The symphylids, also known as garden centipedes or pseudocentipedes, are soil-dwelling arthropods of the class Symphyla (Pocock). The genera most reported as crop pests are Scutigerella Ryder and Hanseniella Bagnal, belonging to the family Scutigerellidae (de Morais and da Silva, 2009). However, it was reported recently that a species in the genus Millotellina Jupeau also infests fIower crops in Colombia (Salazar-Moncada, 2015a).
The symphylids are pests of diverse crops worldwide. Symphylids consume the roots and seedlings of plants, reducing the absorption of nutrients and water from the soil to the plant, which limits plant growth (Soler et al., 2011; Umble et al., 2006). In Colombia, the species Scutigerella immaculata (Newport) has been recorded as a pest of pineapple crops (Ananas comosus) in nurseries, where only three symphylids per plant can reduce root biomass by 67% (Agredo et al., 1988). Similarly, it was found that only five symphylids per plant can reduce plant dry weight by 55% to 68% in fIower crops for eight weeks (Acosta, 2006) such as carnation (Dianthus sp.), rose (Rosa sp.), and chrysanthemum (Dendranthema sp.).
Symphylids control has been complicated because of their high degree of both vertical and horizontal mobility in the ground (Umble and Fisher, 2003). In this regard, they evade the action of commonly used pesticides such as Chlorethoxyfos (organophosphate: O,O-diethyl O-3,5,6-trichloropyridin-2-yl phosphorothioate), Chlorpyrifos (organophosphate: O,O-Diethyl O-3,5,6-trichloropyridin-2-yl phosphorothioate), and Terbufos (organophosphorus: O,O-diethyl S((tert-butylthio)methyl)phosphorodithioate) (Lingenfelter, 2013). The harmful effects of these substances on human and animal health, and its contamination of water bodies have resulted in international calls to restrict the use of these chemicals (Montero and Franco, 2009). Therefore, alternative and effective strategies for the control of symphylids, such as the use of entomopathogenic fungi (Umble and Fisher, 2003; Nicholls, 2008), are needed. The benefits of fungi in biological control have been demonstrated. Acosta (2006) evaluated the pathogenic activity of four fungal isolates - Beauveria bassiana Bassi, Paecilomyces lilacinum Thom, Lecanicillium lecanii Zimm, and Metarhizium anisopliae Sorokin - on different stages of S. immaculata. Results in greenhouse conditions showed that the fungi L. lecanii and M. anisopliae at a concentration of 1×1011 conidia mL-1 reduced S. immaculata numbers by 15% and 17.5%, respectively.
Bio-control and Environmental Microbiology (BIOMA) group, of Universidad de Antioquia, isolated a strain of the fungus Purpureocillium lilacinum (Luangsa-ard et al., 2011), strain UdeA0106. This species was isolated in 2006 in the soil samples from Universidad de Antioquia Farm, municipality of Gómez Plata, Antioquia, Colombia. Preliminary pathogenic tests have shown that the mycelium of this fungus grows on symphylid cuticles, which can reduce symphylid numbers on plants (data not shown). Due to the observed potential of this strain for the control of symphylid density on chrysanthemum crops (Dendranthema grandifIora Tzvelev), this study aimed to present an evaluation of the pathogenicity of strain UdeA0106 under in vitro and greenhouse conditions as a biological control agent to symphylids pest.
MATERIALS AND METHODS
Study sites
In vitro tests were carried out at the laboratories of the University of Antioquia. Field tests were carried out in a commercial greenhouse of the fIower plantation FIores Esmeralda S.A.S.C.I. This plantation is in the department of Antioquia, municipality of La Ceja, Colombia: 6.0367734° N, -75.4248762° W, 2180 m.a.s.l.
Symphylid samples
Symphylids were obtained by trapping (N=40) with beet slices that were placed on soil under ornamental plants for 12 hours. Symphylid adults (Scutigerella immaculata s.l.) were determined by their characteristic of the presence of 12 pairs of legs (Halliday, 2004). Symphylids were harvested early in the morning and stored in Petri dishes that contained soil from the sampling site and maintained at the same humidity and temperature (RH 70% and 19-23 °C). The individuals collected were stored until use in the laboratory of the fIower plantation.
Fungus and symphylid preparation
The mass multiplication of the fungus Purpureocillium lilacinum, strain UdeA0106, and the preparation of the spore solution concentrations used for in vitro and greenhouse pathogenic tests were carried out using the methodology established by Cenicafé (Vélez et al., 1997). It considers rice as a culture medium, then obtains conidia by mechanical separation and subsequently counting in the Neubauer chamber.
The solution concentrations used for inoculation were: 1×105 conidia mL-1, 2×106 conidia mL-1, 1×107conidia mL-1, 1×108 conidia mL-1, and 2×108 conidia mL-1. In sterile Petri dishes without soil, symphylids were immobilized by placing at a low temperature: refrigerated at 7 °C for 30 minutes. The symphylids were then disinfected with 1 mL of sodium hypochlorite (NaOCl 1%) and washed with sterile, distilled water.
In vitro pathogenic test
The test unit was a Petri dish containing ten symphylids previously placed in a sterile lid with a hole in the center to limit the sprinkling of the fungus on the arthropods. Inoculation involved pouring the spore solution on each unit, allowing to contacting with the symphylids for three minutes. The symphylids were then transferred to another sterile Petri dish with a filter paper moistened with sterile distilled water. Each of the spore concentrations was administered three times in duplicate, providing a total of six test units. The Petri dishes were then incubated at room temperature and in the dark for ten days. Periodic reviews were made to keep the filter paper moist in the Petri dish. Normal controls consisted of using symphylids inoculated with sterile distilled water that substituted the spore solution in the procedure described before. Mycosis readings per symphylid were carried out after ten days using a stereoscope. The percentage of mycosis was calculated as ten times the number of symphylids infected with fungus. Mycosis by the strain UdeA0106 was confirmed by direct cultivation on PDA agar.
Pest attack in the greenhouse
This test aimed at determining the number of symphylids per plant (density) that will cause a measurable reduction in plant height and root dry weight to then established the number of symphylids required for the greenhouse-based pathogenic test. Here the test unit was a 40×14 cm cylindrical glass container filled 1,400 g of soil. In each unit, two chrysanthemum seedlings - Anastasia White variety susceptible to this pest - were planted with different densities of symphylids, the negative control consisted of only the seedlings without symphylids (Table 1). The seedlings were irrigated with 140 mL of fertilizer solution to promote growth and to simulate field conditions. The top of each glass cylinder was covered with a white tulle fabric to prevent symphylid escape. The response variables evaluated were the height of the plant and root dry weight. Plant height was measured with a Stanley fIexometer starting from the neck of the root to the terminal bud. Root dry weight was established in eight repetitions with two seedlings by cutting the roots up to the neck of the stem. The roots were washed and dried in an oven at 70 °C for 72 hours and then weighed on an analytical balance. The plant measurements were taken 24 days after infestation with the pest, by which time the symphylid attack symptoms on the plant were visible.
Pathogenic test in the greenhouse
An experimental greenhouse at FIores Esmeralda S.A.S.C.I. was used. It was maintained under the same conditions of the greenhouses involved in commercial fIower production. The soil was subjected to a solarization process for six weeks to guarantee the absence of symphylids; then, it was kept at 19-23 °C and 70% RH (measured with a ground hygrometer HM008). The same test unit was used as previously described for the pest attack in the greenhouse, except 1,000 g of soil was used. Based on the result of the pest attack experiment, five symphylids per 100 g of soil were used. The treatments consisted of administering 100-mL solutions of different concentrations (1×108, 1×105, 1×103 conidia mL-1) at a ratio of 1:10 (inoculated fungus:soil). There were eight repetitions per inoculating concentration. A positive control consisted of seedlings with symphylids, but without the application of strain UdeA0106. The negative control consisted of only the seedlings without symphylids. After 24 days, the soil of each test unit was processed with the modified Berlese method (Ledesma, 1994), which consisted of depositing sub-samples of 100 g of soil on a mesh 1 mm in diameter. This was then covered with a cone with a light source incorporated that produced heat that forced the symphylids to leave the mesh and group on the black base. In addition, a manual review of the soil sample was carried out to ensure all individuals were counted. Response variables measured - plant height and root dry weight - were as described in the pest attack greenhouse experiment.
Statistical analysis
For the in vitro pathogenic test, a Probit analysis (Finney Method [Lognormal Distribution]) was performed to find the mean effective dose of mycelia that produced mycosis in 50% of the symphylid population. For both greenhouse-based tests, a completely randomized experimental design was applied. An analysis of variance and a multiple comparison test were performed for the means with the Tukey test at a 95% confidence level; the statistical analyses above-mentioned were performed in the Biostat Online Software. The response variables in both greenhouse-based tests were plant height and root dry weight. In the pathogenic test, the response variable of a reduction in the number of symphylids was evaluated with an Abbot correction (Abbott, 1925) for symphylid density. Finally, a Probit (Finney, 1977) analysis (Finney Method [Normal Log Distribution]) was performed to find the lethal concentration that killed 50% of the symphylid population (LC50). The data, that did not meet the assumptions of normality of errors and homogeneity of variances, were transformed and processed with non-parametric analyzes of Kruskal-Wallis (Kruskal and Wallis, 1952).
RESULTS AND DISCUSSION
In vitro pathogenic test
It was confirmed that symphylids are susceptible to attack by Purpureocillium lilacinum, strain UdeA0106. The tests results for different concentration of fungus showed a direct relationship between mycosis per symphylid and inoculate concentration. At the lower concentration (1×105 conidia mL-1), there was a lower percentage of mycosis (60%), whereas, at the higher concentration (2×108 conidia mL-1), the percentage of mycosis was maximal (100%).
Pest attack in the greenhouse
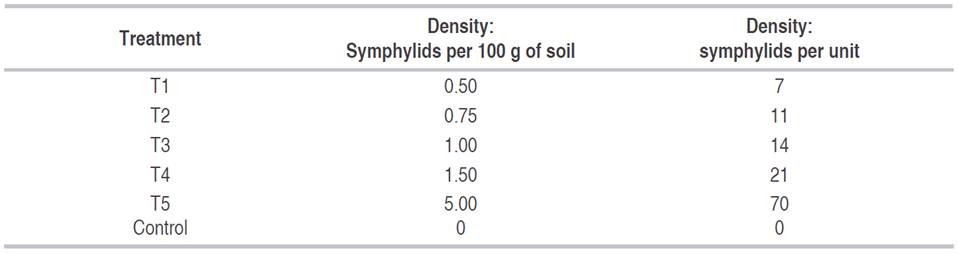
Symphylid density was not significantly different among them (Figure 1). Compared to the control (absence of symphylids), all symphylid densities were associated with a significant (P<0.0001) decrease in plant root dry weight, including an average density as low as 0.5 symphylids per 100 g of soil (Figure 1). The highest density of 5 symphylids per100 g of soil was associated with the minimum average root dry weight; therefore, this symphylid density was used for the greenhouse-based pathogenic test.
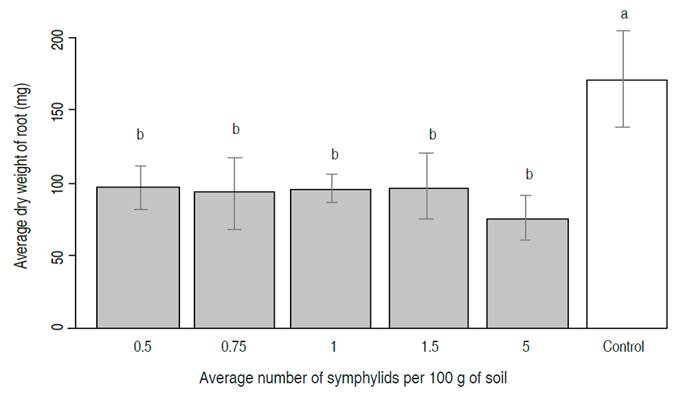
Figure 1 The effect of different symphylid densities on plant root dry weight. Control: no symphylids. Different letters indicate statistically significant differences among symphylid number per 100 g of soil at P=1.85×10-8; F= 12,436; GL= 5; N=8 replicates per treatment. Bars indicate standard deviation.
The threshold at which symphylid density causes economic plant damage is not well established (Umble et al., 2006). In fIower crops, it has been recommended that the mere presence of a symphylid is a sufficient signal to implement a control method (Peña, 1998). A rational pest sampling method in fIower crops has recently been proposed, which established an action threshold when an average density of 0.4 symphylids per 100 g is recorded in three soil sample replicates (Salazar-Moncada et al., 2015b). In this study, it was demonstrated the impact of symphylid infestation on root dry weight of chrysanthemum crops. The findings of this research are consistent with the literature, where an average of 0.5 symphylids per 100 g of soil caused a decrease of 42.82% in root dry weight, quantifying the extent of plant damage caused by this pest.
The height of the plants indirectly relates to the damage of their roots by symphylids (Umble and Fisher, 2003). This was studied in Scutigerella immaculata infestations during the early growth stages of carnation, rose, and chrysanthemum in pots, where the pest significantly affected the plant height at densities of five or greater symphylids per plant (Peña, 1998). However, in this study, this variable did not present significant differences with respect to the control. A possible explanation of this result is that in the pathogenic tests, a tulle fabric was added to the top of each experimental unit that prevented the normal passage of light and promoted some elongation of the stem.
Pathogenic test in the greenhouse
Plant root dry weight showed significant statistical differences between fungus strain UdeA0106 inoculate concentrations. The highest fungus concentrate (1×108 conidia mL-1) produced an average plant root dry weight that was not statistically different from the negative control (i.e., uninfested seedlings: the absence of symphylids and fungus) (Figure 2). Furthermore, this high-test concentration showed the only statistically significant increase in root dry weight compared to the positive control (i.e., infested seedlings: with symphylids and absence of biocontrol) as the application of strain UdeA0106. Conversely, the two lower fungus concentrations showed a significantly reduced average dry weight compared to uninfested negative control seedlings and no significant increase in dry root weight compared to infested positive control seedlings.
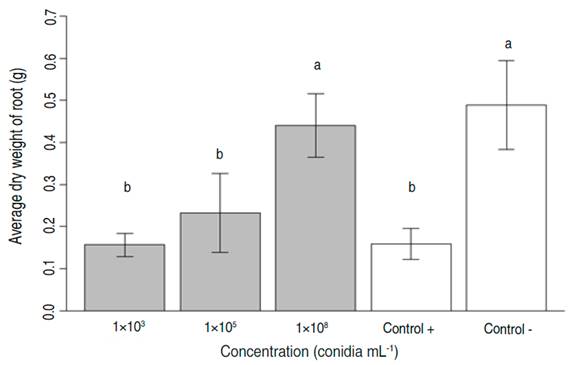
Figure 2 Results of inoculations of Purpureocillium lilacinum, strain UdeA0106, on average plant root dry weight (N=8 replicates).Control + = plants with symplylids and no pathogenic fungus. Control - = plants with no symplylids or fungus. Averages with different letters indicate statistically different means at P=5.75×10-12; F= 38,903; GL= 4; N=8 replicates per treatment. Bars indicate standard deviation.
In general, it was observed that the higher the concentration of fungus strain inoculated, the lower the symphylid density at the end of the test period (Table 2 and Figure 3).
Table 2 Percentage reduction in symphylid density in pathogenic tests in the greenhouse. Percentage reduction is calculated as an average of N=8 replicates.
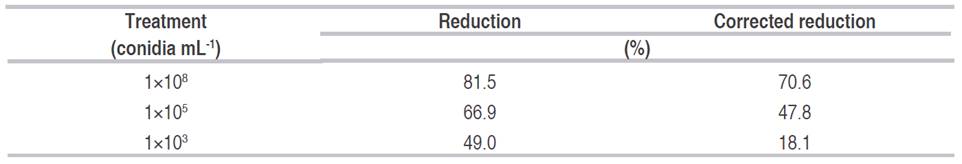
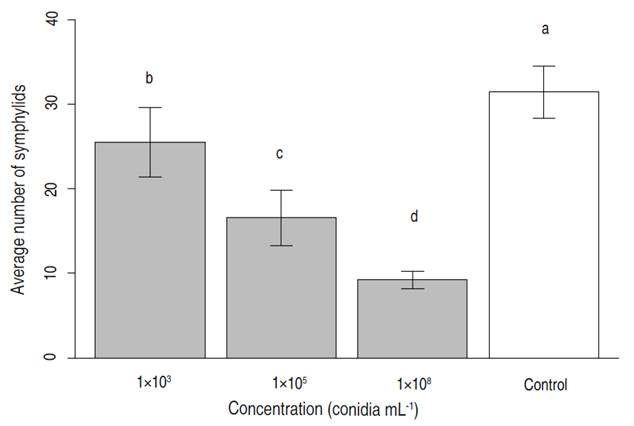
Figure 3 Results of inoculations of Purpureocillium lilacinum strain UdeA0106 on average symphylid density. Control = plants with symphylids and no fungus. Averages with different letters (a, b) indicate statistically different means at P=1.21×10-14; F=104,81; GL=3; N=8 replicates per treatment. Bars indicate standard deviation.
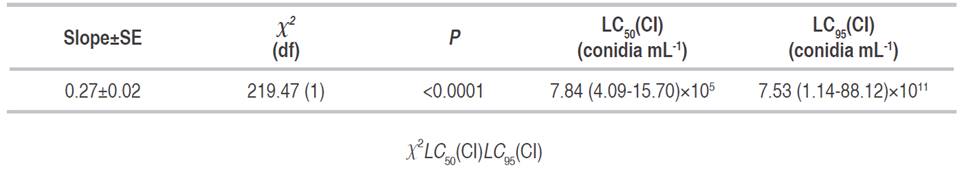
The highest inoculation concentration was associated with the greatest reduction in symphylid density (70.6%). The relationship between mortality and the dose concentration (Normal-log distribution) fitted a Probit regression model (Table 3). The mean (LC50) and 95% (LC95) lethal concentration dosages were established as well.
Table 3 Probit analysis of the relationship between Log10(Dose) and mortality. The median (LC50) and 95% (LC95) lethal concentration dosage and the confidence intervals (CI) of the analysis are indicated.
The pathogenic potential of fungi in the genus Purpureocillium has been demonstrated in the management of various pests; in eggs and J2 stages of the nematodes Meloidogyne incognita-javanica (Cardona et al., 2014) and Globodera rostochiensis (Núñez-Camargo et al., 2012), and in the tick Rhipicephalus microplus (Canestrini) (Angelo et al., 2012). Pathogenic fungi have also been shown as effective when combined with other biological control organisms in the management of Meloidogyne incognita (Huang et al., 2016) and Aphis gossypii Glover (Wakil et al., 2012). In this study, it was demonstrated the effective control of symphylids in flfIower crops, where inoculation with Purpureocillium lilacinum, strain UdeA0106, at a concentration of 1×108 conidia mL-1 reduced symphylid density by 70.6%. Further studies to determine if the cuticular components of symphylids share biochemical characteristics with nematodes and mites, this would enhance the understanding of the pathogenic behavior of both organisms.
This research also showed for the first time that biological control against symphylids is an effective pest management strategy that has the potential to protect the exportation of fIower crops from Colombia. Thanks to this research, this control method is currently being used in the plantation Flores Esmeralda S.A.S.C.I., achieving the complete eradication of their chemical management of this pest and offering compliance with the quality requirements for crop exportation to countries such as the United States. Before the adoption of this method of biological control, symphylid infestations in the fIower plantation were high: 10% of cultivars showed direct pest damage. Twenty-four days after the application of fungus P. lilacinum strain UdeA0106, no signs of plant damage were observed during the subsequent nine weeks. Since 2014 the plantation has not detected significant economic damage due to symphylids.
CONCLUSIONS
Pathogenic tests in field conditions showed that the action of the fungus P. lilacinum, strain UdeA0106, was effective against symphylid associated with plant damage. The highest inoculate concentration significantly reduced pest density. Plants in this treatment developed roots in the same way as the plants in the negative control of uninfested plants with no symphylids and no fungus. The Probit analysis showed a low value (LC50=7.84×105 conidia mL-1) that is advantageous for future commercial formulations since the registered products with fungi as an active ingredient have concentrations between 1×108 and 1×1012 conidia mL-1.