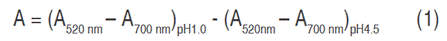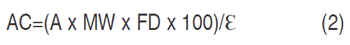Mortiño berry belongs to Ericaceae family and Vaccinium genus. This fruit is called Andean berry or wild berry because it grows spontaneously in the Andean region from 3,400 to 3,800 masl (Prencipe et al., 2014). There is a growing interest on Andean berries due to the useful bioactive phytonutrients that they contain; Vaccinium floribundum is also known as "superfruit" due to its high content of phenolic compounds, predominantly quercetin, hydroxycinnamic acids and cyanidant-3-glucosides (C3G) that support its great antioxidant power (Vizuete et al., 2016). In vitro studies showed an inhibitory effect on the accumulation of lipids, adipogenesis, and anti-inflammatory mediators of phenolic extracts from Mortiño (Schreckinger et al., 2010). On the other hand, a research with other berries has shown the positive effect of phenolic compounds also found in Vaccinium floribundum, for the prevention of diseases such as cancer, diabetes and hyperlipidemia in vitro, in vivo and epidemiological studies (Kianbakht et al., 2014).
The main effect of antioxidants is the capacity of scavenge free radicals. Compounds that are easier to oxidize are often the best antioxidants, since they can donate electrons or hydrogen atoms to reactive free radicals (Castañeda et al., 2009).
High concentrations of anthocyanins are found in the outer layer of mortiño (Skrovankova et al., 2015). These pigments, family of flavonoids are formed by an anthocyanidin aglycone and a sugar. The chemical form of the anthocyanins consists of two aromatic rings A and B linked by a 3-carbon chain; the variation of R1 and R2 groups in B ring originates different anthocyanins with different colors (Trouillas et al., 2016). An increase in hydroxylation produces a blue color while an increase in methoxylation turns to red. The most abundant anthocyanidins could be listed as: Pelargonidin (orange), Cyanidin (orange red), Delphinidin (red), Peonidin (orange red), Petunidin (red) and Malvidin (bluish-red) (Garzón, 2008).
Anthocyanins are soluble in alcohols, acetone, dimethyl sulfoxide and water, due to their polar character. Choosing an extraction method always seeks to maximize the recovery of the pigment, prevent its degradation and reduce the contaminants. Most of the time in aqueous solution anthocyanins could be found in three different states. In pH 1-3 the most abundant form is red flavylium cations, around pH 4-5 the specie is colorless, and in alkaline solution the tautomerized molecule generates a yellow color (Lila et al., 2016).
Anthocyanin rich extracts have been widely investigated for its use as natural dyes (Khoo et al., 2017; Mojica et al., 2017). Furthermore, the food color market is expected to grow from 10 to 15% each year, and the mean trend in this industry is the use of natural colorants in order to produce "clean-labeled" food (Cortez et al., 2017). Antiproliferative and antioxidant properties of anthocyanins have also proved promising results for functional product development. However, anthocyanins are unstable and susceptible to the degradation in presences of factors such as pH, light, temperature, oxygen, solvents, metal ions, among others (Diaconeasa et al., 2015).
Due to the great number of industrial applications and health benefits of these colored compounds, despide the problems of stability derived from their obtaining and purification process. Therefore, the aim of this study was to identify the best conditions to extract anthocyanins from Vaccinium floribundum without affecting the antioxidant capacity of these polyphenols.
MATERIALS AND METHODS
Standards and Chemicals
TPTZ (PubChem CID: 77258; 98%) and Trolox (PubChem CID: 40634; 97%) were purchased from SIGMA; ABTS (PubChem CID: 16240279; 98%) was purchased from ROCHE; absolute ethanol (PubChem CID: 702) was purchased from PHARMCO; methanol (PubChem CID: 887; 99.9%), ferric chloride hexahydrate (PubChem CID: 24810; 100.5%), potassium chloride (PubChem CID: 4873; 99.8%) and sodium acetate trihydrate (PubChem CID: 23665404; 99.9%) were purchased from FISCHER SCIENTIFIC; potassium persulfate (PubChem CID: 24412; 98%) was purchased from LOBACHEMIE; hydrochloric acid (PubChem CID: 313; 37%) was purchased from MERCK.
Sample preparation
Mortiño (Vaccinium floribundum) was purchased at "La Kennedy Market" in Quito, Ecuador. The fruits were chosen in good condition, without surface damage, and a maturity degree between four and five (fruits between purple and dark purple) (Buitrago et al., 2015). These berries were processed in Ultramaxx processor to form a paste, which was then stirred and homogenized using an Ultra-Turrax at 13,000 rpm.
Extraction and quantification of anthocyanins
Approximately 10 g of the paste were weighed in analytical balance (Mettler Toledo, Model AB204 S, 250±0.001) and placed in an Erlenmeyer flask (125 mL) (Oancea et al., 2012). 40 mL of the solvent were added and the mixture was stirred for 20 s using a Magic Clamp Universal Platform, and it was placed on the stove (Binder, Model ED 56), for 4 h at 30 °C and 60 °C according to the experimental design. Then it was filtered using ash-less filter paper (Macherey Nagel MN 640 w, with diameter 125 mm), to obtain the anthocyanin extract.
The treatments were arranged in a CRD with factorial model (23), corresponding to the combination of solvent (methanol and ethanol), concentration (20 and 60%) and temperature (30 and 60 °C). Three repetitions were performed, with a total of 24 experimental units. Ethanol and methanol were acidified with hydrochloric acid (37%) until reaching pH 1 (Garzón, 2008).
The treatments were: E1 (Ethanol 20%, 30 °C), E2 (Ethanol 20%, 60 °C), E3 (Ethanol 60%, 30 °C), E4 (Ethanol 60%, 60 °C), M1 (Methanol 20%, 30 °C), M2 (Methanol 20%, 60 °C), M3 (Methanol 60%, 30 °C), M4 (Methanol 60%, 60 °C).
The quantification of anthocyanins was performed according to the pH differential method (AOAC-2005.02, 2019). For absorbance, Genesys 10 UV Thermospectronic spectrophotometer was used. Samples at pH 4.5 and pH 1 were measured both 520 and 700 nm. A blank sample was also measured (each buffer without the addition of the extract) and final absorbance was calculated with Equation 1:
The concentration of monomeric pigments in the extract was expressed in mg of C3G per 100 g of mortiño (Kuskoski et al., 2005). It was calculated based on the volume of extract and sample weight (Equation 2):
Where:
AC: anthocyanins concentration; A: absorbance; MW: molecular weight; FD: dilution factor; Ɛ: molar absorptivity (MW: 449.2 g mol-1; molar absorptivity coefficient, Ɛ:26,900).
Anthocyanin extracts were protected from light and frozen (-18 °C ) for further analysis. To thaw the sample, it was placed in refrigeration at 4 °C for 24 h.
The data were subjected to analysis of variance (ANOVA) and means were assessed by Tukey test (P<0.05), with Minitab Statistical Software (2018).
Antioxidant capacity of mortiño
To determine the antioxidant capacity the FRAP method and the ABTS method were applied. A CRD with factorial model 22 (solvents: methanol and ethanol; temperatures: 30 and 60 °C) and 3 repetitions for each method. The concentration of both solvents was constant (20%). The data were subjected to analysis of variance (ANOVA) and means were assessed by Tukey test (P<0.05), with Minitab Statistical Software (2018).
The FRAP reagent was prepared by mixing 2.5 mL of TPTZ solution (0.0312 g of TPTZ compound with 40 mM HCl), 2.5 mL of 20 mM FeCl3-6H2O (0.1352 g of FeCl3-6H2O solubilized in 25 mL of distilled water) and 25 mL of 0.3 mM acetate buffer pH 3.6 (0.0061 g of sodium acetate in 200 mL of distilled water). The pH was adjusted to 3.6 with 40 mM HCl and the volume was completed up to 250 mL with distilled water.
The results were expressed in Trolox equivalent (μmol TE 100 g-1 FW), after elaborating a calibration curve of this compound (Trolox, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), which is a water-soluble analogous of vitamin E widely used in the expression of antioxidant capacity studies (Ozgen et al., 2006). The absorbance was read at 570 nm using a MRX Microplate Reader equipment (Dynex Technologies).
The calibration curve by the FRAP method showed the following data: the regression equation y=0.0169X-0.0329; coefficient of determination R2 =0.9990.
The treatments of this stage of the research were: FE1 (ethanol, 30 °C), FE2 (ethanol, 60 °C), FM1 (methanol, 30 °C), FM2 (methanol, 60 °C). The concentration of both solvents was constant (20%).
The cationic radical ABTS is a chromophore generated by an oxidation reaction of ABTS (2,2'-azino-bis-(3-ethyl benzthiazolin-6-sulfonate ammonium)) at a concentration 7 mM with potassium persulfate (2.45 mM) (1:1), incubated at room temperature (25 °C ) and in the dark for 16 h. Once the radical ABTS•+ was formed, it was diluted with ethanol 96% v/v until to obtain an absorbance value of 0.700±0.100 at 730 nm (maximum absorption wavelength) (Cano et al., 2000).
The calibration curve by the ABTS method showed the following data: the regression equation y=1.6928X-0.5273; coefficient of determination R2=0.9964.
For the determination of antioxidant capacity of anthocyanins extracts, 10 μL of the diluted sample with 96% ethanol (1:20) reacted with 990 μL of ABTS solution and the same procedure indicated above was followed. ABTS values were expressed as µmol TE 100 g-1 FW (Garzón et al., 2010).
The treatments were: AE1 (ethanol 20%, 30 °C), AE2 (ethanol 20%, 60 °C), AM1 (methanol 20%, 30 °C), AM2 (methanol 20%, 60 °C).
RESULTS AND DISCUSSION
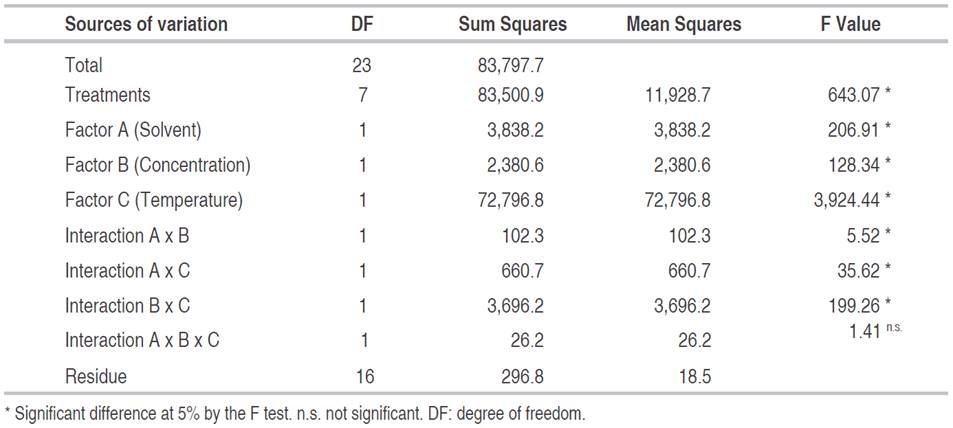
The influence of the solvent type, concentration and temperature on the anthocyanin content determined by differential pH method is showed in Table 1. In the same way, the two-way interactions of the factors influenced the response variable (P<0.05). The coefficient of variation was 1.64%, less than 5%, which means a correct development of the experiment procedure and a good control of the extrinsic variation (Condo and Pazmiño, 2015).
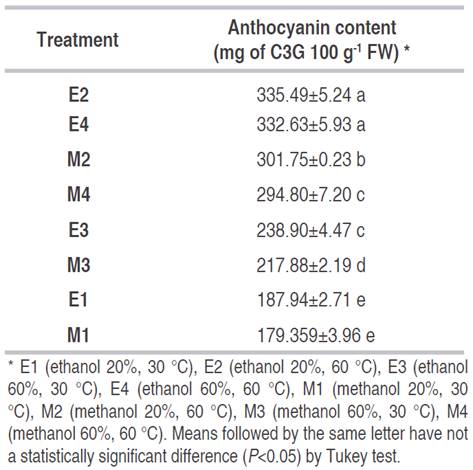
The highest concentration of anthocyanins (Table 2) was obtained (335.49 and 332.63 mg C3G 100 g-1 FW) using ethanol as solvent (20 and 60%) at 60 °C (treatments E2 and E4, respectively). There was no significant difference between the mentioned treatments (P<0.05). Other Vaccinium floribundum studies showed concentrations similar and higher than those obtained in this investigation. Vasco et al. (2009) reported 345 mg of C3G 100 g-1 FW after wetting 0.6 g of lyophilized sample and extracting four times with ethyl acetate (10 mL), and quantification by HPLC. In addition, Garzón et al. (2010) found 329.0±28.0 mg of C3G 100 g-1 FW by the differential pH method working with fresh berry powder sprayed with liquid nitrogen and frozen at 70 °C and extracted with 100% methanol for three times until the solution became colorless. On the other hand, 376.2±49.9 mg of C3G 100 g-1 FW was analyzed by HPLC after the extraction of anthocyanins from the frozen mortiño, using dynamic maceration with 0.6 M HCl in methanol (Prencipe et al., 2014).
Significantly, the temperature increased the amount of anthocyanins extracted from the berries, especially when the solvent was ethanol (Table 2). The best concentration of anthocyanins could be extracted at 60 °C, due to the heat damage of the cellular wall of mortiño outer layer, improving the pigment transference (Gavahian et al., 2018). The same tendency was reported by other studies (Marquez et al., 2014; Spigno et al., 2007). Also, the temperature increment accelerated the diffusion rate and improved the solubility of the required biomolecules.
On the other hand, the possible thermal degradation of anthocyanins can also occur. This effect was not found in this study because the protocol avoided extreme temperatures and times. The thermal degradation of anthocyanin is a first order reaction. Half-life time calculated in anthocyanin concentrates was 16.7 h at 60 °C (Wang and Xu, 2007). In the present research, 60 °C was the maximum temperature used for 4 h. Therefore, low thermal damage was expected. The best solvent in the extraction process was ethanol (Table 2). Other authors also reported the best anthocyanin extraction with ethanol solution at 70% (Fu et al., 2016; Pedro et al., 2016).
The amount of extracted compounds depends on the solvent polarity and the solubility of the substratum (Metrouh-Amir et al., 2015). The hydroethanolic solutions in different concentrations are the most popular options for anthocyanin extraction (Fu et al., 2016). As presented in Table 2, the treatments with ethanol at 20% (E2) and 60% (E4) concentration did not show a significant difference (P>0.05). At 60 °C, the concentration of ethanol did not significantly influence the extraction process. However, a higher concentration of anthocyanins was obtained using methanol at 20%.
The anthocyanin structures have different behaviors in the extraction medium. Flavonols and anthocyanins are soluble in polar solvents, while the glucosides are more soluble in water and the aglycones more soluble in alcohols (Pérez-Gregorio et al., 2010). The solubility of anthocyanins in polar solvents is due to the hydroxyl groups and sugars present in their structure; therefore, they are commonly extracted with methanol or ethanol or with a mixture (Welch et al., 2008). Ethanol has a polarity index of 4.3, methanol of 5.1 and water of 10. Consequently, a better extraction performance with ethanol (the best extraction treatments) could be explained by a lower polarity of the extraction medium (Gupta et al., 1997). In addition, the hydrophobic characteristic of anthocyanins is given by the polyphenolic structure allowing their solubility in organic solvents such as ethanol and methanol (Khoo et al., 2017).
The temperature and polarity of the solvent could affect the antioxidant capacity of the extracts. The difference in antioxidant activity due to the solvents could be explained by the different metabolites extracted depending on their polarity. Compounds with the same polarity are solubilized while the solvent penetrates through the solid phase (Muhamad et al., 2014).
Despite temperature accelerates mass transfer and free more antioxidant compounds, principally anthocyanins that increase the antioxidant power of the concentrate, temperatures higher than 50 °C possibly decompose the heat sensitive antioxidative compounds in more than 8 h of exposition (Spigno et al., 2007). Degradation of anthocyanidin with a 0-dihydroxyl substitution such as cyanidin, delphinidin and petunidin, which are the most susceptible to oxidation, can significantly reduce the antioxidant power (Castañeda-Ovando et al., 2009). However, in the present study, time and temperature combination avoided such problems.
The influence of the type of solvent, extraction temperature and interaction of both factors (P<0.05) on the antioxidant capacity of the extracts was observed with the two methodologies used (Table 3). For both cases, the coefficient of variation was less than 5%, which means a correct development of the experiment and a good control of the extrinsic variation.
Table 3 Analysis of variance of the antioxidant capacity of the treatments by FRAP and ABTS methods.
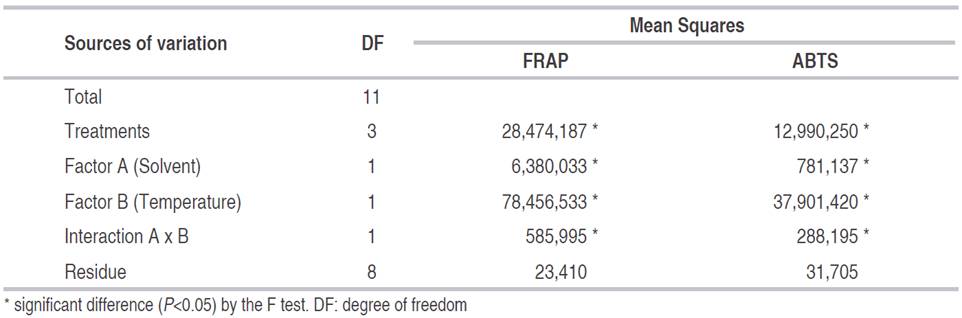
In Table 4, the best antioxidant capacity (19,653.3±256.62 µmol TE 100g-1) was determined with the FRAP method using 60 °C and ethanol at 20% as solvent (treatment FE2). This concentration was higher than 16,140 µmol TE 100 g-1 reported by Gaviria et al. (2009) and Moyer et al. (2002) with values between 12,000 and 15,000 µmol TE 100 g-1. In the ABTS method, the treatments AM2 (methanol, 60 °C) and AE2 (ethanol, 60 °C) had the highest antioxidant capacity (8,458.3±127.45 and 8,258±325.05 µmol TE 100 g-1, respectively), being significantly different from the rest of the treatments (P<0.05). Other study found similar results 8,694±435 µmol TE 100 g-1 (Gaviria et al., 2009). In addition, the higher extraction temperature did not affect the antioxidant capacity since at 60 °C higher values were obtained than at 30 °C (Table 4).
The amount of anthocyanin extracted from Vaccinium floribundum and its antioxidant capacity were greater than in other fruits of the same family, for instance, Vaccinium corymbosum (blueberry) most widely studied and available in other regions (Lee and Wrolstad, 2004).
The inactivation of reactive oxygen species can occur by various routes. The application of different methodologies to evaluate antioxidant activity will explain the mechanisms by which bioactive compounds such as anthocyanins act (Montoya et al., 2012). It is recommended to analyze the antioxidant capacity with at least two methods to achieve more reliable results (Boeing et al., 2014). According to this, in this work, the antioxidant capacity was determined by ABTS and FRAP methods, obtaining by this latter method a greater antioxidant capacity in all treatments. Pearson’s correlation between the two methodologies in this study was 0.898 indicating a highly positive correlation. That is close to results found by other authors 0.94 (Dudonné et al., 2009) and 0.9877 (Montoya et al., 2012).
To analyze the relationship between antioxidant capacity and anthocyanin concentration, the Pearson coefficient was calculated, obtaining the following data: Pearson Coefficient of FRAP vs Anthocyanin Content: 0.973; Pearson Coefficient of ABTS vs Anthocyanin Content: 0.952. Both values represent high positive correlation between these two quantitative variables.
CONCLUSIONS
The highest content of anthocyanins was obtained under extraction conditions of 60 °C with 20 and 60% hydroethanolic solvent solution. The highest antioxidant capacity was also obtained when highest extraction temperature (60 °C) was used, indicating that the thermal effect after 4 h of extraction did not affect the antioxidant power of anthocyanins and this moderate temperature could increase the diffusion rate and solubility without damage of the compounds. The treatment extracted with hydroethanol solution (20%) presented the highest antioxidant capacity by the FRAP method, while by ABTS method with methanol and ethanol treatments at the same concentration (20%), there was not statistical difference between both treatments (AM2 and AE2). The highest temperature tested (60 °C) maximized the extraction of anthocyanins and did not affect the antioxidant activity. As a result, ethanol at 20 and 60%, 60 ºC for 4 h are the best conditions to extract and quantify the anthocyanins of the mortiño fruit.