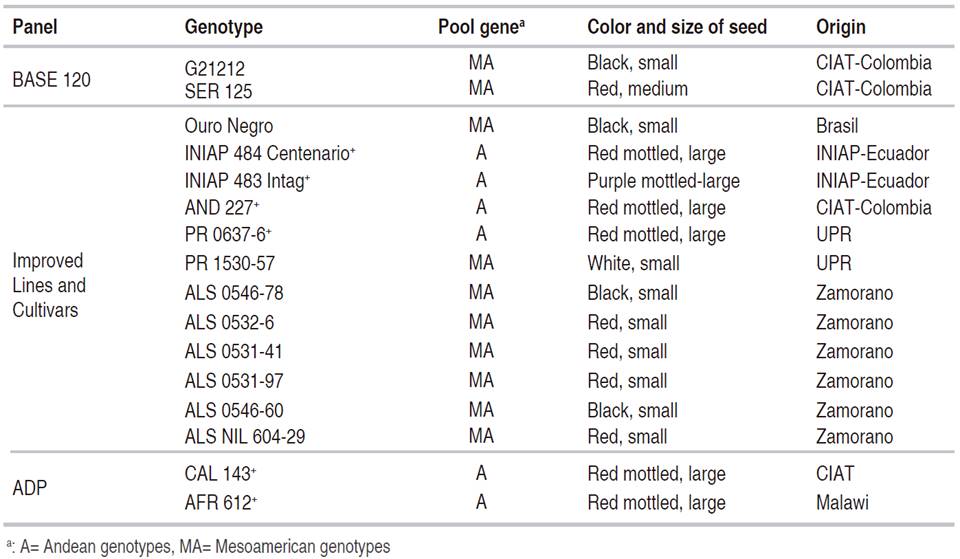
In summary, 16 bean genotypes, from a total of 181 genotypes evaluated, had a mean score ≤3.0; which classified them to be resistant to both isolates of P. griseola (Table 3).
Table 3 Common bean genotypes with resistance to Angular Leaf Spot isolates ALS 9029JD2 and ALS 1146C.
These results demonstrate the genetic vulnerability of most of the bean lines to this disease, which has been mentioned previously by several authors such as Singh and Schwartz (2010), Abadio (2012), E Silva (2008), and Mahuku (2003) among others.
It is important to note that from the 16 bean genotypes identified as resistant (originated from breeding programs in Honduras (EPZ), Puerto Rico (UPRM), Colombia (CIAT), and Ecuador (INIAP)), some of them have resistance to other diseases of economic importance. For example, CAL 143, INIAP 484 Centenario, INIAP 483 Intag, and Ouro Negro showed resistance to several races of Colletotrichum lindemuthianum (Rodríguez-Ortega et al., 2018; Zuiderveen et al., 2016).
Because of the high variability of P. griseola, the improvement for effective and lasting resistance to angular leaf spot requires the introduction of resistance genes of Andean and Mesoamerican origin (Mahuku et al., 2003). Therefore, the different combinations of the resistance genes present in the 16 genotypes identified as resistant in this study should provide wider and long-lasting resistance. Although the groups of lines were evaluated with two highly virulent isolates of P. griseola (races 61:11 and 63:51), the resistant lines should be screened with other endemic isolates of the ALS pathogen. Information about the pathogenic variability of P. griseola is useful to determine the distribution and frequency of pathotypes (races) and to select the best sources of resistance for different geographic regions.
Genetic studies have reported two types of inheritance (qualitative and quantitative) of ALS resistance (Keller et al., 2015; Oblessuc et al., 2012). It is important to investigate the inheritance of the resistance present in these genotypes. This knowledge will help plant breeders optimize the selection of resistant plants. According to Pereira et al. (2015), AND 277, Ouro Negro, and CAL 143 are recognized sources of resistance. Although these lines are not resistant to all isolates of P. griseola, they were resistant to the two races of P. griseola (61:11 and 63:51) from Puerto Rico. Souza et al. (2016) reported that these lines have the Phg 1, Phg 3 and Phg 5 resistance genes respectively.
It is important to continue the evaluation and identification of new sources of resistance to expanding the genetic base of resistance to this disease in order to counter the pathogenic variability identified in P. griseola.
CAL 143 is considered a very important source of resistance to angular leaf spot that is widely used by plant breeding programs in several countries. For example, CAL 143 is a parent of the improved variety INIAP 484 Centenario in Ecuador (Murillo et al., 2012). This variety has shown resistance to thirteen angular leaf spot races identified in that country and its resistance has remained stable in the field until now (unpublished data).
Similar to CAL 143, other bean lines such as BAT332, G5686, MAR 2, MAR3, Mexico54, AND277, Cornell 49-242, and Ouro Negro, among others, are also considered important resistance sources (Souza et al., 2006; Gonçalves-Vidigal et al., 2013). Therefore, it is important to continue with the validation and identification of Quantitative Trait Locus (QTL) or resistance genes in these genotypes, and the molecular markers linked to them, which will facilitate their use by genetic improvement programs through marker-assisted selection.
CONCLUSIONS
Only 16 of the 183 genotypes evaluated were resistant to the two isolates P. griseola (races 61:11 and 63:51). The 16 resistant lines were: G21212, SER 125, AFR 612, Ouro Negro, CAL 143, AND 227, PR 0637-6, PR 1530 -57, ALS 0546-78, ALS 0532-6, ALS 0531-41, ALS 0531-97, ALS 0546-60, ALS NIL 604-29, INIAP 484 Centenario, INIAP 483 Intag. The last twelve lines were developed by breeding programs from CIAT (Colombia), the UPR (Puerto Rico), Zamorano (Honduras), and INIAP (Ecuador). The sources of resistance include genotypes of Andean and Mesoamerican origin. This information can help plant breeding programs to pyramid genes from both gene pools and to generate varieties with long-lasting resistance to this disease.