The genus Capsicum spp. (2n=2x=24) belongs to the Solanaceae family, concentrated mainly in tropical and subtropical regions of America (López-España et al., 2016). It comprises a set of more than thirty species. Capsicum annum, C. chinense, and C. frutescens stand out (Toquica et al., 2003) as the most cultivated because of their high content of vitamins A, C, and Calcium (Nuez Viñals et al., 1996). In Colombia, there is a growing demand for Capsicum species due to their good profitability, production, and export possibilities (Medina et al., 2006). The main producing departments of this cultivar are Valle del Cauca, Santander, Antioquia, Córdoba, and Bolívar - being the latter the largest producer with 1.8 million t in an area of 24,700 ha (Agronet, 2010).
The genetic improvement of this cultivar occurs mainly through conventional techniques, thus generating genotypes with valuable genes related to production and resistance to biotic and abiotic stresses (Regner, 1996). However, there are inherent limitations to sexual reproduction: incompatibility and imprecision. Besides, obtaining pure or homozygous lines is time-consuming since they require at least six cycles of self-fertilization (Cravero et al., 2011). In this context, the production of haploid and doubled haploid plants in vitro is a useful tool since pure lines can be obtained in one generation, thus reducing time and production costs (Maraschin et al., 2005). As an advantage, doubled haploid plants have a great application in genetic and cytogenetic studies (Dwivedi et al., 2015).
Haploid plants are those that have the number of gametophytic chromosomes of their progenitors (Das et al., 2018). Doubled haploid plants are produced through the duplicated chromosome of the haploids (Dwivedi et al., 2015), either spontaneously or artificially induced by antimitotic agents (Badu et al., 2017). After chromosomal duplication, the haploids become 100% homozygous and can be used directly as parental lines in the production of F1 lines (Bal and Abak, 2007).
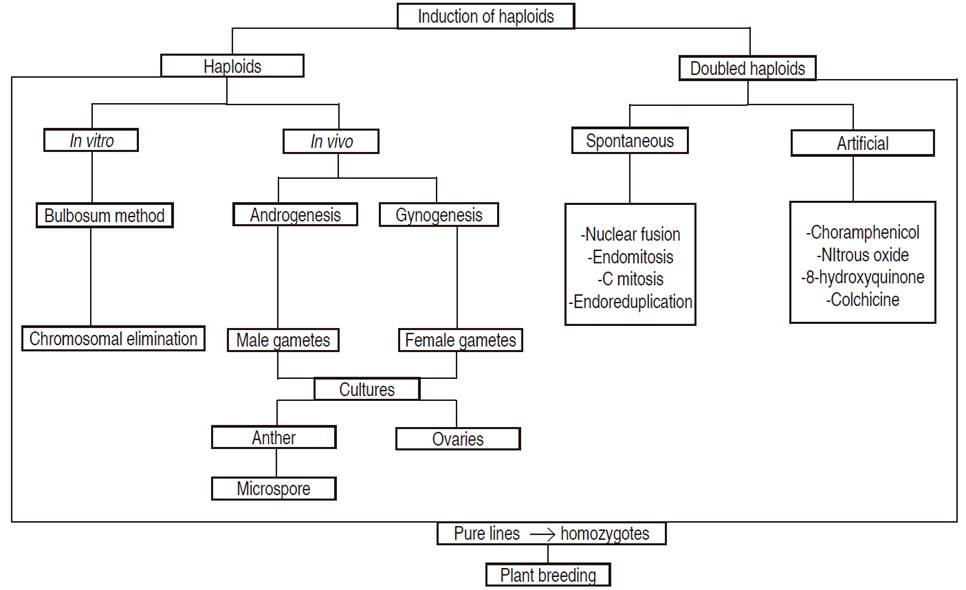
Given the importance of haploids and doubled haploids in the improvement of crops, different artificial strategies have been developed for their production, such as: chromosomal elimination (bulbosum method), hormonal treatment, heat shock methods, induction of gynogenesis (culture of ovaries), and androgenesis involving anthers and microspores, the latter being the most commonly used in pepper cultivars (Das et al., 2018).
Several studies have been focused on the conventional improvement of Capsicum spp. However, new research has emerged using in vitro techniques in the induction of whole plants from androgenesis to generate doubled haploids, which is used to support breeding programs. Therefore, the objective of the present review is to present some scientific achievements in the production of haploids and doubled haploids via androgenesis in pepper crops. The information is useful for those genetic improvement programs that seek to apply techniques that increase the efficiency in obtaining cultivars, shortening the time required to obtain them, and maximizing the use of resources.
GENERALITIES OF HAPLOIDS AND DOUBLED HAPLOIDS INBREEDING
Importance of haploids and doubled haploids
The constant agricultural evolution and the high demand from the growing population for food with nutritional value force improvement programs to accelerate the production of improved varieties with desirable agronomic properties. The main purpose is to seek alternatives that lead to the integration of new methodologies and technologies, such as the production of doubled haploid plants (Barro et al., 2001; Achar, 2002).
The doubled haploid plants make up a very useful tool for the breeder in the generation of new and improved varieties (Tuvesson et al., 2000). Currently, one of the main drawbacks of conventional breeding programs for autogamous species is the long period needed to produce an improved line. Due to several generations of self-pollination are required to achieve homozygous genotypes. Once the new genotypes are obtained, the agronomic and industrial traits of interest are consecutively evaluated until they can finally be delivered to farmers (Jacquard et al., 2009).
Biotechnological techniques involving in vitro culture of anthers, isolated microspores, ovaries, etc., can be used with the same efficacy as conventional methods of self-fertilization or backcrossing (Guzy-Wrobelska and Szarejko, 2003). With the in vitro techniques, it is possible to develop resistant cultivars to various plant diseases; saving time, labor, and space in the experimental field (Wędzony et al., 2009). The evaluation of these methodologies will constitute an important tool for the genetic improvement and for obtaining new varieties with excellent agronomic attributes so that they can be distributed to the farmers in less time.
Brief history of anther culture for haploid and doubled haploid production
Several reports are concluding that duplicate haploids can accelerate the production of new plant varieties of agronomic interest, being them applied in different species (Thomas et al., 2003). Among the most outstanding crops are barley (Hordeum vulgare L.), pepper (Capsicum annuum L.), rice (Oryza sativa L.), tobacco (Nicotiana tabacum L.), and wheat (Triticum aestivum L) (Ferrie, 2007).
The first report on the use of haploid lines in pepper was published by Christensen and Bamford (1943), finding spontaneous haploid plants among the evaluated material. Similarly, in 1945, Toole and Bamford obtained for the first time doubled haploid plants in cultivars of Capsicum L. using colchicine. Subsequently, Campos and Morgan (1958) succeeded in inducing haploids by intraspecific crossing (Dumas de Vaulx and Pochard, 1974). The yield of these haploid lines was similar to their parents, although with a much lower percentage of fertility; these investigations opened the way to use haploids in plant breeding. The next step in the use of haploids was the development of biotechnological techniques such as the cultivation of anthers, developed in the early seventies. Wang et al. (1973) and George and Narayanaswamy (1973) were the first to report the successful regeneration of haploids in pepper from anther culture (Harn et al., 1975). These researches showed embryonic induction, but a successful regeneration of plants was rarely obtained.
In the works carried out by Sibi et al. (1979) and Dumas de Vaulx et al. (1981) in Capsicum, using the cultivation of anthers, the purpose was to make the technique more efficient in terms of obtaining regenerated plants by making certain modifications. Thus, for instance, the changes made by Sibi et al. (1979) consisted of pre-treatment of anthers in cold (4 °C, 48 h) and determination of the state of optimal development of the microspore (medium or late uninucleate), which allowed them to obtain seedlings for each isolated anther. On the other hand, Dumas de Vaulx et al. (1981) exposed the anthers to thermal shock of 35 °C during eight days of darkness, obtaining between 5-10% of regenerated plants of pepper genotypes.
Dolcet-Sanjuan et al. (1997) describe a new protocol using a biphasic system of semi-solid and liquid medium, replacing sucrose with maltose and enriching the atmosphere of the anther culture medium with CO2 for androgenesis in pepper hybrids, where they managed to increase the number of embryos (up to 3,561 per 100 fIowers) and plants (up to 23 per 100 fIowers) with 65% of plants duplicated spontaneously. Time later, Yin et al. (2010) confirm that pretreatment at low temperatures, the combinations of growth regulators, the concentrations of activated carbon, and pre-culture temperatures are critical factors that affect the formation of androgenic embryos.
Over the years, research aimed at the use and production of doubled haploids has focused on the refinement of in vitro culture. It has been seen that the optimization of in vitro factors such as quality of the medium, light intensity, temperature, nutrient replacement, among others, improve the androgenic response of the obtained in vitro-plants. However, it has been shown that this response is highly infIuenced by its different genotypes (Morrison et al., 1986).
Despite the advantages offered by the aforementioned processes, there are numerous reports that argue the recalcitrance of the genus Capsicum to in vitro morphogenesis, which manifests itself in various ways such as the low efficiency of the regeneration systems as well as the low reproducibility of the protocols of regeneration -the high index of deformed somatic embryos, the low rate of germination and/or conversion of somatic embryos into plants (Steinitz et al., 2003). Besides the above, a protocol for a specific genotype for the production of doubled haploids has not been fully established (Irikova et al., 2011). The most critical steps are the production of structures similar to the embryos and the subsequent generation of the plant, the collection of fIower buds in the optimal state, stress treatments, in addition to the environment and the culture conditions (Irikova et al., 2011).
COMPOSITION OF CULTURE MEDIA AND CULTURE CONDITIONS
Basal media are the main culture components since these contain the nutritional requirements for an embryogenic response (Koleva-Gudeva et al., 2007). The most used media for haploid and doubled haploid induction are N6, MS (Murashige and Skoog, 1962), NN (Nitsch and Nitsch), B5, CP (Dumas de Vaulx et al., 1981), among others, including the modified versions. Moreover, sucrose, maltose, and mannitol are the main sources of carbohydrates in these media, due to their nutritional and osmotic effects (Powell, 1990; Thompson et al., 1986). Taşkin et al. (2011) found a higher embryo production and formation in MS media after comparing four types of culture using pepper species. However, other studies using MS and CP basal media for embryogenic formation from anthers found that CP was the adequate medium since normal embryos of Capsicum annum L. were formed in all cases (Luitel and Kang 2013). Interestingly, although basal media are important for achieving a positive androgenic response, most researchers conclude that the frequency of callus and embryo formation depends on the genotype.
On the other hand, growth regulators (e.g., auxins, cytokines, among others) play a fundamental role by greatly affecting the development of the microspore and promoting cell division in embryos and calli (Chen, 1986). Olszewska et al. (2014) explored the effect of anther age and two concentrations of kinetin in the regeneration medium. The modifications assessed by the authors led to a greater androgenic response in Capsicum genotypes compared to the traditionally used protocols for this species. Similar findings were reported by Cheng et al. (2013) while studying the effect of growth regulators and activated carbon on embryogenesis induction in C. annuum L. The authors concluded that the concentration of activated carbon could act as a promoter of embryogenesis in microspore culture. In particular, the addition of antioxidants and activated carbon is often useful with some genotypes since it reduces tissue blackening caused by phenols. However, Vélez Torres et al. (2010) assessed the effect of different concentrations of growth regulators on pepper cultivars and concluded that exogenous regulators determine the successful formation of embryogenesis, while the endogenous hormone levels define the response in vitro.
It has been shown that stress pre-treatments (which vary considerably among species) applied to anthers and microspores induce the sporophytic pathway and inhibit the gametophytic pathway (development of fertile pollen) (Germanà, 2011) and promote the embryogenic potential. Pre-treatments, such as thermal shock (cold, hot), high humidity, water stress, nitrogen deprivation, ethanol, among others, are the most used for the androgenic pathways (Shariatpanahi et al., 2006). Ercan and Ayar Şensoy (2011) studied the androgenic response in pepper cultivars after a pre-treatment with cold (4 °C) and darkness for 24 hours and obtained 44 embryos from 2398 anthers in in vitro culture. Later, Popova et al. (2016) assessed the embryogenic response of C. annuum L. to different lengths of a pre-treatment of low temperature and darkness (during the first eight days). The results indicated that embryogenic efficiency decreases at low temperatures and under photoperiod inhibition. Several studies have shown that the exposure of androgenic cultures to alternating light periods (14/10 h; light/dark; 2,000 lux m-2) generates an artificial environmental signal that regulates pollen morphogenesis in vitro (Reynolds and Crawford, 1997).
MAIN METHODS FOR HAPLOID AND DOUBLED HAPLOID INDUCTION IN Capsicum spp.
There are several techniques for obtaining haploids - in vivo and in vitro methodologies (Figure 1). The most used methods are gynogenesis and androgenesis (Irikova et al., 2016). Gynogenesis involves female gametophytes (ovules and ovaries), and androgenesis uses male gametes (anthers and microspores) to generate haploids. Gynogenesis is the least favored technique due to its low efficiency (Forster et al., 2007). On the other hand, male gametogenesis is one of the most used methods for haploid and doubled haploid production (Kasha and Maluszynski, 2003), and it has been successfully used in more than 200 species, mainly annual plants, including pepper (Mitykó et al., 2006).
Anther culture
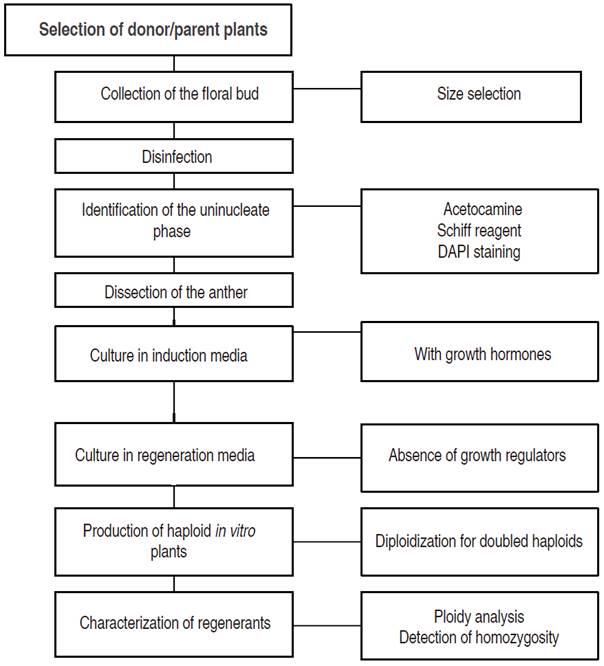
There is no specific protocol or culture condition for Capsicum spp. to induce androgenesis in vitro. However, some common guidelines are provided for plant formation from anther culture (Figure 2). In general, most studies report an optimal floral bud size between 3 and 6 cm in diameter. The buds are washed with distilled water, and surface disinfection is performed with calcium hypochlorite, 70% ethanol, Tween 20, among others. After determining the developmental stage of the microspore through staining with acetocarmine, Schiff reagent or DAPI, among others, those in early or late uninucleate phases are cultured in induction media with plant growth regulators (2,4D, dichlorophen- oxyacetic acid; KIN, kinetin, among others) and maintained under a pre-treatment at approximately 28-35 °C with light (12-18 h) and dark (6-12 h) periods. Following embryo formation, these cultures are transferred to regeneration media (free of growth hormones) to stimulate the formation of roots and shoots. The entire process is conducted under aseptic conditions in a laminar flow cabinet (Reinert and Bajaj, 1977). The most used methods for ploidy analysis of in vitro plants are cytological techniques involving chloroplast counts in guard cells, chromosome counts in somatic cells from root apices, as well as flow cytometry. Besides, isoenzyme and DNA marker-based techniques are also used (Germanà, 2011).
Microspore culture
Similar to the protocol for anther culture, there is no standard condition to induce plant formation from microspores. The explant can come from microspores or immature pollen to obtain haploid plants, with the advantage that embryos can be obtained from heterozygote somatic tissue (Regner, 1996). This methodology is similar to the one for anther culture, which begins with the mechanic isolation of microspores from a single anther using a glass rod. The microspores are then cultured in an isolation medium containing sucrose, ascorbic acid, L-proline, biotin, and nicotinic acid, according to most studies. The microspore is generally suspended using a nylon net and then transferred to liquid or solid culture media without growth hormones (regeneration or multiplication media). Finally, a haploid plant is obtained and, subsequently, a double haploid plant by treatment with colchicine. This protocol is performed under sterile conditions (Das et al., 2018).
The anther culture technique is often the method of choice by most researchers for the production of doubled haploid. The advantage of this procedure is that the nutritional requirements of the anthers are much simpler than those of the isolated microspores (Bajaj, 1990), and it is not as laborious as microspore culture. Despite these advantages, there are several limitations, such as the regeneration of anther wall somatic tissue, the production of mixoploids (in somatic and gametic tissue), and the formation of asynchronous microspores (Kott et al., 1988). Besides, the use of grains in advanced developmental stages is challenging since these suppress the androgenic capacity of the younger grains by releasing toxic substances (Bhojwani and Razdan, 1996).
Another determining factor for the success of anther culture is the selection of the floral buds since pollen in the early uninucleate phase is more sensitive to external stress, and this could hinder embryogenesis (Irikova et al., 2011). According to Kim et al. (2004), the optimal anther phase for embryo production is the early binucleate phase. Likewise, Supena et al. (2006a) determined that a critical factor for C. annuum L. cultivars is the selection of microspores in the late unicellular phase.
The difficulties discussed can be overcome by culturing isolated microspores. This technique allows not only to discard the use of somatic tissue (i.e., the unknown effect of the anther wall) (Mishra and Goswami, 2014) but also to isolate and culture microspores in an adequate phase; thus, contributing to an efficient regeneration. In particular, there are thousands of microspores per anther, which allows obtaining a greater number of haploid plants, and this can be applied at a large scale on a variety of genotypes (Taşkin et al., 2011). Supena et al. (2006b) found a greater rate of spontaneous re-diploidization from isolated microspores compared to cultured anther. These results prompted studies such as the one by Lantos et al. (2009), in which isolated microspore cultures in pepper genotypes were improved by applying wheat ovary co-culture. Similarly, Heidari-Zefreh et al. (2018) improved the efficacy of microspore embryogenesis and, subsequently, the regeneration frequency of C. annuum L. seedlings by applying different concentrations of ascorbic acid in the medium along with a thermal shock. Despite the success of these studies, there is limited available research regarding isolated microspore culture in C. annuum L., mainly since this technique requires more suitable equipment and technical-scientific skills.
Chromosomal duplication of haploid plants
The research focused on the rapid development of improved cultivars using in vitro techniques usually requires first obtaining haploid plants for them to be later diploidized (duplication of chromosomes). Seedlings derived by androgenesis lead to the spontaneous formation of haploids and doubled haploids; however, the percentage of the latter is very low. Due to this fact, it has become necessary to resort to chemical substances, including colchicine (C22H25NO6), widely used for the induction of polyploidy in plants (Urwin, 2014). This substance is an alkaloid extracted from Colchicum autumnale, which inhibits the self-assembly of tubulin, preventing the formation of the microtubules of the spindle, affecting only cells that are in the division, therefore acting as a “mitotic poison” (Badu et al., 2017). The most important advantage of polyploidy is that plants tend to have better performance and morphological characteristics, such as the height and size of plant organs (Hannweg et al., 2016), and the increase in biomass in general (Urwin, 2014).
This mutagenic can be applied to plants in different ways: by aqueous solution submerging the roots (systemic absorption) or by microinjection, in which the solution is incorporated into the in vitro plant by mechanical inoculation. On the other hand, calli can also be imbibed in colchicine before they are planted in the regeneration medium (Dhooghe et al., 2011).
A large number of polyploid induction processes have been reported with colchicine, being a very effective mechanism, but it has also been seen that its use can generate chimerization and death of the tissue with which it has been in contact, so the production of viable polyploid plants decreases in a large proportion (do Rêgo et al., 2009). Because colchicine has negative effects, the use of Oryzalin and other methods that affect mitotic processes, have begun to be used, generating higher rates of viable polyploid seedlings.
Techniques for the chromosomal duplication of haploids in pepper cultivars are generally based on treatment with colchicine. Gémesné Juhász et al. (2001a, 2001b) showed that they could increase the efficiency in 95% of doubled haploid production in pepper cultivars (Capsicum annum L.) by applying colchicine (0.04%) to the regeneration medium. In subsequent investigations, Gémesné Juhász et al. (2001a) indicated that the medium supplemented with colchicine resulted in an effective diploidization of 50-95% explants.
FINAL CONSIDERATIONS
The biotechnology techniques and advancements in molecular biology have enabled the development of useful tools for plant improvement. Among these, the production of haploid and doubled haploid plants is relevant to conventional improvement since it reduces the time required to obtain new varieties. Doubled haploid plants constitute genetically pure lines, which do not exist in conventional cultures and allow to rapidly fix new gene combinations resulting in individuals with greater production, resistance to pests and diseases, and higher tolerance to biotic and abiotic factors. However, the application of these tools must still overcome several key factors, including recalcitrant plants, genotype dependence, among others, that challenge the production of a suitable percentage of endogamic lines. Besides, a better understanding of the cellular, biochemical, and molecular basis of embryo induction through the androgenic pathway is required. Consequently, the current protocols need improvement, and new genotype-independent strategies should be implemented. The processes involved in microspore-induced embryogenesis should be further investigated, mainly, the cell division processes for obtaining haploid plants. The haploid induction technique can be efficiently combined with other plant biotechnology techniques, resulting in important achievements in genetic studies, gene mapping, QTL localization, genomics, and mutagenesis and genetic transformation research. Overall, there is a clear interest in this tool for plant improvement and its future application in several agriculturally important crops.