The identification of phytochemical based strategies for the control of plant pathogens is important since it can be used in sustainable production systems where there are not many possibilities to manage plant-parasitic nematodes. It can also be essential for the development of new nematicides in traditional agriculture (Chitwood, 2002). Nowadays, further research on environmentally friendly biofumigants is suggested (Devi, 2018).
Meloidogyne chitwoodi Golden, O'Bannon, Santo, & Finley is an important pathogen of potato and other crops in the western part of Europe and is also a major pest of potato in the Northwestern states of the United States (Castagnone-Sereno et al., 1999). Pratylenchus penetrans is an obligate plant parasite of a wide range of hosts, mainly in temperate climates. It is one of the principal nematodes infesting ornamental plants and causes serious losses in different crops (Peng and Moens, 2002).
Plant compounds may act as a repellent, attractant, hatching stimulants or inhibitors and nematotoxicants. They can be used as fumigants or introduced in crop rotation programs for nematode control (Chitwood, 2002).
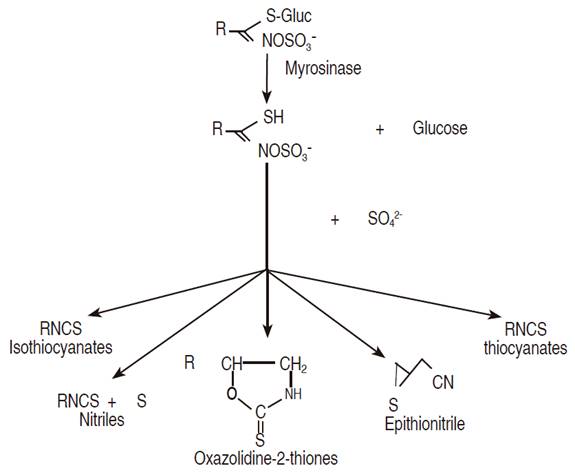
Glucosinolates are a group of allelochemicals that occur in all plants of the order Brassicales or Capparales (Cronquist, 1981), being the Brassicaceae family the most numerous and important group (Fahley et al., 2001). Glucosinolate (GLSs) have sulfur, and that explains its strong flavor (Avato et al., 2013). More than 130 glucosinolates have been identified (Fahley et al., 2001; Kirkegaard et al., 1999) and may be divided into three subclasses comprising aliphatic, phenyl, and indol-3-ylmethyl glucosinolates (Buskov et al., 2002). Isothiocyanates (ITC) and other plant compounds such as nitriles and thiocyanates (Buskov et al., 2002) are released from Brassicaceae when glucosinolates are hydrolyzed by the action of the enzyme myrosinase (Figure 1) (Kirkegaard et al., 1999). These hydrolysis products have shown various bioactive effects against some soilborne diseases and nematodes (Rosa et al., 1997).
The glucosinolate content average is higher on field-grown B. juncea, B. napus, B. campestris, and Eruca sativa, compared to the greenhouse and high tunnel cultivation (Antonious et al., 2009).
Brassica species have shown a significant reduction in nematode numbers of P. neglectus (Potter et al., 1998) and P. penetrans (McFadden et al., 1992) when used as green manures. The same has been observed for M. chitwoodi (Mojtahedi et al., 1993). Other nematode species have shown mortality up to 100% when low doses of glucosinolates from Brassicaceaes have been applied, in vitro; such as in the case of Globodera rostochiensis (Serra et al., 2002), G. pallida (Lord et al., 2011) and M. incognita (Oliveira et al., 2010). There is also a biocidal property with the use of GLSs on Xiphinema index and Heterodera carotae (Avato et al., 2013). Width control spectrum has been found by using these species (Björkman et al., 2011). Other plant compounds as the ones released from Lupinus sp. and several kinds of grass have proved to have a nematicidal effect; mulching with Pennisetum purpureum has been used for the control of M. javanica (Matsumoto et al., 2002) and the application of Sudan's grass extracts has reduced M. hapla juveniles that penetrate lettuce roots (Wildmer and Abawi, 2007). Anastasiadis and Karanastasi (2011) also presented the effectivity of brassica and ryegrass soil amendments on M. incognita and javanica. The Fabaceae Medicago sativa has been found a good control against G. rostochiensis (D'Addabbo et al., 2011).
Considering the potential of these species as possible biocides, the purpose of this trial was to determine the nematicidal effects of plant extracts on plant-parasitic nematodes.
MATERIALS AND METHODS
Extracts
Brassicaceous accessions from a local field were tested: B. fruticulosa subsp. mauritanica, B. fruticulosa, B. tournefortii, B. tournefortii, Sinapis arvensis subsp. arvensis, B. carinata, Raphanus sativus, R. sativus, B. juncea, B. juncea (oriental), B. tournefortii, B. oxyrrhina, B. napus subsp. oleifera, Sinapis alba, Crambe abbyssinica and Crambe hispanica (identified as extract: 1, 2, 3, 4, 5, 7, 8, 9, 10, 11, 13, 15, 17, 18, 20 and 21, respectively, showed in Figure 2). The extracts of Brassicaceae and one extract of Lupinus sp. were obtained with a blend of 3 g of fresh shoot material in 6 mL of phosphate buffer (25 mM, pH 7, with 15 mg L-1 streptomycin), later sieved, centrifuged and used directly to make dilutions. Seven grasses, belonging to Lolium spp., were collected from dried meadows, and these samples were roasted for 3 hours from 160 to 260 °C before extracting the active components. Grasses were identified by letters (A, B, C, D, E, F, and G), as shown in Table 2. Grass A was not heat-treated while G was the most exposed to high temperatures. Grass extracts were made by blending 10 g of dried grass for one hour in 40 mL of extraction buffer.
Dilutions
Extract dilutions were made to test the dose-response of nematode migration activity. The dilutions show doses that could be obtained by practical quantities of crop residue on field. 6 mL from each extract of the stock-solution was used. This volume was diluted to 1/3, where 6 mL was used to make the dilutions 1/4, 1/16, and 1/64. The final dilutions were prepared with the same phosphate buffer (pH 7) as the original extracts. These dilutions were used for the filter plate experiments.
Filter plate experiments
The filter plate experiment was used to test the dose-response of nematode migration activity. There were two plates placed one on top of each other. The upper plate was a 96-wells high filter plate with a small tube underneath. The lower plate was a standard 96-wells plate. The upper plates were filled with cigarette filters. It was pipetted 700 µL of the plant extract solution on these filters, then 200 µL of the nematode suspension (200 P. penetrans and 100 J2 M. chitwoodi nematodes). This proportion was selected because Meloidogyne migrates faster, so there would be more nematodes per well. The plates were covered and stored at 20 °C for 24 hours. After 24 hours, the upper plate was transferred to a new lower plate, and 200 µL of buffer was added. The whole set was place at 20 °C for 24 hours again, so the rest of the nematodes could migrate. The plates were counted again after 48 hours. Four replications per dilution were scored, and four wells with 900 µL of buffer per plate were used as a control. For each well, the number of nematodes was scored. Juveniles and adults P. penetrans were counted separately.
Nematode counting for each dilution was compared and statistically analyzed with STATISTIX for Windows. All data were log(x+1) transformed for an appropriate analysis. Tukey tests were used to compare treatments with significant differences (P<0.05).
RESULTS AND DISCUSSION
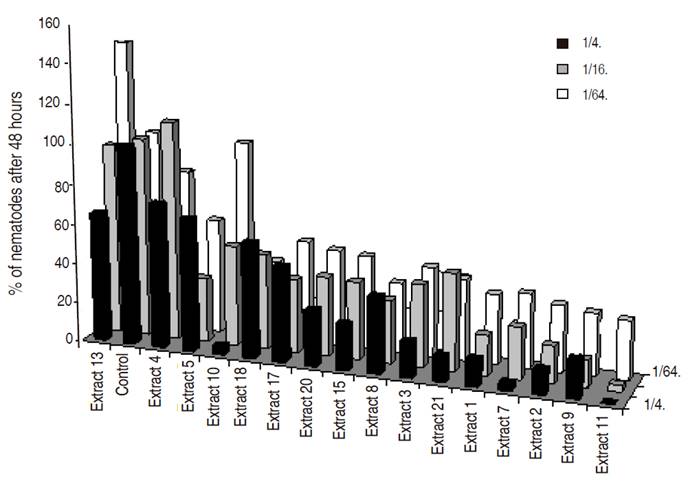
All the Brassicaseous and grass extracts showed nematicidal or repellent effect at a high concentration (1/4 dilution), whereas at lower concentrations (dilutions 1/16 and 1/64) differences were not always observed (Figures 2, 3 and 4). This nematicidal effect was a function of the dilution and time of exposure.
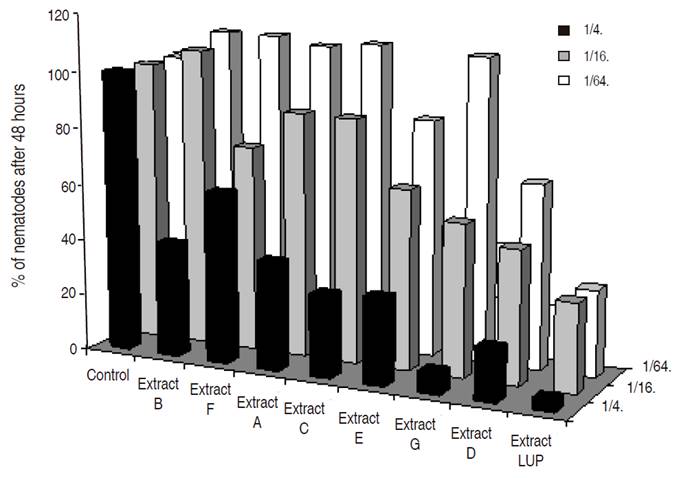
Figure 3 Percentage over the control of total P. penetrans found after 48 hours for the different grass extracts and Lupinus sp. at different concentrations.
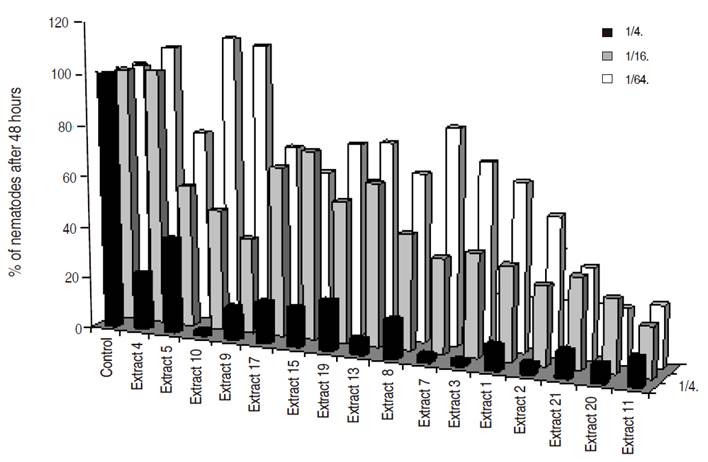
Figure 4 Percentage over the control of total M. chitwoodi found after 48 hours for different Brassicaceae extracts at different concentrations.
Glucosinolates relative slight structural differences could confer deeply different nematicidal effects, confirming that biological activity was a function of the concentration of the product and the chemical properties of the R chain (Serra et al., 2002; Zasada and Ferris, 2003).
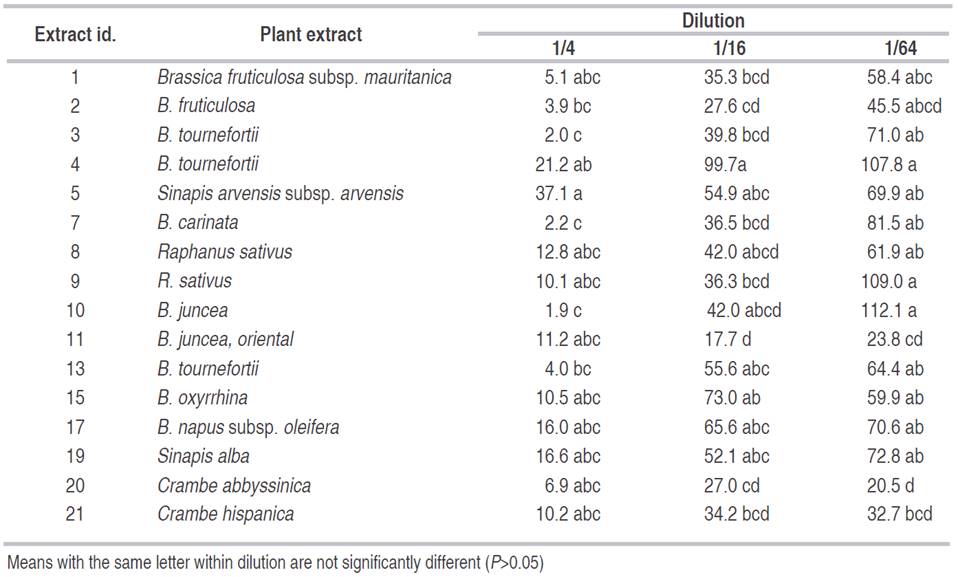
Best nematicidal effects at 1/4 dilution were observed with Extracts 11, grass G and Lupinus for P. penetrans (Tables 1 and 2), and with extracts 10, 3, and 7 for M. chitwoodi (Table 3). At 1/16 dilution the extracts with higher effect were 11, 9, and Lupinus sp. for P. penetrans and extract 11 for M. chitwoodi. At the lower concentration of 1/64 most effective extracts were 2, 11, 9, and Lupinus for P. penetrans, and 20 and 11 for M. chitwoodi.
Table 1 Mean of total nematodes present after 48 hours for different Brassicaceae extracts at different concentrations in P. penetrans corrected over the control.
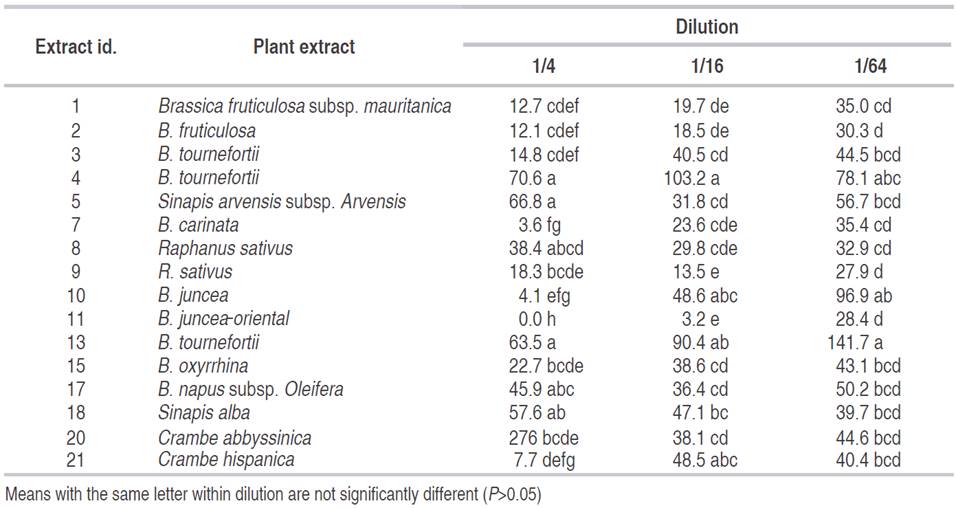
Table 2 Mean of total nematodes present after 48 hours for different plant grass extracts at different concentrations in P. penetrans corrected over the control.
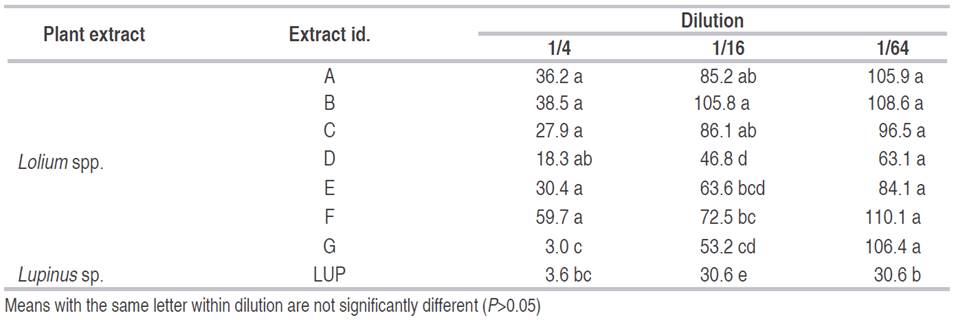
Table 3 Mean of total nematodes present after 48 hours for different Brassicaceae extracts at different concentrations in M. chitwoodi corrected over the control.
In cases where the metabolite had a better effect when used in a lower concentration (e.g., 1/64) could be assumed that its effect did not only depend on the concentration of the plant extract, but it was also related to the optimal dilution at which the compound gave positive results for nematode management.
For P. penetrans control the principal compounds involved are sinigrine and gluconapin according to preliminary studies performed by the Plant Research International in Wageningen. The first one was found in high concentrations in extracts 7 and 11 (Raphanus sativus and Brassica juncea oriental, respectively), while the latter was in excess in extracts 1 and 2 (Brassica fructiculosa both species) (data not shown). In the experience of Lazzeri et al. (1993) with a concentration of 0.05%, sinigrin and gluconapin showed nematicidal effect after 96 hours and 114 hours, respectively, for the control of Heterodera schachtii. Brassica juncea products have also shown a reduction in Globodera pallida (Lord et al., 2011), P. penetrans and M. incognita (Zasada et al., 2009). Ngala et al. (2014) also found nematicidal effects for the same nematode with B. juncea and Raphanus sativus deterred its multiplication.
For M. chitwoodi control showed a clear correlation between the type of glucosinolate so its control effectiveness could not be done easily because most of the extracts controlled the nematode effectively, at least for the lowest concentration (Table 3). However, it could be assumed that several isothiocyanates or the combination of them have detrimental effects on M. chitwoodi populations; therefore, its use could be highly appreciated in crops where this nematode causes economic losses. Further chemical studies of the most promissory plant species are necessary in order to determine specific compounds that have nematicidal or nematostatic effects.
CONCLUSIONS
Glucosinolates present in Brassica juncea and Lupinus sp. showed the most significant nematicidal effect with the lowest dilution (1/4). It seemed that the high concentration of these glucosinolates in the plant tissue is the direct responsible for the biocidal effect of these plants. Given the high effectiveness of most of the tested Brassica spp. for the control of M. chitwoodi and P. penetrans.