Jatropha (Jatropha curcas L.) has not yet captured the necessary interest in its systematic improvement for better plantation yields (Montes et al., 2014). Jatropha enhanced varieties with desirable characteristics under suitable growing conditions are not available, which contributes to risk for the future of their sustainable production (Rao et al., 2017). Jatropha genetic resources could be enhanced through a global exploration, where the introduction of genetic materials, characterization and evaluation could provide essential information for the development of elite varieties through various methods (Morris et al., 2013). The key to a conventional breeding program lies in the availability of genetic variations of a specific desirable characteristic (Varshney et al., 2013). Rosado et al. (2010) found accessions from Asia, Africa and South America with low genetic diversity of Jatropha, while accessions with high genetic variability were found in Guatemala and Chiapas, Mexico.
Currently, elite Jatropha germplasm in the world is not available. Materials that combine several desirable traits are needed to establish large-scale plantations to ensure their economic viability (Singh et al., 2016). Therefore, there is almost no germplasm exchange. Only local materials are used, but these are not interchanged between regions (Rade-Loor et al., 2017). The growth of trees is determined by the infIuence of their genetic composition, the environment and the interaction between them. This interaction must be evaluated in breeding programs to measure plantation improvement in multiple environments. For breeders, it is vital to identify Jatropha materials, where this interaction does not occur. It ensures consistency among cultivar performances within a regional environment. Hence, selection based on a single environment will always be inferior to that based on multiple environments (Senger et al., 2016). According to Basha and Sujatha (2007), identification of promising lines within a germplasm bank of Jatropha may require a study covering typically a period of 5 to 10 years.
Several studies on Jatropha genetic diversity have been carried out to determine the genetic variation in populations (de Azevedo et al., 2017). In Ecuador, the National Institute of Agricultural Research (INIAP), within the Portoviejo Experimental Station (EEP), carried out an extensive national collection to establish a conservation and evaluation program for Jatropha accessions in 2008. The purpose of this germplasm bank was to test accessions, which included the analyses of genetic variability, broad-sense heritability and genetic advantage to establish clonal seed orchards to cover the Jatropha elite materials needed under the local tropical dry forest conditions (Cañadas-López et al., 2017). The information obtained will serve to form a collection of genotypes with desirable characteristics for future use in a genetic improvement program of this species for biodiesel production in Ecuador. The objectives of the present investigation were to evaluate the phenotypic behaviors of 130 Jatropha accessions and to characterize the accessions based on genetic variability of the selected accessions.
MATERIALS AND METHODS
The study was conducted at the Portoviejo Research Station (EEP), which belongs to the National Institute for Agricultural and Cattle Ranching Research (INIAP) (0°6’S, 80°23’W), Lodana Sector, Canton Portoviejo, Province of Manabí, Ecuador. The EEP is located at 47 masl, with a mean annual temperature of 26.3 °C, mean annual precipitation of 809.6 mm, mean relative humidity of 83% and mean number of sunshine hours of 1,159.3 h year-1
(Cañadas-López et al., 2018a; Cañadas-López et al., 2018b). The research area is ecologically classified as a tropical dry forest (Cañadas, 1983).
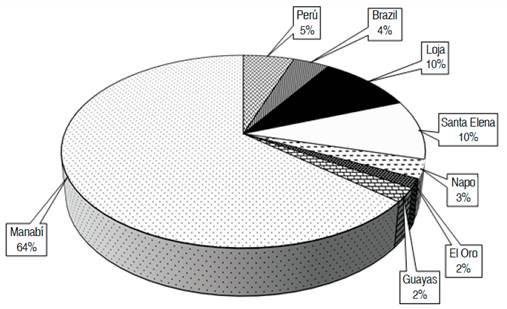
The collection of germplasm consists of 91% of materials from Ecuador (Figure 1), 5% from Brazil and 4% from Peru. After the establishment of the germplasm bank in 2008, the materials brought from abroad were not properly adapted to local conditions.
A total of 130 Jatropha accessions remained in the field. Plus-tree criteria were followed for selection according to Cornelius (1994). The selection was conducted in order to highlight phenotypic characteristics of interest such as the number of fruits per tree (FP), the number of seeds per fruit (SpF), weight of 100 dry seeds (100SW), seed length in cm (SL), seed width in cm (SW), lower standard deviations as regular seed production (RP), high standard deviation as an indicator of irregular seed production (IP), oil content of seeds in percentage (Oil) and seed production in g per tree (SP). The seeds of all Jatropha accessions were separated, cleaned and stored in cotton bags under environmental conditions to establish seed characteristics. The seed lots were dried in a hot air oven at 60 °C until seeds reached a constant weight and a humidity of 8%. Five samples were taken from each seed batch, and 100 randomly selected undamaged seeds (total 500 were measured in length and width). The Soxhelt method was applied to estimate the oil content of 200 seeds with three replications for each seed lots (Cañadas-López et al., 2018). Once the collection was assembled, germplasm cuttings from plus-tree selection planted in 2008 with three replications totaling 12 plants per batch with a spacing of 2x2 m and a germplasm bank was established in EEP-INIAP.
In 2014, Jatropha germplasm was evaluated according to the above indicated variables. Techniques of correspondence analysis were applied. The analysis of cross-tabular data in form of numerical frequencies was particularly helpful since it shows an elegant but straightforward graphical display, which permits a rapid interpretation and understanding of the data (Bortz and Schuster, 2011). A primary indicator of analysis results was the inertia that can be interpreted as the weighted average of squared χ2 distances between the average of the observed variables and the Jatropha accessions of different provenance regions. For this purpose, the software Statistica version 13.3 was applied.
During June 2015 and August 2016, Jatropha accessions described under the above criteria were sown in a system 2x2 m with three replications. Four Jatropha trees were selected from 18 month old, for each replication of each accession and data was recorded with the following morphological characteristics:
Height of plants (cm): it was measured from soil level to the apex and results were reported as average.
Number of branches per plant: The number of branches growing from the main stem in different positions of the nodes, including basal branches were counted.
Number of bunches per plant: Expressed as average number of fIower bunches per plant.
Number of fruits per plant: The average number of fruits per plant.
Number of seeds per fruit: Seed average found within the Jatropha fruit.
Weight of 100 seeds: All batches of seeds were dried under similar temperatures and humidity conditions until reaching a constant weight. Three samples were taken from each batch of seeds and 100 were randomly selected to measure their weight.
Seed yields: The weights of the seeds were established with a precision balance. After removing the pulp from the yellow fruits, the extracted seeds were dried in a convection oven at 60 °C for two days.
Analysis of variance and mean comparison were carried out with Statistica Software, version 13.3. Both genotypic and phenotypic correlation coefficients were calculated according to Johnson et al. (1955). Variability, genetic advance as a mean percentage, phenotypic and genotypic variance, phenotypic variation coefficient (PCV) and genotypic variation coefficient (GCV) were calculated for seed oil content (Yoshida et al., 2007). Accession heritability was estimated by dividing the variance of the measurements into components between accessions and within accessions. For the genotypic variance, the difference between and within the selected accessions was calculated. In addition, the variance within accessions has been defined as phenotypic variance. Subsequently, the genotypic variance was divided by the phenotypic variance to obtain the broad-sense heritability. Genetic advance (%) was estimated as the difference between the genotypic mean of the accessions and the genotypic mean of the population.
RESULTS AND DISCUSSION
Phenotypic behavior of 130 Ecuadorian Jatropha accessions - evaluation 2008-2014
Figure 2 shows the first two dimensions from the correspondence analysis of the 130 Jatropha accessions and their nine variables. A very large proportion (96.2%) of the inertia is displayed along the first dimension. SP and FP were the main contributors to this dimension. These variables are opposed by a cluster of the variables SL, SW, SpF and 100SW. The second axis explains only 2.1% of the inertia. In this case, the main contributors were regular seed production (RP) and oil content (Oil). These were primarily opposed to IP and a lower extent, to the variables mentioned above that form a cluster. The accessions located close to the variables SP and FP were CP041, CP052, CP037, CP054, CP060, CP122, CP118, CP120, and CP121.
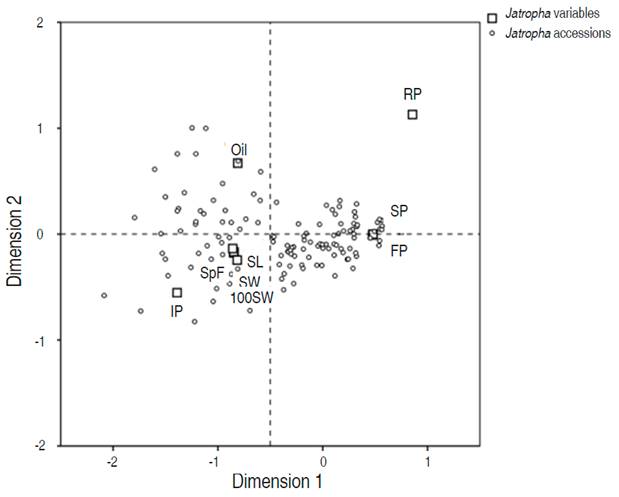
Figure 2 Dimensions 1 and 2 of the correspondence analyses of 130 Jatropha accessions, highlighting ten relevant variables. Number of fruits per tree (FP), number of seeds per fruit (SpF), 100-seed weight (100SW), seed length (SL), seed width (SW), regular seed production (RP), irregular seed production (IP), oil content (Oil) and seed production (SP).
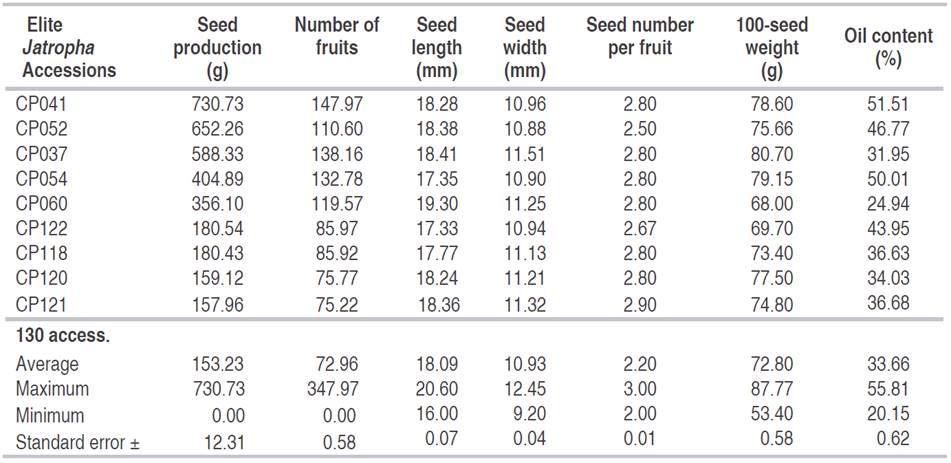
The highest production of seed and fruits from the 130 tested accessions of Jatropha are shown in Table 1, which are characteristics of 9 elite Jatropha accessions. The oil content, another important production factor, was opposite to the factors SP and FP. The values of the oil content of the nine highlighted accessions were indeed inconsistent and vary around the overall average of all tested accessions.
Table 1 The Jatropha accessions with the highest seed production and the number of fruits, and summary statistics of 9 accessions and 130 accessions tested.
In Ecuador, under tropical dry conditions, Cañadas-López et al. (2017) established for three Jatropha accessions a production of 0.70-0.75 t ha-1 year-1 for a 7-year-old plantation. The height of the Jatropha dry seed production variation was infIuenced by a factor unrelated to the water availability in the soil. At the same time, Cañadas-López et al. (2018) observed a production of 316.46 g tree-1 year-1 (791.15 t ha-1 year-1) for the Jatropha accession CP041 of an eight-year-old. It would mean that Jatropha’s productivity could be lower than 1000 kg ha-1 year-1 under dryland conditions in Ecuador (Cañadas-López et al., 2020). These productions registered in Ecuador were below the production variability ranges reported by Openshaw (2000) between 0.4 to 12 t ha-1 year-1. Thus, Jatropha breeding programs are an important task in improving the dry seed production on marginal lands.
Yong et al. (2010) under the effects of four fertilizers in Sigapour observed an average number of fruits per plant of 81 (240 g (T240) of Osmocote® Plus). Joshi et al. (2011) registered 33 fruits per tree and a fruit yield per plant of 27 in 2009. These trees were sprayed with Ethrel (growth regulator) 150 ppm in the year 2008. Mohapatra and Panda (2010) registered for 18 Jatropha accession from India, an average of 77 fruits per tree. Comparable results were observed for the 130 Ecuadorian Jatropha accessions under dry forest conditions with an average of 75 fruits per tree.
Jatropha has a three-locular ovary and generally the fruit has three seeds (Divakara et al., 2010). In some Mexican Jatropha genotypes, four seeds per fruit are found under normal growth conditions (Makkar and Becker, 2009). For Jatropha accessions coming from six different countries, Nietsche et al. (2014) reported ranges between 2.63 to 2.96 seeds per fruit. In the present research, the number of seeds per fruit was established with an average of 2.20 seeds per tree and was lower to the other authors.
The average of 130 Jatropha accessions was 36.68% (±0.62) of oil content for the Jatropha accessions in Ecuador under dry forest conditions. Carels (2009) mentioned that 35% of oil seed content could be seen as a good Jatropha accession. Superior oil contents were found by Martínez-Díaz et al. (2017) with a Jatropha oil seed variation from 42.35% to 55.39% for different Mexican Jatropha populations. In Colombia, the percentage of Jatropha oil seed content varied between 37.2% and 40.1% (Montenegro et al., 2014).
Ginwal et al. (2005) established that in India the 100-seed weights were highly variable and these were under strong genetic control in comparison to environmental infIuence. Wani et al. (2012) found the 100-seed weight between 49 to 69 g in the Andhra Pradesh State, India. While for Malaysian Jatropha accessions, Shabanimofrad et al. (2013) registered weights from 44 to 77 g. The 100-seed weight of this study was 74.49 g, which is in the range of the other reports.
Nietsche et al. (2014) reported a seed length of 18.43 mm and 18.56 mm seed width for 15 Jatropha accessions and argued that a lower availability of water periods infIuences in seed characteristics such as seed shape. Under dry conditions, the cell turgor pressure is infIuenced to increase cell expansion and carbohydrate accumulation. In the present investigation, it was possible to observe greater lengths (18.93 mm) and narrower widths (10.93 mm) of Jatropha seeds, although the seed ellipsoid shape prevailed.
Genetic association in growth and production characteristics of nine elite Jatropha selected accessions, evaluations in 2015 and 2016
There is a strong correlation (genotypic and phenotypic) between seed length and width. In contrast, there is no significant relationship (genotypic and phenotypic) between the seed width and 100-seed weight, nor between 100-seed weight and oil content (Table 2). The morphology characterization of seeds from Ecuadorian Jatropha accessions at EEP is the first step to determine the population’s genetic variability. However, biotic and abiotic factors infIuence on the seed size (Valdés-Rodríguez et al., 2018).
Table 2 The correlation coefficient between seed length, seed width, 100-seed weight and oil content for nine selected Jatropha accessions, EEP-INIAP, Manabí-Ecuador.
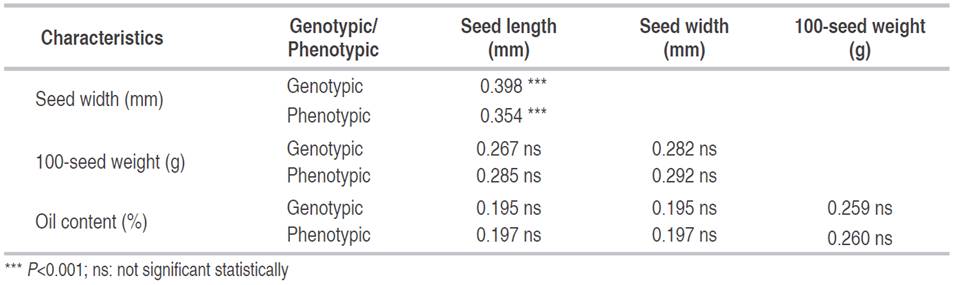
The degree of correlation coefficient at genotype level was greater than its corresponding phenotypic coefficient correlations for seed width and seed length parameters (Table 2). This trend was congruent with those obtained by Valdés-Rodríguez et al. (2018) in Mexico. Nevertheless, the oil seed content was not related to any other variables analyzed. These results were in contrast with those found by Rao et al. (2008), who argued that the seed weight could be considered as an important trait for the early selection of seed sources.
Genetic association in the characteristics of growth and production of nine elite Jatropha selected accessions
The maximum variation was observed for the Jatropha tree height followed by dry seed production and the number of fruit tree-1 (Table 3). At the same time, the minimum variance was detected in seed length, seed width, the weight of 100-seed weight, oil content, and the number of seeds per tree. The phenotypic variation coefficient was similar to the genotypic variation coefficient. The lowest genotypic variance was recorded for the seed number fruit-1 (8.41-10.30) and seed weight (9.81-13.87). Broad-sense heritability of seed length was 88%, 100-seed weight was 86% and of oil content 80% (Table 3). The genetic advance ranged from 3.81% (oil content) to 19.09% (seed length) for the nine tested Jatropha accessions.
Table 3 Genetic variable estimations for seed and oil traits in INIAP Jatropha accessions, EEP-INIAP, Manabí-Ecuador.
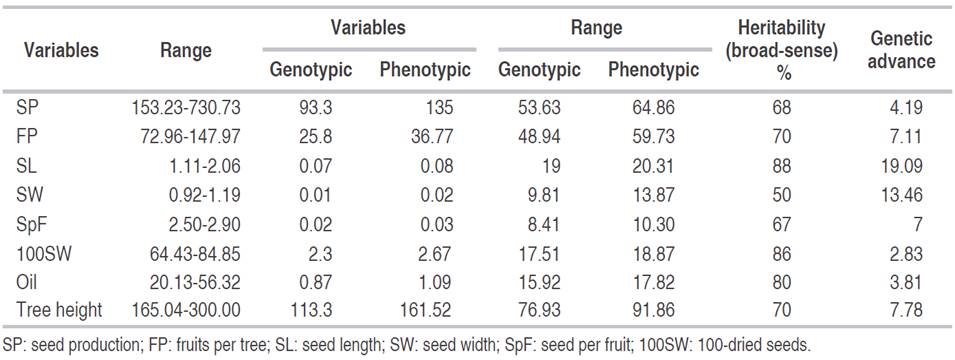
A close correspondence was observed between the genotypic and phenotypic variance coefficient for all Jatropha accessions studied. Since the variation depends on the unit’s magnitude measuring of the traits, the coefficient of variation is independent of its unit of measure. It is useful for comparing populations (Martin and Montes, 2015). For dry seed production, tree height and the FP of the Ecuadorian Jatropha accessions, a high genotypic and phenotypic coefficient of variation was found. These data can be compared with the results obtained by Rao et al. (2008) for production (54.90-55.26) and tree height (26.71-28.43). This fact indicates that the selection can be applied to the traits to isolate a more promising line. The same results were reported by Martin and Montes (2015). Nevertheless, the high PCV and GCV for the studied morphological characters of the Jatropha tree indicated that environmental infIuences on the expression of these traits were minor (Tefera et al., 2003).
Seed length, 100-seed weight and oil content showed a moderate coefficient of genetic and phenotypic variability. According to Patil (2010), this reasonable variation could be improved by exhaustive selection. The characteristics of seed length and 100-seed weight displayed a low coefficient of variance, indicating the need of other resources with high variability of these characteristics.
However, characters that exhibit maximum heritability and high genetic advances as a mean could be used as a powerful selection tool. Such traits are controlled by the additive genes and are less infIuenced by the environment (Ahsan et al., 2015). For all Jatropha characteristics, a high broad-sense heritability was observed in the present study. In observed populations, the variation is due to both genetic and environmental factors, because genetic variability is the only inheritable from generation to generation. Heritability alone does not give a clear idea about the expected genetic gain in the succeeding generation and therefore, it should be considered as a whole with the genetic advances (Alves et al., 2013).
Hence, the combination of high broad-sense heritability and high genetic advantage will provide a clear basis on the reliability of that particular trait in the selection of Jatropha varieties (Hernández-Velasco et al., 2016; Ortiz-Olivas et al., 2017) It was not observed in the present investigation on the elite EEP-INIAP Jatropha accessions.
CONCLUSION
In an environment of global warming and food security problems due to human population growth, priority should be given to plants that are not edible, but that can be used for the production for biodiesel. Jatropha's potential as an organic biodiesel raw material in promoting socio-economic development and meeting energy demands as the "Jatropha for Galápagos" Project does, which needs superior Jatropha materials. However, the results of this study highlight the low phenotypic diversity of the physically dry seed variability within the Jatropha EEP-INIAP germplasm bank under tropical dry forest conditions. It could be because 72% of the material collected comes from the Manabí Province. The Jatropha vegetative propagation form, mostly diffused in Manabí, is by stakes, which could have caused the lack of variability, refIecting a common descendant. There is an urgent need to improve the germplasm resource by obtaining new accessions, mainly from countries considered as centers of origin of the species to promote the genetic diversity necessary to develop a genetic improvement program for the Jatropha plantations.