Mango (Mangifera indica L.) is one of the five tropical fruits with the highest consumption worldwide (Caballero et al., 2015). Tommy Atkins is the most exported variety in Ecuador with 65% of total mango exports. Tommy Atkins mango has high resistance to handling; therefore, it has potential for the development of minimally processed products (Chiumarelli et al., 2011).
Refrigerated minimally processed mango is a good option on the market; and it responds to the need of a modern world where less time is available to prepare food (González-Aguilar et al., 2008). Industrialization of mango could contribute to the development of Ecuadorian agroindustry, through the creation of small and medium-sized enterprises (Cedeño and Cerón, 2018), and its contribution to GDP, which currently represents 7%, and is lower compared to Colombia (10%), Chile (13%), and Uruguay (12.4%) (Fiallo, 2017).
Postharvest mango losses vary between 20 and 50% (Dávila, 1998; Singh et al., 2013). The most common problems of mango agroindustry are weight loss, mechanical damage and attack of bacteria and fungi. Physicochemical properties such as color, firmness, among others, are affected by metabolic disorders caused by cutting (Tovar et al., 2001). Therefore, it is necessary to use techniques to preserve the quality attributes of minimally processed mango.
Fresh and minimally processed fresh-cut products are naturally contaminated by microorganisms of several sources, including the farm environment, post-harvest handling and processing (Abadias et al., 2008). The microflora associated with raw fruits mostly includes yeasts and moulds (Burnett and Beuchat, 2000; Tournas, 2005).
Edible coatings are an alternative to preserve the quality and freshness of minimally processed products and prolong their shelf-life. The application of coatings creates a semipermeable gas and water vapor barrier that reduces the speed of respiration and dehydration of the coated products and creates conditions similar to foods subjected to modified atmospheres (Chiumarelli et al., 2011). The most common polymers in the preparation of edible coatings are proteins, polysaccharides and lipids. Among the polysaccharides, cassava starch has been widely used due to its availability and relative low cost (Santacruz et al., 2015; Souza et al., 2012; Kampeerapappun et al., 2007; Flores et al., 2007). Edible coatings based on only polymers or in combination, have been applied to different fruits, e.g., cassava starch in fresh-cut pineapple (Bierhals et al., 2011), cassava starch and citric acid in fresh-cut Tommy Atkins mango (Chiumarelli et al., 2010), mixtures of starch and chitosan in guava (Bezerra et al., 2015), ascorbic acid and N-acetyl-cysteine in bananas (Palacín, 2012), and modified cassava starch in tomato (Hernández et al., 2011).
Chitosan has achieved considerable interest in the industry due to its biodegradability, biocompatibility and non-toxicity properties (Dash et al., 2011). Chitosan solutions exhibit good coating formation capacity and antimicrobial activity, making them potentially useful for antimicrobial biopolymer development (Dutta et al., 2009). The antimicrobial effect of chitosan could be the result of changes in cell permeability produced by the electric charge of chitosan (Devlieghere et al., 2004), molecular weight and degree of deacetylation (Zheng and Zhu, 2003). The pH and type of acid where chitosan is dissolved, as well as storage conditions may also influence antimicrobial properties (Begin and Van Calsteren, 1999; Leceta et al., 2013).
Edible coatings can be used with food additives acting against enzymatic browning, microbial growth and texture loss. The use of essential oils (EO) or active ingredients of essential oils such as carvacrol, carvone, cinnamaldehyde, citral, p-cimene, eugenol, limonene, menthol and thymol have been particularly prominent because they extend the shelf-life of food (Sung et al., 2013). Perdones et al. (2012) reported minimal changes in the physicochemical and microbiological characteristics of strawberries coated with chitosan and lemon EO for 15 days in storage at 4 °C compared to uncoated fruits or fruits coated only with chitosan. Another additive used together with edible coatings is salicylic acid. It delays the ripening of fruits, probably due to the inhibition or action of ethylene biosynthesis (Srivastava and Dwivedi, 2000). Salicylic acid has been used to control the aging by cooling of pears (Asghari et al., 2007), strawberries (Babalar et al., 2007), grapes (Asghari et al., 2009) and fresh-cut Sindrhi mangoes (Moradinezhad, 2020). There are no studies of the use of cassava starch with the addition of either cinnamaldehyde or thymol for the preservation of fresh-cut Tommy Atkins mango.
The present work aimed to study the use of edible coatings based on cassava starch together with salicylic acid, cinnamaldehyde and thymol, as well as chitosan, for the preservation of fresh-cut Tommy Atkins mango stored in refrigeration conditions. Analyses of instrumental texture (penetration force), titratable acidity, color and microbiological analysis (fungi and yeast) were performed on stored mango.
MATERIALS AND METHODS
The chitosan (molecular weight 149 kDa, deacetylation degree 95%) was donated by the Public University of Navarra (Pamplona, Spain). Tommy Atkins mangoes were purchased at a local market in the city of Manta, Ecuador. Mangoes with a degree of ripening of two (Báez, 1998) were selected according to the size and without damage.
The selected mangoes were washed, manually peeled and cut into 8.0x1.5 cm slices. These slices were immersed in the corresponding coating solution (chitosan, C; starch+salicylic acid, SSA; starch+cinamaldehyde+thymol, SCT) and dried at room temperature (approx. 25 °C). Mango samples with no coating were used as control samples. The mango slices (approx. 100 g) were then placed on polyurethane trays and coated prior to storage at 8 °C and 90% relative humidity.
Coating preparation
Coating based on either starch or chitosan were prepared by the casting technique. The chitosan coating was prepared using a chitosan solution 1% (w/v), using citric acid solution 1% (w/v) as solvent. Tween 20 at 1% (w/v), glycerol 0.5% (w/v) and glucose 0.5% (w/v) were added to the solution before homogenization with an ultraturrax (Politron, Switzerland) at 11,000 rpm for 4 min.The starch coating was prepared according to Santacruz et al. (2015). A solution of cassava starch 0.5% (w/v) was heated to 90 °C for 5 min. Tween 20 at 1% (w/v), glycerol 0.5% (w/v) and salicylic acid 2 mmol L-1 were added to the hot solution. Once the solution reached room temperature, glucose 0.5% (w/v), cinnamaldehyde 0.15% (w/v) and thymol 0.15% (w/v) were added. Finally, the solution was homogenized as described previously.
Physical-chemical characterization
Weight loss. It was calculated by weighting the fruit at 0 day and after each storage time. Measurements were performed in triplicate and the results were reported as percentage of weight.
Instrumental texture. Penetration analyses were performed according to Castro et al. (2014). Analyses were performed using a Shimadzu texturometer (EZ LX Model, Japan). A stainless-steel probe of 3 mm diameter and 8 cm length was used. The probe was introduced into the fruit at 15 mm depth with a velocity of 10 mm s-1. The maximum force (penetration force) resulting from three measurements was reported.
Soluble solids. The fruit was disintegrated using a domestic blender, followed by a filtration on a piece of cloth. The filtered juice was analyzed by a digital refractometer (KRÜSS, Germany) according to the AOAC method (1990), the results of three measurements were reported as °Brix.
Titratable acidity. Titratable acidity was determined by titration with 0.01 M NaOH solution according to the AOAC method (1984), the results of three measurements were reported as percentage of citric acid.
Color analysis. The color of mango pulp was determined using a Konic Minolta (Japan) colorimeter in a L*, a*, b* scale. Color measurements were expressed based on the chromaticity parameters a* (green [-], red [+]) and b* (blue [-], yellow [+]). Measurements were made in triplicate.
Microbiological analysis. Fungi and yeasts counting were performed on mango samples at 0, 4, 8, and 12 days of storage. 10 g of sample were used to mix with 90 mL of KCl solution 0.1% (w/v). The inoculum was prepared by mixing 1 mL of the previous solution with 9 mL of distilled water. Counting of fungi and yeasts were made according to NOM-111-SSA1-1994 (Norma Oficial Mexicana, 1994). Three repetitions were performed for each sample.
Statistical analysis. The results were analyzed by means of ANOVA and a Tukey test, using a significance of 5% by the statistical package InfoStat, Professional Version 2016. Measurements of the previous analyses were performed in triplicate along 12 days of storage.
RESULTS AND DISCUSSION
Instrumental texture. The results of the instrumental texture revealed that the maximum penetration force decreased for coated and uncoated mango samples along the 12 days of storage with values ranging from 6.0 to 0.48 N (Figure 1).
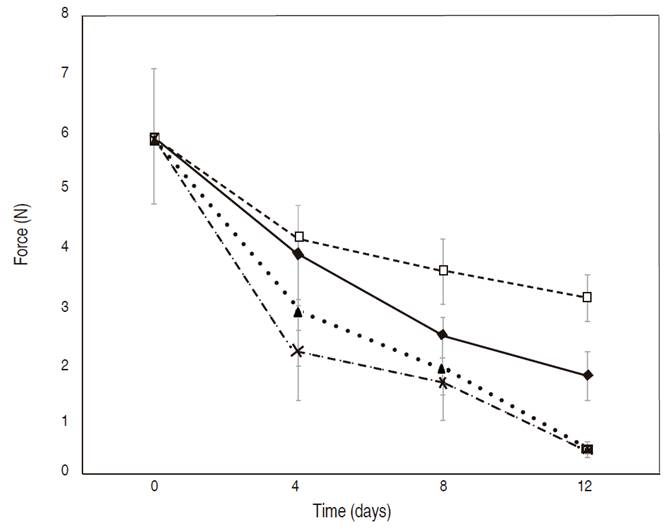
Figure 1 Maximum penetration force on fresh-cut Tommy Atkins mango with and without edible coating for 12 days at 8 °C and 90% relative humidity. □ chitosan (C), ▲ cassava starch+ salicylic acid (SSA), X cassava starch+cinamaldehyde+thymol (SCT) and ♦ uncoated
There was no statistically significant difference in penetration force between the samples at 4 day of storage. However, after 8 and 12 days of storage, penetration force was higher for samples coated with chitosan, while the lowest penetration force was recorded for mango samples coated with salicylic acid (SSA) and cinamaldehyde+thymol (SCT). The penetration force for the chitosan-coated (C) mango sample is maintained probably due to the reduced respiration rate (Cissé, 2015). Similar results were found by Zhu et al. (2008), using chitosan at different concentrations in 'Tainong' cv mangoes.
Weight loss. There was no difference on the weight loss of both samples coated with starch (SSA and SCT) during the whole storage time (P<0.05). Uncoated sample had the lowest weight loss followed by samples coated with chitosan. Castro et al. (2017) showed lower weight loss for papayas coated with chitosan and uncoated fruits compared to papayas coated with starch. The presence of compounds like cinnamaldehyde, thymol or salicylic acid into the coating, could accelerate a weight loss by osmotic dehydration (Vega et al., 2007).
Soluble solids. The results showed no significant difference in soluble solids content between the uncoated and coated mango samples along storage (P<0.05). Samples had a soluble solids content of approximately 9.5 °Brix at 0 day and after 12 days of storage had a value of 8.5 °Brix (Table 1). A decrease in soluble solids content was found by Bueno et al. (2005), in minimally processed pineapple stored at 5 °C. The reduction of soluble solids is probably due to the respiratory process which may lead to high consumption of organic substrates, i.e. sugars (Kader et al., 2002).
Table 1 Titratable acidity, soluble solids and weight of fresh-cut Tommy Atkins mango coated with either chitosan (C) or starch+salicylic acid (SSA), starch+cinnamaldehyde+thymol (SCT), stored for 12 days at 8 °C and 90% relative humidity.
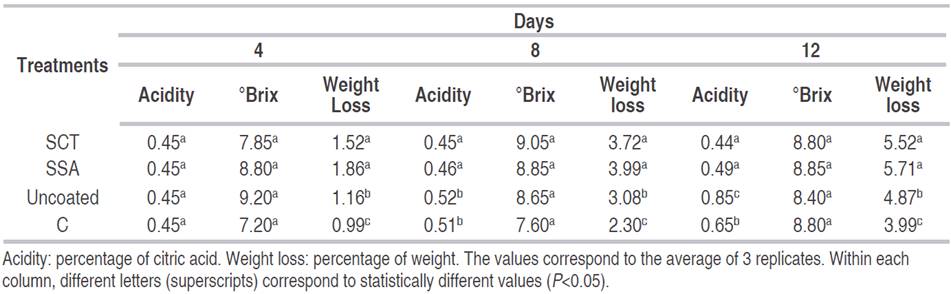
Titratable acidity. The results showed that fruits coated with chitosan and uncoated samples presented a significant difference compared to samples treated with SSA and SCT (Table 1). Acidity values were higher for uncoated sample with value of 0.45% for 0 day, reaching 0.85% for 12 day, followed by chitosan treatment with 0.45% for 12 day and 0.65% for 12 day of storage. The lowest values were for samples treated with salicylic acid, which presented values of 0.45% for 0 day and 0.49% for 12 day. Samples treated with cinamaldehyde+thymol, showed acidities of 0.45% for 0 day and 0.44% for 12 day of storage.
High values of titratable acidity of uncoated sample may be due to the production of organic acids by growing of microorganisms (Russo et al., 2014). Similar behavior was found by Bueno et al. (2005) for minimally processed pineapple stored at 5 °C. Low values of titratable acidity for fruits treated with starch edible films is another indicator of ripening, as organic acids are also used during respiration (Freire et al., 2005). Additionally, starch edible coating may reduce the level of O2 inside the packages of mango slices, leading to minimal effect on the physical and chemical changes of mango during storage in low O2 atmospheres (Freire et al., 2005; Rathore et al., 2007). The chitosan treatment showed greater values of acidity in relation to the other treatments throughout the storage period. Chitosan films are more selectively permeable to O2 than to CO2, maintaining the conditions of the coated fruit similar to an uncoated sample and promoting the production of metabolites that lead to an increase of acidity (Kweon et al., 2001).
Color.Table 2 shows the results of color of mango slices during storage. There was not statistically difference for L*, a* and b* values between coated and uncoated samples. The only exception was the L* value of the sample treated with SSA after 12 days of storage, which was smaller and statistically different to the other samples. At those days of storage, L* value decreased by 10% for SSA samples and by 7.9% for SCT samples, while the uncoated sample increased 9%. The decrease in the L* value means that mango pulp became less bright during storage. There was not difference between a* and b* values among samples.
Table 2 Changes in L*, a* and b* values of fresh-cut Tommy Atkins mango coated with C, SSA or SCT, stored for 12 days at 8 °C and 90% relative humidity.
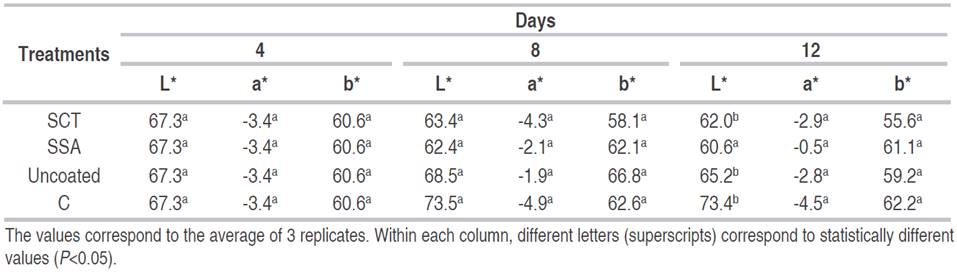
Other authors (Robles et al., 2013) used antioxidant edible coatings for Kent mango cubes. They found at the end of storage that coated samples lost only 2.5% of the initial L* value compared to 7% loss in samples with no edible coating. Edible coatings based on polysaccharides and antioxidants have been used to delay browning in freshly cut apples maintaining L* values throughout storage (Lee et al., 2003; Fontes et al., 2008). Chiumarelli et al. (2011) reported that cassava starch coatings offer effective maintenance of color characteristics in cut mango samples due to the combined effect of the coating and citric acid. Although edible polysaccharide coatings such as starch are a good gas barrier (Dussan et al., 2014), in the present study, SSA or SCT did not achieve an effective browning delay.
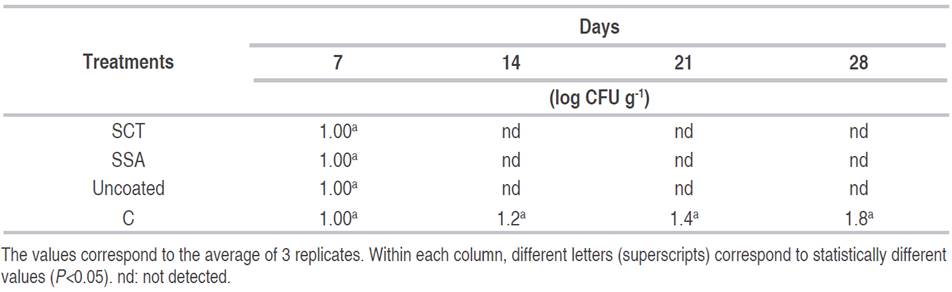
Microbiological analysis. The results of the microbiological analysis (Table 3) show that all the treatments inhibited the development of fungi and yeasts in comparison with the uncoated samples, which showed an increase in the colonies from 1 log CFU g-1 at 0 day to 1.8 log CFU g-1 at 12 day of the study. Studies show chitosan at concentrations lower than 1% affects the sporulation of Botrytis cinerea and Penicillium expansum (Liu et al., 2007). The effect of chitosan on the germination of Rhizopus stolonifer spores has been previously reported at concentrations ranging from 1 to 2 mg mL-1 (Hernández et al., 2007; Hernández et al., 2008). Besides, Badawy and Rabea (2009) reported that chitosan applied in concentrations of 2 to 4 mg mL-1 can control Botrytis cinerea infections in tomato fruits. Bautista et al. (2003) showed that chitosan coatings control anthracnose in papaya fruits and inhibits the growth of fungi such as Fusarium oxysporum, R. stolonifer, Penicillium digitatum and C. gloeosporioides at 3% (Bautista et al., 2003; Bautista et al., 2004). The efficacy of cinamaldehyde in inhibiting the growth of fungi of the genera Aspergillus and Penicillium has been demonstrated by several authors. López et al. (2007a) found that P. islandicum and A. flavus were completely inhibited by cinamaldehyde-fortified in vapor phase. Tunc et al. (2007) found that cinamaldehyde is one of the strongest growth inhibitors of Penicillium notatum. Antimicrobial polypropylene films incorporating 2% cinamaldehyde also showed complete inhibition of A. flavus, Penicillium comuna, P. expansum, Penicillium nalgiovense, Penicillium roqueforti, and P. islandicum (López et al., 2007b). Plotto et al. (2003) reported that carvacrol, thymol, and citral compounds showed inhibition of mycelial growth of Botrytis cinerea, Alternaria arborescens, and Rhizopus stolonifer. Essential oils may affect stages of fungal development such as germination of spores and development of mycelium.
CONCLUSIONS
The use of edible chitosan-based coatings reduces textural and weight loss changes in mango slices stored in refrigeration, compared to coated samples with starch and uncoated samples. There was no difference in soluble solids between coated and uncoated samples during the whole storage, whereas differences in color were observed after the 8 day. The starch-based coatings reduce the changes of acidity during storage. Chitosan and starch-based coatings inhibits the growth of fungi and yeasts on mango slices. Further studies could examine the solubilization of chitosan in other acids, as well as sensory analysis of coated samples for consumer acceptability