The Brazilian legal Amazon has a total area of, approximately, 5.0 million km2, corresponding to about 60% of the Brazilian territory. In 2018, it had a cumulative deforested area corresponding to 708,301 km2, equivalent to about 17% of the entire Brazilian Amazon forest. Approximately 13% of the area is occupied by agriculture, with an estimated area of 43,092,115 ha occupied by pasture (Miranda et al., 2019).
In these pastures, it is common to use fire to control weeds and to stimulate pastures regrowth, arguing the ashes can increase the soil fertility. However, about 90 days after burning, soil fertility levels begin to decrease (Santana et al., 2013). Thus, the benefits of this process are short-lived, increasing soil exposure to erosive processes.
The conversion of Amazon rainforest to pasture through slash and burn, affects the physical, chemical, biological and even mineralogical properties of the soil. It is aggravated by the general characteristics of the soils formed in the region, which have extreme poverty in phosphorus available (P); high acidity; high aluminum saturation (Al3+); low Cation Exchange Capacity (CEC); macro and micronutrient poverty and susceptibility to compaction and erosion (Vale-Júnior et al., 2011).
The effect of temperature caused by burning on soil chemical properties leads to an increase in pH, CEC, in addition to calcium (Ca), magnesium (Mg), potassium (K) and phosphorus available (P). The rise in temperature increase the pH value and decrease the CEC. Regarding nutrients, if the burning temperature rises in excess, Ca, Mg, K and P decrease (Giovannini et al., 1990).
The exposure of the soil to extreme temperatures (>600 °C) also causes auterations in mineralogy and texture of the soil, increasing the amount of sand and a decrease in silt and, mainly, clay. Regarding mineralogy, with increasing temperature, the peaks for gibbsite in the sand fractions can gradually decrease in intensity, and disappear completely at a temperature above 600 °C (Ketterings et al., 2000).
In pastoral systems, the topsoil is sensitive to changes in management, mainly due to the compacting of the surface caused by heavy animals grazing in extensive systems. Also, the microbial activity of the soil and the contributions of plant material affect the dynamics of soil properties on the surface in relation to the soil in depth (Boeni et al., 2014).
Studies about chemical changes in the superficial layer of the soil (0-0.05 m) under forest subjected to burning, showed an increase in the values of pH, electrical conductivity, organic carbon and exchangeable bases, caused by the addition of ash from the fires (Iglesias et al., 1997). Alterations may also occur in fertility, in which the soil has higher concentrations of P, Mg and K, after being subjected to high temperatures; however, a decrease in the density of microfungi could occur (Copogna et al., 2009). Other alterations in the superficial layer (0-0.05 m) can be observed in relation to the increase of the sand fraction and decrease of the silt and clay fractions (Ketterings et al., 2000).
Because of the bad aspects that fire can cause in soil properties, some alternatives can be used to suppress its use, including mowing (weed cutting practice). The permanence of plants considered weeds, can contribute to the vegetation cover in areas with deficient pastures, in order to protect the soil (Campos et al., 2019).
Weeds can protect to the soil in degraded pastures, as the plants intercept the direct splash of rain promoting infiltration, increasing water retention and dissipating runoff (Lewis et al., 2013). Studies show lower losses of nutrients, such as N, P and K, in cultivation systems in presence of weeds (Lenka et al., 2017).
Regarding soil quality, the non-removal of weeds contributed positively to Ca and CEC. In addition, it raises the total organic carbon content by 0-0.03 m (Araujo-Junior et al., 2011). Therefore, manual weed control in areas with deficient pasture can be an efficient alternative for maintaining soil quality without the use of fire, which can promote deleterious changes in edaphic characteristics.
Due to the high use of fire in pasture systems in the Amazon studies of the fertility levels of these soils are necessary. In this sense, this study aimed to evaluate the effect of prescribed burning and mowing on vegetation cover of U. brizantha pastures and soil fertility in pastures located in the Brazilian Amazon.
MATERIALS AND METHODS
Site description
The study was conducted in Itupiranga county (05°08'20" S, 49°19'25" W), Pará state, which is part of the Brazilian Amazon. The soil is a Latossolo Vermelho-Amarelo (Embrapa, 2009), Ferralsols (FAO, 2015). The regional climate is Am in the transition to Aw (Köppen and Geiger, 1954). The region has an average annual temperature of 26.35 °C and has a dry season between May and October, and a wet season between November and April (Lisbôa, 2017).
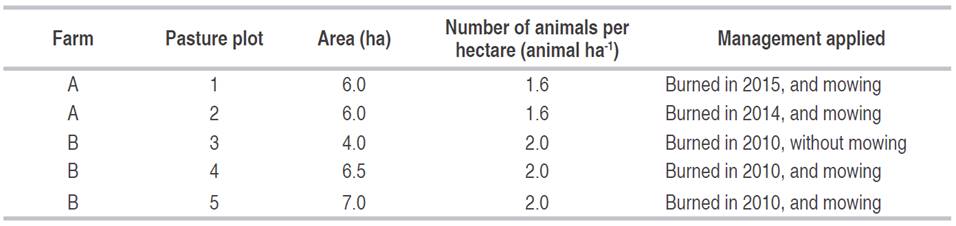
Five pasture plots were evaluated in two contiguous farms (A and B), as well as a native (original) forest fragment between them, as reference. In the areas where the pastures were located, a previous native forest was slashed and burned for pasture formation in 1993 and was seeded with U. brizantha cv. Marandu. The forest in question is classified as a land-based equatorial broadleaf forest. The pastures never received any type of improvement or fertilizer and only were subjected to slash-and-burn agriculture and mowing until the year 2015 (Table 1).
Table 1 Management applied in five areas of U. brizantha pasture in Itupiranga county, Pará state, Brazil.
Data were collected, and analyzed in 2015 during the dry season (July). Vegetation cover (U. brizantha, weeds, and bare soil, %) was estimated using a quadrat (Martha- Junior et al., 1999), which consisted of 2.0 m2 wooden square that contained a checkered mesh of string with 80 small squares. From each plot, 12 samples were recorded. Weeds were considered any other species than U. brizantha, including Eupatorium sp., Cenchrus sp., Cynodon sp., and Crotalaria sp.
Soil sampling and analysis
Soil samples were collected from 0-0.05 m under the soil surface. To cover as much as possible the entire area studied in each pasture area and in the forest area, four composite samples (obtained from three simple samples) were taken in each area (12 samples in each plot). Together with the pasture, soil samples from a natural (original) forest area that has never been altered were also collected, as a reference. In the laboratory, roots were manually removed from the soil samples before they were passed through a 2 mm sieve. The soil samples sieved were analyzed for: pH in water, in the ratio 1:2.5; Al3+, Ca2+, Mg2+ and Na+, extracted with KCl at 1 mol L-1, at the ratio 1:10; Al3+, by titration with 0.025 mol L-1 NaOH; Ca2+ and Mg2+ by atomic absorption spectrophotometry; Na+ by flame photometry; K+ and P available by extraction with Mehlich-1 (HCl 0.05 mol L-1 + H2SO4 0.0125 mol L-1) at the ratio 1:10; and H + Al, by Ca (OAc)2 at 0.5 mol L-1, adjusted pH 7.0, at the ratio 1:15, titrated with 0.0606 mol L-1 NaOH (Embrapa, 2011).
Statistical analysis
According to Monroe et al. (2016), Fontes et al. (2014), Rocha-Junior et al. (2014) and Lisbôa et al. (2016), the data was analyzed by ANOVA analysis. A randomized design with four replicates (four composite samples), which are considered as pseudo-replication in studies that involve data collection in the field. Each area (pastures and forest) was considered to be a fixed-effect treatment due to several sources of variation as manual control of weeds and burning.
The normality of the data was checked (Shapiro and Wilk, 1965). Also, a cluster analysis was conducted based on the vegetation cover data to identify groups in terms of pasture similarity. These data were subjected to analysis of variance (ANOVA) and a post hoc test (Tukey, P<0.05). The soil fertility data were combined based on the groups obtained by the cluster analysis and compared with the reference area (forest) by ANOVA and a post hoc test (Tukey, P<0.05). A principal component analysis (PCA) was performed to analyze relationships among the vegetation cover variables and soils fertility data. All the analyses were performed using R, version 3.0.1 (R Core Team, 2013).
RESULTS AND DISCUSSION
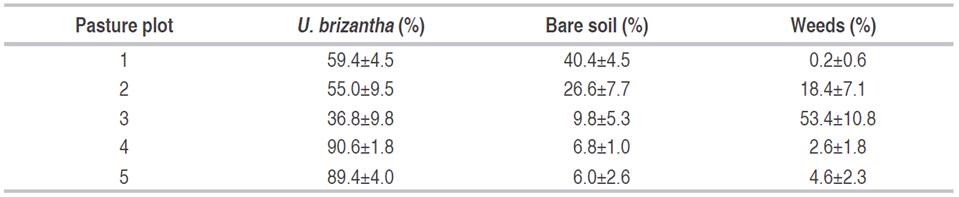
Descriptive statistics of the vegetation cover variables are presented in Table 2.The highest percentages of U. brizantha cover were presented in pastures of the plot 4 (90.6%) and the plot 5 (89.4%); the highest percentage of bare soil occurred in pasture of the plot 1 (40.4%) and the highest percentage of weed cover was 53.4% in pasture of the plot 3.
Table 2 Vegetation cover and bare soil percentage in five pastures in Itupiranga county, Pará state, Brazil.
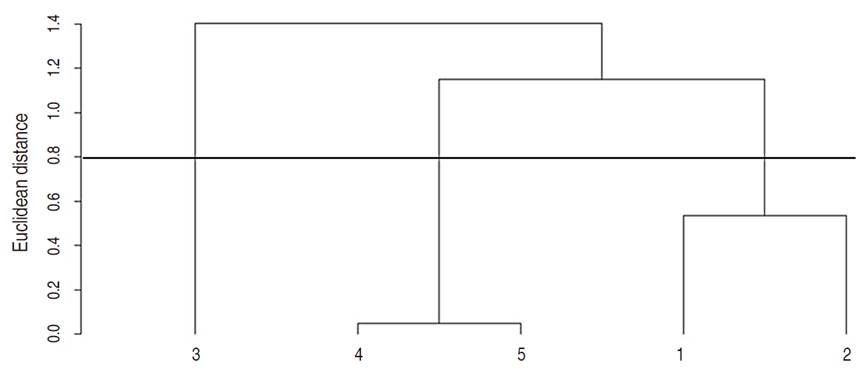
The vegetation cover variables were subjected to a cluster analysis based on Euclidean distances by the complete method. Three groups were identified from the plots evaluated (Figure 1): Group I consisted of pasture plots 1 and 2 (Farm A), Group II contained pasture plots 4 and 5 (Farm B), and Group III only contained pasture plot 3 (Farm B).
Vegetation cover data were combined based on their clusters and subjected to an ANOVA (Table 3). Group II had the highest average U. brizantha cover (90%), followed by Group I (57.2%) and III (36.8%). The absence of burning after 2010, and the practice of weed control, caused the high percentage of U. brizantha cover in Group II. The manual control of weeds in this group favored the domination by the species U. brizantha. The morphological and physiological characteristics of pastures such as a fibrous root system and C4 photosynthesis make them strong competitors, resulting in an important edaphic benefit protecting the soil surface and preventing moisture loss (Morris et al., 1993; Heringer and Jacques, 2002).
Table 3 Vegetation cover variables averages (%) in three groups of U. brizantha pasture in Itupiranga county, Pará state, Brazil.
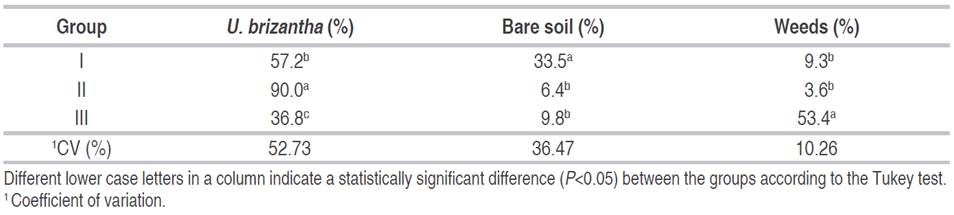
Group I had the highest average percentage of bare soil (33.5%), followed by Groups II (6.4%) and III (9.8%). Group I had the highest average percentage of bare soil because burning between 2014 and 2015 affected vegetation cover and caused an increase in bare soil. Burning is a quite common practice among Brazilian farmers since, theoretically, it increases soil fertility by adding nutrients from ash. However, this benefit is temporary because the soils are exposed to leaching by rainfall, triggering a low soil fertility and increase aluminum saturation, which affects plant growth (Heringer and Jacques, 2002; Santana et al., 2013).
Group III had the highest average weed cover (53.4%), followed by Group I (9.3%) and II (3.6%). Group III (only contained pasture plot 3) had the highest average weed cover because it received no weed control, as opposed to Santos et al. (2019), who considered mowing as an inefficient activity for weed control. In this study, this method proved to be effective, since Group I and Group II showed less weeds compared to Group III.
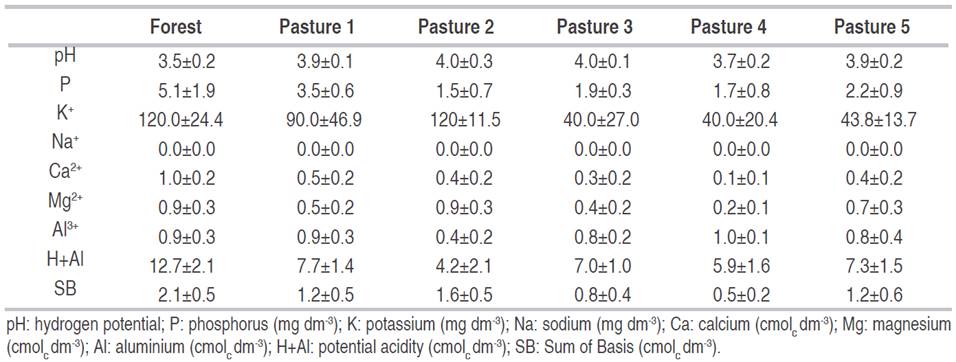
Table 4 shows the fertility data of five pastures and forest plots in the areas studied. Soil pH varied from 3.5 to 4.0. P varied from 1.5 mg dm-3 (pasture 2) to 5.1 mg dm-3 (forest). K+ varied from 120 mg dm-3 (forest) to 40 mg dm-3 (pasture 3 and 4). The highest value of Ca2+ was in forest soil (1.0 cmolc dm-3), and the smallest value was in pasture 4 soil (0.1 cmolc dm-3). The Mg2+ values varied from 0.2 cmolc dm-3 (pasture 4) to 0.9 cmolc dm-3 (forest and pasture 4). Al3+ was another element that presented values bellow 1.0 cmolc dm-3 and varied from 0.4 cmolc dm-3 (pasture 4) to 1.0 cmolc dm-3 (pasture 4). Higher values of H+Al were observed in forest soil (12.7 cmolc dm-3).
The soils of all studied areas presented low levels of fertility according to Prezotti and Guarçoni (2013) classification. Soil pH values were considered low and acid. The values of Al3+ were medium, whereas H+Al values were high. The soils also presented low values for the sum of bases, except forest which had median values. The soil under the pastures presented low values of K+, Ca2+, and Mg2+.
Zenero et al. (2016) found pH values in Amazon soils of 3.9, from 0 to 0.06 m of depth, which demonstrates this soil can be extremely acid. Soil pH is determined by the concentration of hydrogen ions (H+), and it is influenced by acid (H+, Al3+, Fe2+, or Fe3+) and base-forming cations (Ca2+, Mg2+, K+, and Na+) in the soil. Acidic conditions occur in regions with higher amounts of precipitation. High precipitation causes leaching of base-forming cations and decreasing of soil pH. Natural acidic soils are commonly found in forest soils (McCauley et al., 2017).
The action of fire on the chemical and physical properties of the soil, combined with the contribution of the nutrients present in the ash, may have contributed to the increase in pH and H+Al in the pasture areas, regarding the soil forest (original) (Giovannini et al., 1990; Iglesias et al., 1997; Zenero et al., 2016).
Regarding the Phosphorus content, the soil under forest showed better conditions in terms of Phosphorus availability for the crops, in relation to the soils under pasture (Prezotti and Guarçoni, 2013). These results are expected for Ferralsols, which present low fertility because of its source material, high weathering, removal of SiO2, and accumulation of Al2O3 in its mineral phase, with a predominance of kaolinite and oxides of iron and aluminum (Araujo-Junior et al., 2011).
Relating to the availability of P in soils, fire can have a great influence on the bond between P and clay minerals. Considering that the Mehlich-l extraction is based on the principle of dissolution of minerals containing P (Corrêa, 1993), studies relate to the retention of P in mineral surfaces with temperature are important. With that in mind, Ketterings et al. (2000) evaluated the temperature caused by fire over mineralogy and soil texture. The authors observed that the temperature by the fire influenced the clay, decreasing its quantity, mainly, by 0-5 cm, including the reduction of clay minerals such as gibbsite (Al(OH)₃). This mineral is important in the adsorption of P in the soil, and the decrease in that nutrient (P) is related to the decrease in the mineral (Al(OH)₃). Despite this, a dense canopy cover, as in the forest area, protects soil against loss of P as runoff or leaching.
The soil analysis data were combined into their respective groups and compared to each other and the forest area (Table 5). Soil pH value was smaller in forest soil (3.5) than the other pasture soils. There were no differences in Al3+ values between the areas. Forest soil presented the highest values for H+Al, P (5.12 mg dm-3), K+ (120 mg dm-3), Ca2+ (0.96 cmolc dm-3), Mg2+ (0.85 cmolc dm-3), and SB (2.12 cmolc dm-3).
Table 5 Soil fertility data of forest soil and three pastures groups, in Itupiranga county, Pará state, Brazil.
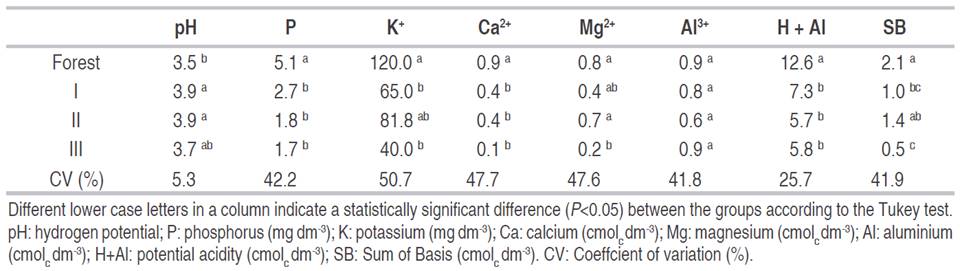
As it was demonstrated in Table 5, soil pH value was higher in pastures soils, along with ash deposition, which can contribute to raising pH values, this fact may be related to the loss of the OH groups of the clays, caused by the temperature rise by burning, which contributes to the formation of oxides of various elements derived from the rupture of carbonates (Giovannini et al., 1990). Also, the ashes from the fire, are mainly composed of Ca, Mg, K, Si, phosphates, and carbonates (CaCO3). In spite of the short benefit of ash reported by the literature, the low solubility of this mineral allows its persistence for more than three years after the fire, keeping the pH moderately alkaline in superficial horizons, in normally acidic soils (Iglesias et al., 1997).
The forest presented the highest values of macronutrients (P, K+, Ca2+, and Mg2+). This fact demonstrates the important role that trees play absorbing available nutrients from lower depths and distribute it to the soil surface via litterfall. Besides, as in the forest area, it takes up available nutrients from lower depths and redistributes them to the soil surface vialitterfall, the decomposition of straw on the soil surface can increase the availability of nutrients, favoring plants(Campos et al., 2019). Kautz et al. (2013) explained the nutrient accumulation in the Ap horizon as a turnover and long-term accumulation of nutrients acquired from the subsoil and translocated in the shoot and root systems, predominantly as a result of litter mineralization in the Ap horizon. As the forest area has a huge diversity of tree species, they can have roots at different depths, which means greater access to nutrients compared to pasture.
As discussed before, the absence of fire after 2010 until 2015 and the practice of weed control caused a high percentage of U. brizantha cover in Group II, and this can benefit vegetation growth. Roots can reach high depth as in U. brizantha, and the high rhizosphere extension can assist them in the access to water and nutrients and provide greater shoot growth (Kautz et al., 2013).
The PCA results are presented in Figure 2. Vegetation cover from the forest area was not collected. The values of Ca2+ and Mg2+ were not included in this analysis to avoid multicollinearity, instead of that, they were summed as Ca2++Mg2+, for the same reason, the sum of basis was not included (Hair et al., 2005).
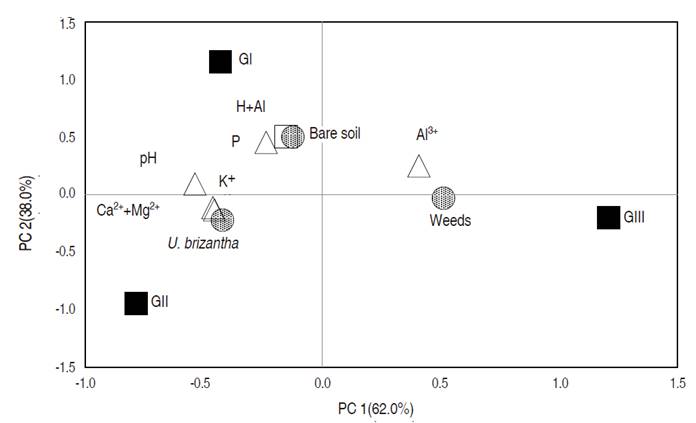
Figure 2 Principal component analysis of vegetation cover and soil fertility in U. brizantha pastures in Itupiranga county, Pará state, Brazil.
Principal component 1 (PC 1) explained 62.0% of the variability, and principal component 2 (PC 2) explained 38.0%. Group I, which had a high percentage of uncovered soil, was associated with the values of H+Al and P. This demonstrates that these soils have low natural fertility, which can be aggravated by burning pastures. With the most recent use of fire, 2014 and 2015, the low growth of forage in the pastures of this group indicates that, perhaps, this area has not yet recovered, with less soil coverage. In turn, the lack of cover can result in the worsening of soil quality, due to the lack of nutrient cycling, accumulation of nutrients and organic matter and leaching of nutrients. In addition, the lack of liming and soil fertilization, also aggravate the issue of acidity (Kautz et al., 2013; Santos et al., 2015).
Group II, where later burning occurred in 2010, there was the highest U. brizantha cover, and less weeds, was better related to exchangeable soil bases (Ca2++Mg2+, and K+). According to Crespo et al. (2015), an important way of nutrients entry in pasture soils is the litter. The biomass of pasture roots can contribute to the nutrients deposition in these systems; after this, nutrients return to the soil, and subsequently recycled.
PCA results for Group III had a better association for weeds and Al3+. This group presented the highest aluminum values and the lowest sum of base values. Under these conditions, cultivable plants have severe restrictions on their establishment, which may decrease their presence in the area, increasing the presence of weeds. In the absence of soil correction, with the lime application, acidification causes an increase in species regarded as agricultural weeds, and a reduction in productivity (Goulding, 2016). Weed species identified in the present study are commonly found in soils with low fertility and high acidity (Gazziero et al., 2006; Brighenti et al., 2010; Moreira and Bragança 2011; and Costa el al., 2011).
CONCLUSIONS
There was similarity between the pasture areas, reducing the five pastures to three groups. The highest percentage of uncovered soil was observed in Group I, which was associated with more recent pastures burning.
The highest percentage of U. brizantha was observed in Group II, which was favored by the later burning, in relation to the other pastures, and with the practice of mechanical weed removal. This group had better levels of K, Mg and sum of bases.
As expected, the highest percentage of weeds was observed in Group III, where there was no control for their proliferation. This group had a greater relationship with the high levels of Al3+ in the area.
Adopting the least use of fire on the pastures combined with the mechanical control of weeds, can be the best alternative for maintaining the soil cover and bringing benefits for quality surface layer of the soil.